Abstract
Introduction:
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders in women. PCOS comprises a broad spectrum of anomalies, including hyperandrogenism, chronic anovulation, obesity, and infertility. Insulin resistance and its compensatory hyperinsulinemia play a key role in the pathogenicity of PCOS. This study compares the effects of 2 types of insulin sensitizer drugs, metformin and pioglitazone, on clinical, metabolic, and endocrine characteristics of women with PCOS.
Methods:
In this randomized clinical trial, 56 women with PCOS (ages 20–49 years) were treated orally with either metformin (500 mg 3 times daily) or pioglitazone (30 mg daily) for 3 months. Clinical (body weight, blood pressure [BP], and body mass index) and laboratory indices (fasting blood sugar [FBS], serum triglyceride [TG], cholesterol, low-density lipoprotein, high-density lipoprotein, insulin, testosterone, and dehydroepiandrosterone [DHEA]) were measured before and after therapy. Data were analyzed by Chi-square and McNemar's tests.
Results:
Significant decreases were seen after treatment with metformin in extent of hair loss (P = 0.008), wrist circle (P = 0.011), weight (P = 0.047), diastolic BP (P = 0.023), and DHEA (P = 0.035). A significant decrease in TG was seen with pioglitazone treatment (P = 0.047). In both groups, significant decreases in acne, menstrual disturbance, FBS, and serum insulin were seen.
Conclusion:
There is a significant amelioration of endocrine and metabolic indices with pioglitazone in PCOS patients. Although we were not able to recommend one treatment regime over the other, pioglitazone offers a useful, alternate treatment in women with PCOS who are not able to tolerate metformin.
Key words: Insulin resistance, metformin, pioglitazone, polycystic ovary syndrome
INTRODUCTION
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders, affecting 5–10% women of reproductive age.[1] PCOS is a heterogeneous condition characterized by chronic anovulation, hyperandrogenism, ovarian dysfunction, and polycystic ovaries.[2] PCOS is the most common cause of female infertility and is also associated with increased risks of diabetes mellitus, cardiovascular disorders, and endometrial cancer.[3] Although not yet fully understood, insulin resistance and its compensatory hyperinsulinemia are thought to play a key role in the pathogenicity of PCOS.[2,3] The incidence of insulin resistance in PCOS is substantially higher in weight- and age-matched women without PCOS [4] and is a consistent feature of PCOS in both normal and overweight women. Insulin resistance also appears to play a significant role in the pathogenesis of the hyperandrogenism and infertility of PCOS.[3,5] Women with anovulation, hyperinsulinemia, and hyperandrogenism are at greater risk of developing diabetes with an age of onset 30 years earlier than in the general population.[6] Hyperinsulinemia increases the risk of cardiovascular disease both directly and by its impact on lipid metabolism.[5,7] Early control of blood glucose, insulin, and lipids, before the onset of menopause, is therefore of importance.[8]
Medications which improve insulin sensitivity offer new therapeutic approaches to the treatment of PCOS.[4,6] These medications include the oral antidiabetic agents such as metformin and pioglitazone. Although both medications reduce insulin resistance, they differ in their mechanisms of action.[9] Metformin is a biguanide, which decreases hepatic glucose production, circulating insulin and intestinal glucose absorption, and improving peripheral tissue utilization of glucose. Pioglitazone is a peroxisome proliferator-activated receptor gamma agonist which enhances the ability of muscles to metabolize glucose and improves insulin sensitivity without hypoglycemia or alteration of body fat distribution.[6,7,8,9,10,11] However, few studies are available that compared the clinical and metabolic effects of these medications. In this study, we evaluated the clinical and metabolic effects of metformin versus pioglitazone among women with PCOS.
