Abstract
Background:
Primary hyperparathyroidism (PHPT) is characterized by inappropriately elevated serum parathyroid hormone (PTH) level despite elevated serum calcium. Insulin resistant is the basic pathophysiology, behind the higher prevalence of diabetes mellitus in patients with PHPT. However, the improvement in insulin resistance (IR) after curative parathyroidectomy (CPTX) has not been established yet, as the study results are conflicting.
Materials and Methods:
In this prospective interventional study, ten patients with mild PHPT (Group 1) and another ten patients with moderate to severe PHPT (Group 2) were undergone CPTX. The IR was assessed by homeostasis model assessment-IR (HOMA-IR), quantitative insulin sensitivity check index (QUICKI), fasting plasma glucose (FPG), and fasting serum insulin (FSI), before and 3 months after CPTX.
Results:
There was no significant change of FPG and FSI, before and after CPTX in Group 1 (P = 0.179 and P = 0.104) and Group 2 (P = 0.376 and P = 0.488). Before surgery, HOMA-IR was higher, and QUICKI was significantly lower, in both Group 1 (P = 0.058 and P = 0.009) and Group 2 (P = 0.023 and P = 0.005) as compared to published normal reference mean, with no significant difference between the groups. Three months after surgery HOMA-IR increased further and QUICKI remained unchanged as compared to baseline, in both Group 1 (P = 0.072 and 0.082) and Group 2 (P = 0.54 and 0.56), but statistically insignificant.
Conclusion:
IR remained unchanged after CPTX in mild as well as moderate to severe PHPT. Asymptomatic PHPT with abnormal IR should not be used as criteria for parathyroidectomy.
Key words: Homeostasis model assessment-insulin resistance, insulin resistance, parathyroidectomy, primary hyperparathyroidism, quantitative insulin sensitivity check index
INTRODUCTION
Primary hyperparathyroidism (PHPT) is characterized by inappropriately elevated serum parathyroid hormone (PTH) level despite elevated serum calcium.[1] Classically, PHPT was described as disease of “bones, stones, abdominal groans, psychic moan, and fatigue overtones”.[2] But nowadays with the use of routine biochemical testing, more and more asymptomatic patients of PHPT are being diagnosed.[2] Besides hypercalcemia-related complications, PHPT is also associated with increased risk of cardiovascular disease, hypercoagulable state, and metabolic syndrome.[3,4,5] High prevalence of diabetes mellitus (DM) in patients with PHPT is well established [6,7] and higher prevalence of PHPT in patients with DM has also been demonstrated.[8] Insulin resistant is the basic pathophysiology behind the DM in PHPT. However, the improvement of insulin resistance (IR) after curative parathyroidectomy (CPTX) has not been established yet as the study results are conflicting. There are few studies, which demonstrated the improvement of IR after CPTX in patients with PHPT.[9,10] But at the same time, few studies showed that there was no improvement in IR and metabolic risk factors after CPTX in patients with PHPT.[11,12,13] The present study was designed to observe the effect of CPTX on peripheral IR in patients with PHPT of varying severity.
MATERIALS AND METHODS
Study design and study population
In this prospective interventional study, 20 patients with PHPT of varying severity were recruited consecutively, irrespective of their age and gender. Ten patients with mild PHPT (Group 1) were studied at Henry Ford Hospital, Detroit, Michigan, USA. Another ten patients with moderate to severe PHPT (Group 2) were studied at Post Graduate Institute of Medical Education and Research, Chandigarh, a tertiary care center in northern India. Patients with mild PHPT were asymptomatic, whereas the patients with moderate to severe PHPT were symptomatic, had higher PTH, calcium level and had evidence of bone disease on X-rays and/or nephrolithiasis.[14] Patients with established diagnosis of DM, family history of DM in first-degree relatives and taking medications known to affect carbohydrate metabolism were excluded from the study. The study was conducted between February 2008 and October 2009. The respective institutional review and ethical committee approved the study and written informed consent was obtained from each patient.
