Abstract
Background:
Reference intervals for thyroid hormone during pregnancy need to be gestational age, method, and population specific and there is need to establish trimester-specific thyroid levels for the different population across the world. The aim of this study was to establish trimester-specific reference range for thyroid hormone during pregnancy in a tertiary care center in Haryana.
Materials and Methods:
A total of 1430 pregnant women were recruited for the study. Participants having any history of chronic illness, goiter on physical examination, thyroid illness in the past or present, consuming thyroid medications, family history of thyroid illness, presence of anti-thyroid peroxidase antibody, poor obstetrics history were excluded from the study and reference population was identified to calculate serum free triiodothyronine (FT3), free thyroxine (FT4) and thyrotropin (TSH) for each trimester of pregnancy.
Results:
The 2.5–97.5th percentiles for FT3, FT4, and TSH obtained in this study were 2.53–4.54 pg/ml, 0.88–1.78 ng/ml and 0.37–3.69 μIU/ml in the first trimester, 2.0–4.73 pg/ml, 0.91–1.78 ng/ml and 0.54–4.47 μIU/ml in the second trimester, 2.01–4.01 pg/ml, 0.83–1.73 ng/ml, and 0.70–4.64 μIU/ml in the third trimester of pregnancy. Mean TSH increased and mean FT3 decreased significantly with the progression of gestational period. FT4 decreased from trimester 1–3rd, but the decrease was nonsignificant from 2nd to 3rd trimester.
Conclusions:
Existing results for trimester-specific reference intervals for thyroid hormones are inconsistent and cannot be extrapolated due to differences in ethnicity, maternal iodine status, laboratory assay method, and rigor for selection of reference population. Thus, establishment of reference intervals in each region is of great importance.
Key words: Pregnancy, thyroid function test, trimester-specific
INTRODUCTION
Pregnancy has a profound impact on the thyroid gland and thyroid function. Pregnancy is a state of stress for the thyroid, resulting in hypothyroidism in women with limited thyroidal reserve or iodine deficiency. There is a wide geographic variation in prevalence of hypothyroidism during pregnancy and it varies from 2.5% from the West to 11% from India and correct diagnosis and treatment of thyroid dysfunction is important to prevent both maternal and fetal complications.[1,2,3] Early maternal thyroid insufficiency, even subclinical hypothyroidism (SCH) and isolated hypothyroxinemia have the potential to impair fetal neurodevelopment.[4,5,6] Pregnancy is associated with a number of physiological and hormonal changes that result in significant but reversible alterations in thyroid function tests (TFTs). Production of thyroid hormones and iodine requirement each increases by approximately 50% during pregnancy. The various other changes in TFT during pregnancy include increased in serum free thyroxine (FT4) associated with reciprocal decrease in thyrotropin (TSH) due to thyrotropic activity of human chorionic gonadotrophin during the first trimester, increased sialylation of thyroid hormone mediated by oestrogens and reduced clearance of thyroxine-binding globulin which results in increased levels of total T4 and T3.[7] Therefore, nonpregnant reference intervals should not be used in pregnancy as they can mislead on the diagnosis and treatment of thyroid disorders during pregnancy. Establishment of reference intervals in each trimester of pregnancy is thus of great importance, and these values should be used to diagnose thyroid disorders during pregnancy. However, many factors such as ethnicity, age, manufacturer's methodology, iodine status, and rigor for selection of the reference population and calculation method may affect the establishment of reference intervals for TFTs. Since reference intervals needs to be gestational age, method, and population specific, there is need to establish trimester-specific thyroid levels for different population across the world. In India, limited data's are available over trimester-specific thyroid hormones level during pregnancy and none from Haryana.[8,9,10,11,12] The aim of this study was to establish trimester-specific reference range for thyroid hormone during pregnancy in a tertiary care center in Haryana which is an iodine sufficient area of India.