Side effects of pioglitazone include mild to moderate edema, weight gain, minor reductions in hemoglobin and hematocrit, myalgia, and temporary increases in liver enzymes.[8,11] Metformin is generally well-tolerated but occasionally causes gastrointestinal side effects including vomiting, abdominal bloating, diarrhea, and nausea.[6,7,10]
METHODS
This randomized clinical trial was carried out in the Obstetrics and Gynecology Clinic of Imam Reza Hospital, Kermanshah, Iran, from May 2012 to April 2013. The study was approved by the Ethics Committee of Kermanshah University of Medical Sciences and registered under code IRCT201112078323N1 in the Iranian Center of Clinical Trials (IRCT.ir). Informed consent was obtained from all patients. Before commencement, 56 subjects aged 20–49 years were randomly divided into 2 treatment groups and matched according to age, weight, presence of hirsutism, and history of menstrual disorders.
PCOS was diagnosed according to the revised Rotterdam 2003 criteria,[12] i.e. the presence of at least 2 of the following 3 criteria: (1) Oligo/anovulation, (2) clinical or biochemical signs of hyperandrogenism including hirsutism, acne, or increased serum testosterone, and (3) polycystic ovaries by vaginal ultrasound.[13] Exclusion criteria were diabetes mellitus, abnormal liver function tests, known cardiac or renal disease, smoking, age <20 years, adrenal disorders including congenital adrenal hyperplasia and Cushing's syndrome, thyroid disorders, and hyperprolactinemia.[3,6,10]
Twenty-eight subjects in metformin group were treated with metformin 500 mg, 3 times daily, and twenty-eight in pioglitazone group were treated with pioglitazone 30 mg daily for 3 months. All patients received comprehensive clinical examinations, including height, weight, blood pressure, waist circumference, and body mass index (BMI). All clinical and laboratory examinations were repeated at the end of the 3-month treatment period.
Metabolic and endocrine laboratory parameters included fasting serum insulin, blood glucose (fasting blood sugar [FBS]), low-density lipoprotein (LDL), high-density lipoprotein (HDL), cholesterol (Chol), triglyceride (TG), free testosterone, and dehydroepiandrosterone (DHEA). FBS and lipids were measured by enzymatic methods (Roche Hitachi). Free testosterone and DHEA were by enzyme-linked immunosorbent (ELISA) assays (IBL, USA). Insulin was measured by ELISA (Roche, Germany). Statistical analysis was performed using IBM SPSS software, version 16.0. Qualitative variables (e.g. clinical parameters such as acne and menstrual patterns) were assessed with the Chi-square test. Quantitative variables were assessed by the independent t-test. A P < 0.05 was considered statistically significant.
RESULTS
Average ages of the metformin and pioglitazone groups were 27.5 ± 3.68 and 27.57 ± 5.91 years, respectively. Average BMI before and after treatment was not significantly different. Waist circumference was significantly reduced in the metformin group compared with pioglitazone group (P = 0.011). The majority of women had oligomenorrhea with both clinical and laboratory evidence of hyperandrogenism. Table 1 shows qualitative clinical parameters of PCOS before and after treatment.
Table 1.
Treatment comparisons. Qualitative clinical variables
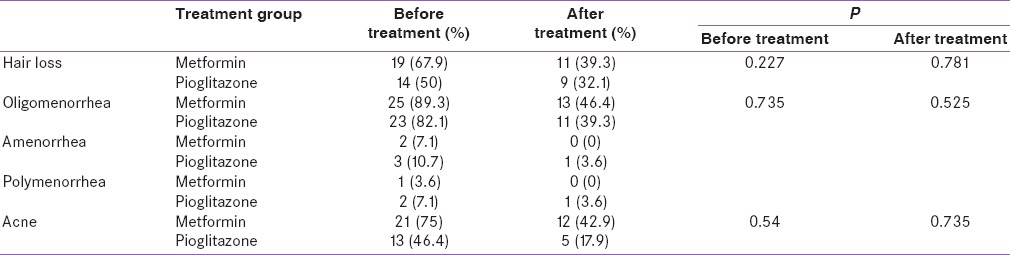
Hair loss is decreased in both groups. This reached statistical significance, however, only in the metformin group. Irregular menstrual patterns and acne significantly decreased in both groups. Table 2 shows quantitative clinical variables before and after treatment.
Table 2.