Method
PHPT was diagnosed by the presence of serum albumin-adjusted hypercalcemia along with inappropriately raised or nonsuppressed serum PTH level, on at least two occasions. The reference range for calcium and intact PTH (iPTH) level was 8.4–10.2 mg/dl and 10–75 pg/ml, respectively. All patients underwent CPTX as per NIH guideline.[14,15] The clinical and biochemical diagnosis of PHPT was confirmed by surgery, followed by histopathological examination of the parathyroid gland. All patients were re-evaluated 3 months after CPTX. Cure of PHPT was defined as normalization of serum calcium and >50% reduction.
The IR was assessed by two well-established methods of estimating IR by homeostasis model assessment of IR (HOMA-IR) and insulin sensitivity by quantitative insulin sensitivity check index (QUICKI). HOMA-IR and QUICKI were measured in all patients at baseline and 3 months after CPTX.
In all patients, fasting serum insulin (FSI) and plasma glucose were measured, 7 days prior and 3 months after surgery from the same sample. Fasting plasma glucose (FPG) was measured by glucose oxidase/glucose hexokinase by Dimension Vista System (Siemens, USA) and FSI (reference value <14 µIU/ml) was measured by chemiluminiscence assay (Centaur XP, Siemens, USA). HOMA-IR was calculated by using following formula, HOMA-IR= (FSI [µIU/ml] × FPG [mmol/L])/22.5.[16,17] QUICKI was calculated by using following formula, QUICKI = 1/(log [I0] + log [G0]), where I0 is the fasting insulin, and G0 is the fasting glucose.[18] The reference normal value for HOMA-IR and QUICKI were 2.06 ± 0.14 (0.7–6.5) and 0.382 ± 0.007, respectively.[17,18]
Serum iPTH, 25(OH) Vitamin D (Vitamin D) were measured by chemiluminescence assay and serum albumin, calcium; creatinine was measured in the institute laboratory by standard methods. Unfortunately, Vitamin D could not be measured after surgery in the Indian patients because of the financial constraints.
Statistical analysis
All continuous data were expressed as mean ± standard deviation unless otherwise specified. Data analysis was performed using Statistical Package for the Social Sciences (SPSS) software, version 13 (IBM). Outcomes were analyzed by change of IR and insulin sensitivity before and after surgery. The data were checked for skewness. Paired t-test, ANOVA followed by post hoc multiple comparisons were used where applicable and a P < 0.05 was set as statistically significant.
RESULTS
Baseline data of study population
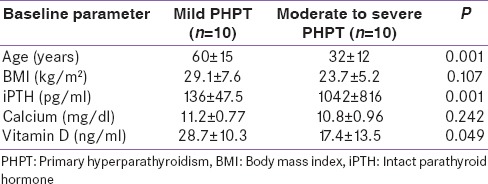
Relevant demographic, biochemical parameter of mild (Group 1) and moderate to severe (Group 2) PHPT groups are compared in Table 1. There were two men in the mild (1:4) and 4 men in the moderate to severe PHPT group (2:3). All patients had a single parathyroid adenoma, except one patient in Group 2 had a double adenoma. Indian patients with moderate to severe PHPT were younger (P = 0.001), had lower body mass index (P = 0.107) and higher iPTH level (P = 0.001) as compared to the American patients with mild PHPT [Table 1]. However, serum calcium (P = 0.242) and Vitamin D (P = 0.049) were lower in Indian patients as compared to American patients [Table 1]. Among the patients with moderate to severe PHPT, seven patients had nephrolithiasis and two had fracture bone as compared to none of the patients with mild PHPT.
Table 1.
Baseline data of the study population
Following surgery, serum Calcium (Ca2+) was normalized in all patients. iPTH was normalized in patients with mild PHPT and reduced by >50% in Indian patients with moderate to severe PHPT (P < 0.001), but remained higher than normal after 3 months [Table 2]. Serum Vitamin D also improved significantly (P = 0.012), after CPTX in the US patients with mild PHPT (28.7 ± 10.3 vs. 37 ± 12.21 ng/ml).