MATERIALS AND METHODS
This was a cross-sectional study conducted at the Department of Endocrinology and Antenatal Clinic in the Department of Obstetrics and Gynecology at Postgraduate Institute of Medical Sciences Rohtak. A total of 1430 pregnant women were recruited for the study and out of which these 461, 469, and 500 pregnant women were in first, second, and third trimester, respectively. After explaining the purpose of the study, informed consent was obtained from each patient and the study was approved by the institutional ethical review board. All women who were carrying a healthy singleton uncomplicated intrauterine pregnancy and consuming iodized salt were recruited in the study. On enrollment of participants, detailed history was enquired and participants were subjected to relevant general physical examination and findings were recorded on a predesigned performa. Physical examination included the presence or absence of goiter and general and systemic examinations. Gestational age was calculated from the 1st day of the last normal menstrual period and gestational age ≤12, >12–28 and >28 weeks comprised the first, second, and third trimesters of pregnancy. Participants having any history of chronic illness, goiter on physical examination, thyroid illness in the past or present, consuming thyroid medications (current and past), family history of thyroid illness, presence of anti-thyroid peroxidase antibody (TPO antibodies), poor obstetrics history included 3 or more abortions were excluded from the study and reference population was identified to calculate serum free triiodothyronine (FT3), FT4, and TSH for each trimester of pregnancy.
Estimation for FT3, FT4, TSH, and anti-TPO was done using the electrochemiluminescence (ECL) technique using commercially available kits by Advia Centaur CP analyzer system and immulite 1000. The analytical sensitivities for FT3, FT4, TSH, and anti-TPO were 0.2 pg/mL, 0.1 ng/dL, 0.010 μIU/mL, and 7 IU/mL, respectively. Intra-assay coefficients of variation for FT3, FT4, TSH, and anti-TPO were 3.8%, 2.20%, 5.2%, and 5.6%, respectively. Laboratory reference range for FT3, FT4, and TSH were 2.3–4.2 pg/mL, 0.89–1.76 ng/dL, and 0.35–5.5 mIU/L, respectively. Normal range for TPO antibody was <35 IU/mL and value greater than or equal to indicate elevated anti-TPO in serum.
According to the aims and objectives of the study, the data were compiled and entered into MS Excel and analyzed, using appropriate statistical tests in Statistical Package for Social Sciences version 20 (SPSS Inc. Chicago IL). For descriptive statistics, frequencies, percentages, mean with standard deviations, and median of different variables were calculated. To assess the difference between categorical variables “Chi-square test” was used. Independent sample t-test was used to compare the means of two separate sets of independent samples. For comparison of means of more than two samples - analysis of variance test was used. The P values were two-tailed and probability level of significant difference was set at <0.05.
RESULTS
A total of 1430 antenatal mothers were included in this study with mean age of 23.89 ± 3.24 years (range 17–38 years). Of these 204 were between 16.0 and 20.0 years of age, 857 were 21.0–25.0 years of age, 324 were 26.0–30.0 years of age, 43 were 31.0–35.0 years of age, and 2 were 36.0–40.0 years of age, respectively. The distribution of the women based on their 1st, 2nd, and 3rd trimesters was 461, 469, and 500, respectively. Mean gestational age of pregnant women in 1st, 2nd, and 3rd trimester were 8.58 ± 2.11, 19.47 ± 4.47, and 34.36 ± 3.52 weeks, respectively. Out of 1430, 537 women were primigravida and 893 were multigravida. History of recurrent abortion (taken as equal to and more than three abortions) was present in 39 (2.7%) pregnant women. History of thyroid illness in the family was present in 18 (1.2%). Goiter was present in 133 (9.3%). AntiTPO antibody was positive in 315 (22%) and out of these 128 were positive in the first trimester, 102 in the second trimester, and 85 in the third trimester. Both goiter and AntiTPO antibody were present in 80 (5.59%) of pregnant women. Overt hypothyroidism was found in 6 (1.3%) women in the first trimester, 7 (1.5%) in the second trimester and 5 (1%) in the third trimester of pregnancy while overt hyperthyroidism was present in 2 (0.4%) in the first trimester, none in the second trimester, and 2 (0.4%) in the third trimester. There was strong relationship between the presence of goiter and TPO antibody positivity in all the three trimester (P < 0.001). Anti-TPO antibody was significantly associated with thyroid dysfunction (P < 0.001). No significant association was found between TPO antibodies with maternal age.