Treatment comparisons. Quantitative clinical variables
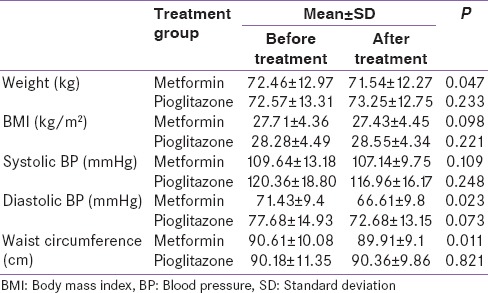
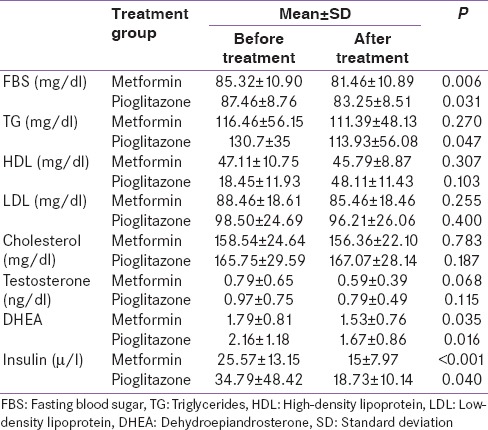
Mean body weight was slightly increased in the pioglitazone group but significantly reduced in the metformin group (P = 0.047). Table 3 shows metabolic and endocrine parameters before and after treatment with metformin and pioglitazone. Serum testosterone and DHEA decreased after treatment with both medications. However, this reduction was statistically significant only for DHEA. FBS and fasting insulin significantly decreased in both groups.
Table 3.
Metabolic and endocrine laboratory parameters before and after treatment
DISCUSSION
Hyperinsulinemia is considered an important pathogenic factor in PCOS, a common female endocrine disorder.[10] Therapies which reduce insulin resistance are therefore of potential clinical value in this disorder.[11] In this study, we compare the efficacy of pioglitazone versus metformin in the treatment of PCOS. Metabolic, endocrine, and clinical outcomes associated with treatments have been compared. Although significant differences between groups were found for some parameters, no regimen proved superior to the other in this study using the treatment regimens specified here.
In this study, the doses of metformin and pioglitazone were 500 mg 3 times daily and 30 mg daily, respectively. Dosages in different studies have varied. Intervention and follow-up times have also shown differences ranging from 12 weeks to 12 months.[9,10,14,15,16] The study showed that treatment with metformin and pioglitazone reduces hyperinsulinemia and hyperandrogenemia and finally remedy menstrual cycle, hair loss, and acne in women with the PCOS. In the present study, BMI was not significantly decreased in both groups, which contradicts the results of Li et al.[10] In this study, waist circumference decreased significantly in the metformin group. Velazquez et al. found that after treatment with metformin, reductions in waist circumference occurred.[17] However, in other studies, changes in waist circumference were not observed after treatment with metformin.[18,19] In our study, the pioglitazone group showed no significant change in waist circumference which was consistent with results from studies that were done by Romualdi et al.[14] Nevertheless, Ortega-González et al. reported that waist circumference is significantly increased after administration of pioglitazone.[15,16] Waist circumferences >35 inches in women indicate abnormal metabolic and hormonal function which is one of the risk factors of cardiovascular disease.[20] Acne is clearly ameliorated after treatment in both groups. Amelioration of menstrual disorders in both groups also was significant. Most studies agree on the effects of both metformin and pioglitazone in the treatment of menstrual disorders and acne.[10,14,15,16] After treatment, FBS and fasting insulin levels decreased significantly in both groups. In Glueck et al. study, the combination of metformin and pioglitazone reduces insulin resistance, glucose, and insulin levels in patients who did not respond to metformin alone.[21] In this study, blood lipid levels did not change after treatment with metformin. In the pioglitazone group, only the TG levels decreased significantly, and the amount of this reduction was very low. In Naka et al. study, treatment with metformin reduces LDL levels, but treatment with pioglitazone increase LDL and Chol and reduces HDL.[16] The clinical significance of changes in lipid levels by pioglitazone is unknown. In this study, testosterone levels did not change significantly after treatment in both groups, but DHEA decreased in both groups and the decrease was greater in the pioglitazone groups. In Romualdi et al. study, treatment with pioglitazone had no effect on free and total testosterone and sex hormone-binding globulin levels;[14] however, clinical symptoms of hyperandrogenism, such as acne and hirsutism, were clearly ameliorated. These results indicate that in addition to the effect on insulin metabolism, pioglitazone may act through other mechanisms to improve clinical symptoms in patients. Strong association between hyperandrogenism and hyperinsulinemia represents the hypothesis that stimulatory effect of insulin on ovarian androgen production is affected by some genetic predisposition.[22] Limitations of this study include the small sample size and short duration of treatment with pioglitazone and metformin. Further studies with more patients are needed to confirm the effect of these medications in PCOS patients.