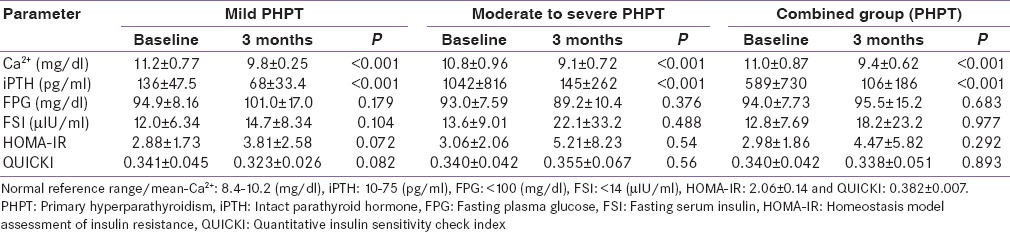
Table 2.
Parameters before and after parathyroidectomy
Insulin resistance
FPG was similar before and after surgery in mild and moderate to severe PHPT [Table 2]. There was no significant difference in FPG between the groups before (P = 0.59) and after (P = 0.085) CPTX. There was no significant (P = 0.683) difference in FPG of the combined group before and 3 months after CPTX [Table 2]. FSI was also similar before and after CPTX in mild as well as in moderate to severe PHPT [Table 2]. FSI was higher preoperatively in moderate to severe PHPT as compared to mild PHPT (P = 0.669) and increased postoperatively in both the groups (P = 0.27), but could not reach to statistical significance [Table 2]. FSI did not differ significantly (P = 0.977) in the combined group before and after CPTX [Table 2].
Homeostasis model assessment of insulin resistance
Before surgery HOMA-IR was higher, both in patients with mild PHPT (P = 0.058) and with moderate to severe PHPT (P = 0.023) as compared to published normal reference mean (2.06 ± 0.14),[17] with no significant difference (P = 0.84) between the groups [Table 2]. Three months after CPTX HOMA-IR increased further in all the groups, with no significant difference within and between the groups [Table 2]. Consequently, the postoperative HOMA-IR was also significantly higher than normal reference mean for the mild (P < 0.001) and moderate to severe (P = 0.004), as well as in the combined PHPT group (P < 0.01) [Figure 1 and Table 2]. Although HOMA-IR was numerically higher in patients with moderate to severe PHPT as compared to mild PHPT, both before and after CPTX, the difference did not reach statistical significance [Table 2].
Figure 1.
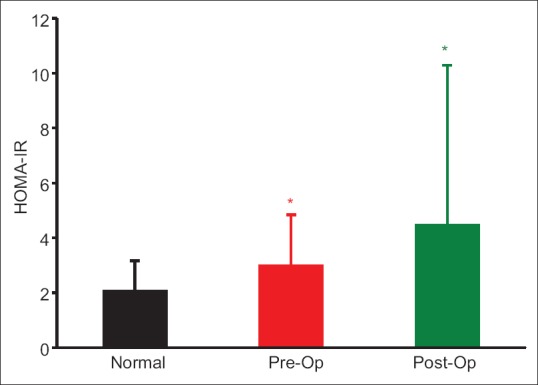
Comparison of homeostasis model assessment of insulin resistance of combined group (primary hyperparathyroidism) with the normal reference mean (2.06 ± 0.14), (FNx01P < 0.01). There was no significant difference between the pre- and post-operative homeostasis model assessment of insulin resistance (P = 0.292)
Quantitative insulin sensitivity check index
Before surgery QUICKI was significantly lower both in mild (P = 0.009) and moderate to severe (P = 0.005) PHPT groups, as compared to published normal reference mean (0.382 ± 0.007),[18] with no significant difference (P = 0.96) between the groups [Table 2]. Three months after CPTX, QUICKI almost remained unchanged in the mild (P = 0.082), moderate to severe (P = 0.56), and combined PHPT groups (P = 0.893) [Table 2]. Postoperative QUICKI was significantly lower than normal reference mean in the mild (P < 0.001) and combined PHPT groups (P < 0.001) but not in the patients with moderate to severe PHPT (P = 0.132) [Figure 2]. There was no difference in QUICKI between the mild and moderate to severe PHPT group before and after CPTX (P = 0.307).