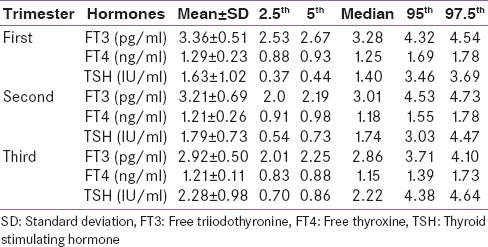
After applying the exclusion criteria, 160 women in the first trimester, 161 in the second trimester, and 126 in the third trimester, i.e., a total of 447 women were excluded from the study. The remaining 983 women constituted the reference population with 301 in the first trimester, 308 in the second trimester, and 374 in the third trimester, respectively. The mean ± standard deviation, median, 2.5th, 5th, 95th, 97.5th, percentiles for FT3, FT4, and TSH were determined in each trimester of pregnancy. The reference intervals for each trimester are shown in Table 1. Analysis of these parameters between each trimester showed significant variation. FT3 significantly decreased with the progression of gestational period (P = 0.001). FT4 decreased significantly from trimester 1st to 2nd (P = 0.024), but the decrease from trimester 2nd to 3rd was nonsignificant (P < 0.071). Mean TSH value significantly increased from trimester 1st to 2nd (P < 0.001) as compared to trimester 2nd to 3rd (P = 0.024).
Table 1.
Trimester-wise values for mean±standard deviation, median, 2.5th, 5th, 95th and 97.5th centiles for free triiodothyronine, free thyroxine and thyroid stimulating hormone from reference population
When trimester specific cut-off values for TSH as defined by endocrine society,[13] that is, 0.1–2.5 mIU/L, 0.2–3.0 mIU/L, and 0.3–3.0 mIU/L, respectively, in first, second, and third trimester of pregnancy were used, SCH was noted in 99 (21.5%) women in the first trimester, 74 (15.8%) in the second trimester, and 129 (25.8%) in the third trimester of pregnancy, respectively. When the trimester-wise 2.5th and 97.5th percentile of TSH derived from the reference population in this study was applied to the total population, the number of women with SCH were decreased from 99 (21.5%), 74 (15.8%), and 129 (25.8%) to 31 (6.7%), 50 (10.7%), 40 (8%) in 1st, 2nd, and 3rd trimester, respectively. It means 68 (14.8), 24 (5.1%), and 89 (17.8%) pregnant females would have been misclassified as having SCH or we can say that over treated if we would have applied endocrine society guidelines on our study population.
DISCUSSION
In this cross-sectional study, we have established gestational age-specific reference intervals for TFT in pregnant women after using rigorous exclusion criteria, i.e. any history of chronic illness, goiter on physical examination, thyroid illness in the past or present, consuming thyroid medications (current and past), family history of thyroid illness, presence of anti-TPO, poor obstetrics history included 3 or more abortions. The 2.5th–97.5th percentiles for FT3, FT4, and TSH obtained in this study were 2.53–4.54 pg/ml, 0.88–1.78 ng/ml and 0.37–3.69 µIU/ml in the first trimester, 2.0–4.73 pg/ml, 0.91–1.78 ng/ml and 0.54–4.47 µIU/ml in the second trimester, 2.01–4.01 pg/ml, 0.83–1.73 ng/ml and 0.70–4.64 µIU/ml in the third trimester of pregnancy. Mean TSH increased and mean FT3 decreased significantly with the progression of gestational period. FT4 decreased from trimester 1st to 3rd, but the decrease was non-significant from 2nd to 3rd trimester.
Several studies across the globe are available on assessment of TFT among pregnant women with marked heterogeneity like the difference in study design with some being cross-sectional while others being longitudinal, use of rigorous exclusion criteria to define reference population and laboratory method used to estimate T3, T4, and TSH [Table 2].[10,11,12,14,15,16,17,18,19,20,21,22,23,24,25] Soldin et al.[15] showed that FT4 decreased from the 1st to 3rd trimester (P < 0.001), but there was no significant difference between the second to the third trimester. TSH increased from 1st to 3rd trimester but remains static between 2nd and 3rd trimester. Similar changes were observed in this study where mean TSH increased and mean FT3 decreased significantly with the progression of the gestational period while FT4 decreased from trimester 1st to 3rd but the decrease was nonsignificant from 2nd to 3rd trimester. Another cross-sectional study by Thevarajah et al.[16] showed that mean TSH levels decreased during the first trimester and then increased significantly (P < 0.05) in the second and third trimester similar to our study and mean FT3and FT4 values declined from the first trimester that the decrease was significant in the second and third trimesters (P < 0.05).