CONCLUSION
Both metformin and pioglitazone are effective in the treatment of women with PCOS. Although we were not able to recommend one treatment regime over the other, pioglitazone does offer a useful, alternate treatment in women with PCOS who are not able to tolerate metformin.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hu L, Shen H, Wu QF, Tian L, Hu MH. Treatment of polycystic ovarian syndrome with insulin resistance by insulin-sensitizer. Clin Exp Obstet Gynecol. 2014;41:288–92. [PubMed] [Google Scholar]
- 2.Valsamakis G, Lois K, Kumar S, Mastorakos G. Metabolic and other effects of pioglitazone as an add-on therapy to metformin in the treatment of polycystic ovary syndrome (PCOS) Hormones (Athens) 2013;12:363–78. doi: 10.1007/BF03401302. [DOI] [PubMed] [Google Scholar]
- 3.Cho LW, Kilpatrick ES, Keevil BG, Coady AM, Atkin SL. Effect of metformin, orlistat and pioglitazone treatment on mean insulin resistance and its biological variability in polycystic ovary syndrome. Clin Endocrinol (Oxf) 2009;70:233–7. doi: 10.1111/j.1365-2265.2008.03309.x. [DOI] [PubMed] [Google Scholar]
- 4.Katsiki N, Hatzitolios AI. Insulin-sensitizing agents in the treatment of polycystic ovary syndrome: An update. Curr Opin Obstet Gynecol. 2010;22:466–76. doi: 10.1097/GCO.0b013e32833e1264. [DOI] [PubMed] [Google Scholar]
- 5.Ziaee A, Oveisi S, Abedini A, Hashemipour S, Karimzadeh T, Ghorbani A. Effect of metformin and pioglitazone treatment on cardiovascular risk profile in polycystic ovary syndrome. Acta Med Indones. 2012;44:16–22. [PubMed] [Google Scholar]
- 6.Ibáñez L, López-Bermejo A, Díaz M, Enríquez G, Del Río L, De Zegher F. Low-dose pioglitazone, flutamide, metformin plus an estro-progestagen for non-obese young women with polycystic ovary syndrome: Increasing efficacy and persistent safety over 30 months. Gynecol Endocrinol. 2010;26:869–73. doi: 10.3109/09513590.2010.487589. [DOI] [PubMed] [Google Scholar]
- 7.Roe A, Hillman J, Butts S, Smith M, Rader D, Playford M, et al. Decreased cholesterol efflux capacity and atherogenic lipid profile in young women with PCOS. J Clin Endocrinol Metab. 2014;99:E841–7. doi: 10.1210/jc.2013-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinaixa M, Rodriguez MA, Samino S, Díaz M, Beltran A, Mallol R, et al. Metabolomics reveals reduction of metabolic oxidation in women with polycystic ovary syndrome after pioglitazone-flutamide-metformin polytherapy. PLoS One. 2011;6:e29052. doi: 10.1371/journal.pone.0029052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du Q, Wang YJ, Yang S, Wu B, Han P, Zhao YY. A systematic review and meta-analysis of randomized controlled trials comparing pioglitazone versus metformin in the treatment of polycystic ovary syndrome. Curr Med Res Opin. 2012;28:723–30. doi: 10.1185/03007995.2012.681636. [DOI] [PubMed] [Google Scholar]
- 10.Li XJ, Yu YX, Liu CQ, Zhang W, Zhang HJ, Yan B, et al. Metformin vs thiazolidinediones for treatment of clinical, hormonal and metabolic characteristics of polycystic ovary syndrome: A meta-analysis. Clin Endocrinol (Oxf) 2011;74:332–9. doi: 10.1111/j.1365-2265.2010.03917.x. [DOI] [PubMed] [Google Scholar]