Figure 2.
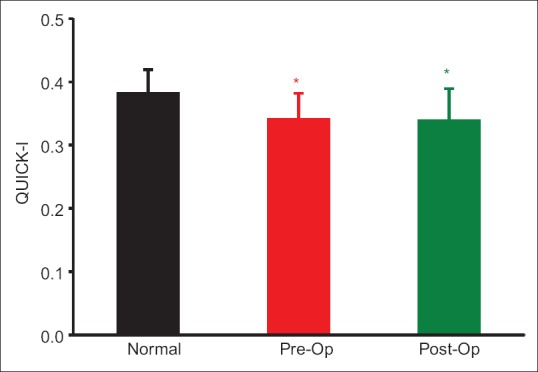
Comparison of quantitative insulin sensitivity check index of the combined group (primary hyperparathyroidism) with the normal reference mean (0.382 ± 0.007), (FNx01P < 0.01). There was no significant difference between the pre- and post-operative quantitative insulin sensitivity check index (P = 0.893)
DISCUSSION
Exact pathophysiology of IR in PHPT has not well established yet. The proposed pathogenetic factors and mechanisms responsible for the IR in PHPT are hypercalcemia, high PTH, low phosphate, and body weight.[19,20,21,22,23] Most likely the disturbance in carbohydrate metabolism is caused by interplay of all these three biochemical abnormalities.[19,20,21,22,23] Hypercalcemia stimulates insulin secretion, impairs peripheral insulin sensitivity, and suppression of gluconeogenesis.[24,25,26] In addition, there is also reduced binding of insulin to its peripheral receptors due to receptor downregulation.[27] In animal models, PTH administration has been shown to cause a reduction in ATP content in pancreatic islets leading to impaired insulin release.[28] Hypophosphatemia in PHPT is also known to cause impaired peripheral glucose utilization and decreased insulin responsiveness.[21,22]
This study also showed hyperinsulinemia in the presence of hypercalcemia in both group of PHPT. Postoperatively, serum calcium normalized but serum insulin levels did not change significantly at 3 months. According to our study serum, calcium level did not correlate with HOMA-IR and QUICKI, before and after CPTX, as observed in previous studies.[20,29] According to our study, PTH level reduced significantly after CPTX, but it did not correlate with serum insulin, HOMA-IR, and QUICKI, pre- or post-operatively in either group. The studies regarding the effect of PTH on hyperinsulinemia and IR are conflicting.[30,31] In our study postoperatively, insulin level increased, and IR either increased numerically or remained unchanged. However, these did not achieve statistical significance, even though the PHT level decreased significantly after CPTX.
Like the previous study,[32] our study also showed that HOMA-IR was significantly higher in patients with mild and moderate to severe PHPT, compared to normal reference mean (P < 0.01). But at the same time, few study showed that HOMA-IR remained unchange compared to baseline values even after 18 months without parathyroidectomy.[33] Kautzky-Willer et al.[34] assessed insulin sensitivity in PHPT, before and 3 months after CPTX by oral and intravenous glucose tolerance test. Even after 1 year of surgery, there was no improvement in insulin sensitivity. In contrast to our study, they showed decreased fasting blood glucose, serum insulin. These contrasting results may be due small sample size, degree of hypercalcemia in patients (mean calcium 12 mg/dl) and use of intravenous and oral glucose tolerance test to assess insulin sensitivity.