Table 2.
Trimester specific reference range for thyroid function test in various studies
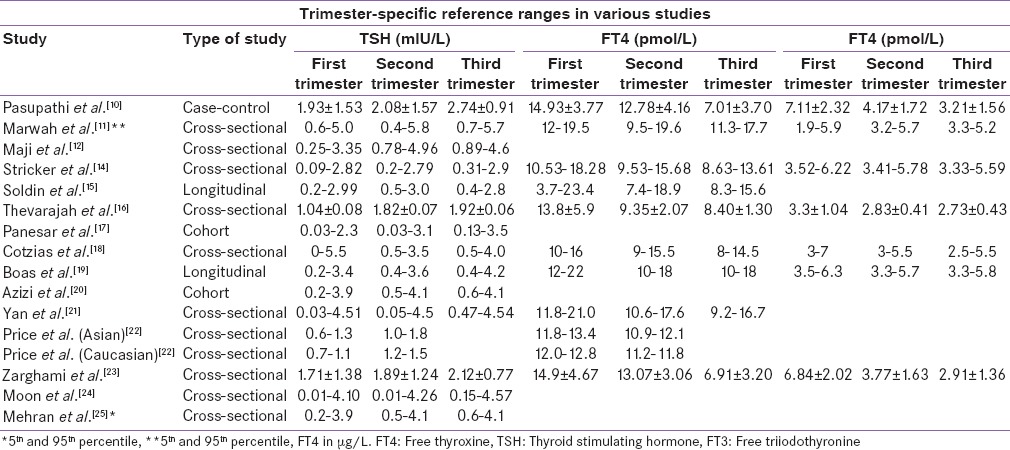
However, the values of TSH reported from these studies are quite different from values reported in the present study as well as from some of the Indian studies. Normal upper limit of TSH in pregnancy has been a subject of debate since a long time. The endocrine society guidelines for thyroid dysfunction in pregnancy published in 2012 have lowered the upper limit of reference range for normal TSH and suggested 0.1–2.5 mIU/L, 0.2–3.0 mIU/L and 0.3–3.0 mIU/L, respectively, in first, second, and third trimester of pregnancy.[13] However, the American Association of Clinical Endocrinology and the Endocrine Society Consensus panel recommended that 4.5 mIU/L should be maintained as the upper limit of normal. They reasoned that although some individuals within the range of 2.6–4.5 mIU/L may have subclinical thyroid disease, there was a lack of evidence of adverse outcome in this group.[26] This was supported by a recent study from Reh et al.[27] in a large cohort of first cycle IVF patients from 2005 through 2008. Although lowering the TSH threshold to 2.5 mIU/L would result in a nearly five-fold increase in the number of women being classified as hypothyroid, the lack of differences in maternal clinical outcomes must be considered in the current controversy regarding the relative merits of lowering the upper limit of normal of TSH. When trimester-specific cut-off values for TSH as defined by Endocrine society guidelines, that is, 0.1–2.5 mIU/L, 0.2–3.0 mIU/L and 0.3–3.0 mIU/L, respectively, in first, second, and third trimester of pregnancy were used, SCH was noted in 99 (21.5%) women in the first trimester, 74 (15.8%) in the second trimester, and 129 (25.8%) in the third trimester of pregnancy, respectively. When the trimester-wise 2.5th and 97.5th percentile of TSH derived from the reference population in this study was applied to the total population, the number of women with SCH were decreased from 99 (21.5%), 74 (15.8%), and 129 (25.8%) to 31 (6.7%), 50 (10.7%), 40 (8%) in 1st, 2nd, and 3rd trimester, respectively. It means 68 (14.8), 24 (5.1%) and 89 (17.8%) pregnant females would have been misclassified as having SCH or we can say that over treated if we would have applied endocrine society guidelines on our study population. Similar observations made by Marwaha et al.[11] in their study from India where the prevalence of SCH decreased to 6.6% from 14.4% when the trimester-wise 95th percentile of TSH from the reference population in their study was applied to the total population.