- 11.Haydardedeoglu B, Simsek E, Kilicdag EB, Bagis T. Metabolic and endocrine effects of metformin and metformin plus cyclic medroxyprogesterone acetate in women with polycystic ovary syndrome. Int J Gynaecol Obstet. 2009;105:32–5. doi: 10.1016/j.ijgo.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 12.Geller DH, Pacaud D, Gordon CM, Misra M. of the Drug and Therapeutics Committee of the Pediatric Endocrine Society. State of the art review: Emerging therapies: The use of insulin sensitizers in the treatment of adolescents with polycystic ovary syndrome (PCOS) Int J Pediatr Endocrinol. 2011;2011:9. doi: 10.1186/1687-9856-2011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdel Fattah G, Al Mohammady M, Hamed DA. Combined pioglitazone–metformin and clomiphene citrate versus metformin and clomiphene citrate in induction of ovulation in women with clomiphene citrate-resistant polycystic ovary syndrome. Middle East Fertil Soc J. 2014;19:27–33. [Google Scholar]
- 14.Romualdi D, Guido M, Ciampelli M, Giuliani M, Leoni F, Perri C, et al. Selective effects of pioglitazone on insulin and androgen abnormalities in normo-and hyperinsulinaemic obese patients with polycystic ovary syndrome. Hum Reprod. 2003;18:1210–8. doi: 10.1093/humrep/deg264. [DOI] [PubMed] [Google Scholar]
- 15.Ortega-González C, Luna S, Hernández L, Crespo G, Aguayo P, Arteaga-Troncoso G, et al. Responses of serum androgen and insulin resistance to metformin and pioglitazone in obese, insulin-resistant women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:1360–5. doi: 10.1210/jc.2004-1965. [DOI] [PubMed] [Google Scholar]
- 16.Naka KK, Kalantaridou SN, Kravariti M, Bechlioulis A, Kazakos N, Calis KA, et al. Effect of the insulin sensitizers metformin and pioglitazone on endothelial function in young women with polycystic ovary syndrome: A prospective randomized study. Fertil Steril. 2011;95:203–9. doi: 10.1016/j.fertnstert.2010.06.058. [DOI] [PubMed] [Google Scholar]
- 17.Velazquez EM, Mendoza S, Hamer T, Sosa F, Glueck CJ. Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metabolism. 1994;43:647–54. doi: 10.1016/0026-0495(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 18.Morin-Papunen L, Rantala AS, Unkila-Kallio L, Tiitinen A, Hippeläinen M, Perheentupa A, et al. Metformin improves pregnancy and live-birth rates in women with polycystic ovary syndrome (PCOS): A multicenter, double-blind, placebo-controlled randomized trial. J Clin Endocrinol Metab. 2012;97:1492–500. doi: 10.1210/jc.2011-3061. [DOI] [PubMed] [Google Scholar]
- 19.Açbay O, Gündogdu S. Can metformin reduce insulin resistance in polycystic ovary syndrome? Fertil Steril. 1996;65:946–9. [PubMed] [Google Scholar]
- 20.Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: A consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010;95:2038–49. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- 21.Glueck CJ, Moreira A, Goldenberg N, Sieve L, Wang P. Pioglitazone and metformin in obese women with polycystic ovary syndrome not optimally responsive to metformin. Hum Reprod. 2003;18:1618–25. doi: 10.1093/humrep/deg343. [DOI] [PubMed] [Google Scholar]
- 22.Lerchbaum E, Schwetz V, Giuliani A, Obermayer-Pietsch B. Influence of a positive family history of both type 2 diabetes and PCOS on metabolic and endocrine parameters in a large cohort of PCOS women. Eur J Endocrinol. 2014;170:727–39. doi: 10.1530/EJE-13-1035. [DOI] [PubMed] [Google Scholar]