However, the study results are conflicting regarding the improvement of IR after CPTX. Few studies revealed the improvement of IR after CPTX in patients with PHPT.[9,10]
According to this study, there is IR in patients with mild as well as moderate to severe PHPT in the absence of DM, as reported previously.[6,7,19,34] In addition, we also demonstrated that there is no significant improvement in HOMA-IR or QUICKI following CPTX, either in mild or in moderate to severe PHPT. According to our study, severity of IR also does not correlate with the severity of PHPT or PTH level. The therapeutic role of CPTX in symptomatic patients with moderate to severe PHPT is well established but at the same time, there is some doubt regarding role of CPTX in asymptomatic patients with mild PHPT.[14] At presents, the patients with asymptomatic mild PHPT undergo CPTX as per NIH guideline.[15,35] The significant clinical implication of this study is, asymptomatic mild PHPT with abnormal glucose metabolism should not be used as criteria for CPTX. Further study with larger sample size and longer duration of follow-up is needed, for better evaluation of the role of parathyroidectomy for improvement of IR in patients with PHPT.
Limitation
The major limitation was, small sample size and short period follow-up. A longer duration of follow-up may be needed for better evaluation of improvement in IR.
CONCLUSION
Peripheral IR (HOMA-IR) and insulin sensitivity (QUICKI) remained unchanged after CPTX in mild as well as moderate to severe PHPT. Both the HOMA-IR and QUICKI do not correlate with the severity of PHPT or PTH level. Asymptomatic mild PHPT with abnormal glucose metabolism should not be used as criteria for CPTX. However, further study is needed for the confirmation of our study result.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Shah VN, Bhadada S, Bhansali A, Behera A, Mittal BR. Changes in clinical & biochemical presentations of primary hyperparathyroidism in India over a period of 20 years. Indian J Med Res. 2014;139:694–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Mundy GR, Cove DH, Fisken R. Primary hyperparathyroidism: Changes in the pattern of clinical presentation. Lancet. 1980;1:1317–20. doi: 10.1016/s0140-6736(80)91783-3. [DOI] [PubMed] [Google Scholar]
- 3.Erem C, Kocak M, Hacihasanoglu A, Yilmaz M, Saglam F, Ersoz HO. Blood coagulation, fibrinolysis and lipid profile in patients with primary hyperparathyroidism: Increased plasma factor VII and X activities and D-Dimer levels. Exp Clin Endocrinol Diabetes. 2008;116:619–24. doi: 10.1055/s-2008-1065365. [DOI] [PubMed] [Google Scholar]
- 4.Procopio M, Barale M, Bertaina S, Sigrist S, Mazzetti R, Loiacono M, et al. Cardiovascular risk and metabolic syndrome in primary hyperparathyroidism and their correlation to different clinical forms. Endocrine. 2014;47:581–9. doi: 10.1007/s12020-013-0091-z. [DOI] [PubMed] [Google Scholar]
- 5.Mendoza-Zubieta V, Gonzalez-Villaseñor GA, Vargas-Ortega G, Gonzalez B, Ramirez-Renteria C, Mercado M, et al. High prevalence of metabolic syndrome in a mestizo group of adult patients with primary hyperparathyroidism (PHPT) BMC Endocr Disord. 2015;15:16. doi: 10.1186/s12902-015-0014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor WH. The prevalence of diabetes mellitus in patients with primary hyperparathyroidism and among their relatives. Diabet Med. 1991;8:683–7. doi: 10.1111/j.1464-5491.1991.tb01678.x. [DOI] [PubMed] [Google Scholar]