As serum TSH levels fluctuate with ethnic variations, it remains a concern, although studies that address this aspect are scant. It has been seen that there is considerable variation in serum TSH and thyroid hormone concentrations in healthy subjects, in whom the inter-individual variability is much greater than the intra-individual variability. Each individual working point and set point of the hypothalamic-pituitary-thyroid axis is determined by genetic and environmental factors and therefore, thyroid hormone values for different ethnicities should be established separately.[28] Price et al.[22] have proved that there were significant differences in thyroid related biochemical markers between Asian and western Caucasian nonpregnant women. They postulated sensitivity of hypothalamic-pituitary-thyroid axis as a cause for these ethnic differences. Another study on US population done to compare reference intervals in the various ethnic groups reported significant ethnic differences in the established first trimester reference interval data.[29] The same analysis also showed the highest prevalence of thyroid autoantibodies (nearly 18%) and increased TSH concentration in the Asian Group. Peeters et al.[30] described the differences in TSH bioactivity due to genetic variability such as C/G single nucleotide polymorphisms (SNPs) in the gene regulating the TSH receptor. Thus, reference intervals for TFTs need to be, not only trimester and method specific but population specific also.
In a study by Mehran et al.,[25] the serum of 152 iodine-sufficient Iranian pregnant women with viable, singleton pregnancies without thyroid autoantibodies or history of thyroid disorder or goiter were selected for analysis. Reference intervals were defined as 5th and 95th percentiles. The TSH reference intervals showed that lower TSH reference limit of 0.2 occurred in the first trimester in comparison with the 2nd and 3rd trimesters of 0.5 and 0.6, respectively. The upper reference range of TSH of 3.9 in the first trimester increased to 4.1 in the second trimester and remained unchanged in the last trimester. Moon et al.[24] established trimester and assay specific 2.5th–97.5th levels of TFT in 531 pregnant Korean women using ECL immunoassay. The TSH reference intervals were 0.01–4.10, 0.01–4.26, and 0.15–4.57 mIU/L for the first, second, and third trimester, respectively. The level of TSH observed in these Korean women was almost identical to what we have observed in this study using ECL assay.
Few studies from India had reported trimester-specific thyroid hormone values with no data available from Haryana, India. Deshwal et al.[8] in their study reported that FT3, FT4 decreased, and TSH increased with the progression of the gestational period as is observed in this study. Another study carried out by Kumar et al.[9] from India evaluated 124 pregnant women using radioimmunoassay showed an increase in TSH progressively with each trimester while serum triiodothyronine (T3) and thyroxine (T4) values increased from first to second trimester and declined from second to third trimester. This pilot study showed that the range of T3 was 1.7–4.3 nmol/L in second trimester and 0.4–3.9 nmol/L in third trimester, T4 as 92.2–252.8 nmol/L in second trimester and 108.2–219.0 nmol/L in third trimester, and TSH as 0.1–5.5 µlU/L in second trimester and 0.5–7.6 µlU/L in third trimester of pregnancy, respectively. In a case–control study by Pasupathi et al.[10] TFT of 75 pregnant women was compared with 75 randomly selected non-pregnant healthy females of childbearing age. The mean FT3, FT4, and TSH were significantly lower in the pregnant group. FT4 strongly declined during the third trimester, whereas FT3 decreased in the second and the third trimesters relative to nonpregnant women. In each trimester, the mean TSH levels of pregnant women were lower than the mean level of nonpregnant and it increases progressively through the three trimesters of pregnancy. The TSH levels observed during 1st, 2nd, and 3rd trimester were 1.93, 2.08, and 2.74 mIU/L, respectively as compared to TSH level of 2.54 mIU/L among nonpregnant women. However, the limitation of these studies was there small sample size and these studies estimated TFT by radio-immunoassay as compared to this study where much larger population was studied and TFT was estimated by ECL technique. A cross-sectional study by Marwaha et al.[11] on 541 pregnant Indian women using ECL method revealed significant decreased in FT4 with advancing pregnancy, while no significant difference was seen in values of FT3 or TSH between the trimesters. The 5th and 95th percentiles values were used to determine the reference ranges for FT3, FT4, and TSH and the observed reference values include FT4 (pmol/L) 12.0–19.45, 9.48–19.58, 11.32–17.7, FT3 (pmol/L) 1.92–5.86, 3.2–5.73, 3.3–5.18, TSH (mIU/L) 0.6–5.0, 0.44–5.78, 0.74–5.7 in 1st, 2nd, and 3rd trimester, respectively. In this study, we also estimated thyroid hormone levels using ECL method as used in a study carried out by Marwaha et al. but we observed different thyroid hormone values. However, the strength of the present study being its bigger sample size, i.e., 983 women and using 2.5th and 97.5th percentile to define reference range for thyroid hormone as compared to a sample size of 541 women and 5th and 95th percentile used by Marwaha et al. Similar observation were made by Maji et al.[12] in their study of 402 healthy pregnant women. The reference intervals for TSH were 0.25–3.35 lIU/ml for the first trimester; 0.78–4.96 lIU/ml for the second trimester, and 0.89–4.6 lIU/ml for the third trimester.