- 7.Khaleeli AA, Johnson JN, Taylor WH. Prevalence of glucose intolerance in primary hyperparathyroidism and the benefit of parathyroidectomy. Diabetes Metab Res Rev. 2007;23:43–8. doi: 10.1002/dmrr.637. [DOI] [PubMed] [Google Scholar]
- 8.Taylor WH, Khaleeli AA. Prevalence of primary hyperparathyroidism in patients with diabetes mellitus. Diabet Med. 1997;14:386–9. doi: 10.1002/(SICI)1096-9136(199705)14:5<386::AID-DIA362>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Cvijovic G, Micic D, Kendereski A, Milic N, Zoric S, Sumarac-Dumanovic M, et al. The effect of parathyroidectomy on insulin sensitivity in patients with primary hyperparathyroidism – An never ending story? Exp Clin Endocrinol Diabetes. 2015;123:336–41. doi: 10.1055/s-0035-1549906. [DOI] [PubMed] [Google Scholar]
- 10.Ajala O, Thondam S, Khaleeli A. Insulin resistance before and after parathyroidectomy in primary hyperparathyroidism. Endocr Abstr. 2009;19:120. [Google Scholar]
- 11.Rudman A, Pearson ER, Smith D, Srivastava R, Murphy MJ, Leese GP. Insulin resistance before and after parathyroidectomy in patients with primary hyperparathyroidism – A pilot study. Endocr Res. 2010;35:85–93. doi: 10.3109/07435801003724503. [DOI] [PubMed] [Google Scholar]
- 12.Ishay A, Herer P, Luboshitzky R. Effects of successful parathyroidectomy on metabolic cardiovascular risk factors in patients with severe primary hyperparathyroidism. Endocr Pract. 2011;17:584–90. doi: 10.4158/EP10321.OR. [DOI] [PubMed] [Google Scholar]
- 13.Bhadada SK, Bhansali A, Shah VN, Rao DS. Changes in serum leptin and adiponectin concentrations and insulin resistance after curative parathyroidectomy in moderate to severe primary hyperparathyroidism. Singapore Med J. 2011;52:890–3. [PubMed] [Google Scholar]
- 14.Eigelberger MS, Cheah WK, Ituarte PH, Streja L, Duh QY, Clark OH. The NIH criteria for parathyroidectomy in asymptomatic primary hyperparathyroidism: Are they too limited? Ann Surg. 2004;239:528–35. doi: 10.1097/01.sla.0000120072.85692.a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Consensus development conference statement. J Bone Miner Res. 1991;6(Suppl 2):S9–13. doi: 10.1002/jbmr.5650061406. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 18.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 19.Prager R, Schernthaner G, Niederle B, Roka R. Evaluation of glucose tolerance, insulin secretion, and insulin action in patients with primary hyperparathyroidism before and after surgery. Calcif Tissue Int. 1990;46:1–4. doi: 10.1007/BF02555816. [DOI] [PubMed] [Google Scholar]
- 20.Ljunghall S, Palmér M, Akerström G, Wide L. Diabetes mellitus, glucose tolerance and insulin response to glucose in patients with primary hyperparathyroidism before and after parathyroidectomy. Eur J Clin Invest. 1983;13:373–7. doi: 10.1111/j.1365-2362.1983.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Lang R. Hypophosphatemia and glucose intolerance: Evidence for tissue insensitivity to insulin. N Engl J Med. 1980;303:1259–63. doi: 10.1056/NEJM198011273032203. [DOI] [PubMed] [Google Scholar]
- 22.Marshall WP, Banasiak MF, Kalkhoff RK. Effects of phosphate deprivation on carbohydrate metabolism. Horm Metab Res. 1978;10:369–73. doi: 10.1055/s-0028-1093393. [DOI] [PubMed] [Google Scholar]
- 23.Grey AB, Evans MC, Stapleton JP, Reid IR. Body weight and bone mineral density in postmenopausal women with primary hyperparathyroidism. Ann Intern Med. 1994;121:745–9. doi: 10.7326/0003-4819-121-10-199411150-00003. [DOI] [PubMed] [Google Scholar]
- 24.Prager R, Kovarik J, Schernthaner G, Woloszczuk W, Willvonseder R. Peripheral insulin resistance in primary hyperparathyroidism. Metabolism. 1983;32:800–5. doi: 10.1016/0026-0495(83)90110-5. [DOI] [PubMed] [Google Scholar]
- 25.Saxe AW, Gibson G, Gingerich RL, Levy J. Parathyroid hormone decreases in vivo insulin effect on glucose utilization. Calcif Tissue Int. 1995;57:127–32. doi: 10.1007/BF00298433. [DOI] [PubMed] [Google Scholar]
- 26.Shaw JH, Croxon M, Holdaway I, Collins JP, Wolfe RR. Glucose, fat, and protein kinetics in patients with primary and secondary hyperparathyroidism. Surgery. 1988;103:526–32. [PubMed] [Google Scholar]
- 27.Prager R, Schernthaner G, Kovarik J, Cichini G, Klaushofer K, Willvonseder R. Primary hyperparathyroidism is associated with decreased insulin receptor binding and glucose intolerance. Calcif Tissue Int. 1984;36:253–8. doi: 10.1007/BF02405326. [DOI] [PubMed] [Google Scholar]
- 28.Perna AF, Fadda GZ, Zhou XJ, Massry SG. Mechanisms of impaired insulin secretion after chronic excess of parathyroid hormone. Am J Physiol. 1990;259(2 Pt 2):F210–6. doi: 10.1152/ajprenal.1990.259.2.F210. [DOI] [PubMed] [Google Scholar]
- 29.Yasuda K, Hurukawa Y, Okuyama M, Kikuchi M, Yoshinaga K. Glucose tolerance and insulin secretion in patients with parathyroid disorders. Effect of serum calcium on insulin release. N Engl J Med. 1975;292:501–4. doi: 10.1056/NEJM197503062921003. [DOI] [PubMed] [Google Scholar]
- 30.Harter HR, Santiago JV, Rutherford WE, Slatopolsky E, Klahr S. The relative role of calcium, phosphate and parathyroid hormone in glucose and tolbutamide mediated insulin release. J Clin Invest. 1976;58:359–67. doi: 10.1172/JCI108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fadda GZ, Akmal M, Premdas FH, Lipson LG, Massry SG. Insulin release from pancreatic islets: Effects of CRF and excess PTH. Kidney Int. 1988;33:1066–72. doi: 10.1038/ki.1988.112. [DOI] [PubMed] [Google Scholar]
- 32.Procopio M, Magro G, Cesario F, Piovesan A, Pia A, Molineri N, et al. The oral glucose tolerance test reveals a high frequency of both impaired glucose tolerance and undiagnosed Type 2 diabetes mellitus in primary hyperparathyroidism. Diabet Med. 2002;19:958–61. doi: 10.1046/j.1464-5491.2002.00809.x. [DOI] [PubMed] [Google Scholar]
- 33.Ayturk S, Gursoy A, Bascil Tutuncu N, Ertugrul DT, Guvener Demirag N. Changes in insulin sensitivity and glucose and bone metabolism over time in patients with asymptomatic primary hyperparathyroidism. J Clin Endocrinol Metab. 2006;91:4260–3. doi: 10.1210/jc.2005-2825. [DOI] [PubMed] [Google Scholar]
- 34.Kautzky-Willer A, Pacini G, Niederle B, Schernthaner G, Prager R. Insulin secretion, insulin sensitivity and hepatic insulin extraction in primary hyperparathyroidism before and after surgery. Clin Endocrinol (Oxf) 1992;37:147–55. doi: 10.1111/j.1365-2265.1992.tb02299.x. [DOI] [PubMed] [Google Scholar]
- 35.Bilezikian JP, Potts JT, Jr, Fuleihan Gel H, Kleerekoper M, Neer R, Peacock M, et al. Summary statement from a workshop on asymptomatic primary hyperparathyroidism: A perspective for the 21st century. J Clin Endocrinol Metab. 2002;87:5353–61. doi: 10.1210/jc.2002-021370. [DOI] [PubMed] [Google Scholar]