CONCLUSION
To conclude with, in this study we established the trimester-specific FT3, FT4, and TSH hormones range in pregnant women from a tertiary care center in Haryana India. Application of nonpregnant reference intervals to the interpretation of TFT in pregnant women has the potential to result in misclassification of patient test results. Existing results for trimester-specific reference intervals for thyroid hormones are inconsistent and cannot be extrapolated due to differences in ethnicity, maternal iodine status, laboratory assay method, and rigor for selection of reference population. Thus, the establishment of reference intervals in each region is of great importance.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rajput R, Goel V, Nanda S, Rajput M, Seth S. Prevalence of thyroid dysfunction among women during the first trimester of pregnancy at a tertiary care hospital in Haryana. Indian J Endocrinol Metab. 2015;19:416–9. doi: 10.4103/2230-8210.152791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosso L, Martínez A, Rojas MP, Margozzini P, Solari S, Lyng T, et al. Frequency of subclinical thyroid problems among women during the first trimester of pregnancy. Rev Med Chil. 2012;140:1401–8. doi: 10.4067/S0034-98872012001100004. [DOI] [PubMed] [Google Scholar]
- 3.Wang W, Teng W, Shan Z, Wang S, Li J, Zhu L, et al. The prevalence of thyroid disorders during early pregnancy in China: The benefits of universal screening in the first trimester of pregnancy. Eur J Endocrinol. 2011;164:263–8. doi: 10.1530/EJE-10-0660. [DOI] [PubMed] [Google Scholar]
- 4.LaFranchi SH, Haddow JE, Hollowell JG. Is thyroid inadequacy during gestation a risk factor for adverse pregnancy and developmental outcomes? Thyroid. 2005;15:60–71. doi: 10.1089/thy.2005.15.60. [DOI] [PubMed] [Google Scholar]
- 5.Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–55. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 6.Pop VJ, Brouwers EP, Vader HL, Vulsma T, van Baar AL, de Vijlder JJ. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: A 3-year follow-up study. Clin Endocrinol (Oxf) 2003;59:282–8. doi: 10.1046/j.1365-2265.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 7.Gyamfi C, Wapner RJ, D’Alton ME. Thyroid dysfunction in pregnancy: The basic science and clinical evidence surrounding the controversy in management. Obstet Gynecol. 2009;113:702–7. doi: 10.1097/AOG.0b013e3181996fe5. [DOI] [PubMed] [Google Scholar]
- 8.Deshwal VK, Yadav A, Gogoi JB. Comparison of FT3, FT4 and TSH levels in Pregnant Women in Dehradun. J Acad Ind Res. 2013;2:239–44. [Google Scholar]
- 9.Kumar A, Gupta N, Nath T, Sharma JB, Sharma S. Thyroid function tests in pregnancy. Indian J Med Sci. 2003;57:252–8. [PubMed] [Google Scholar]
- 10.Pasupathi P, Chandrasekhar V, Kumar US. Thyroid hormone changes in pregnant and non-pregnant women. Thyroid Sci. 2009;4:1–5. [Google Scholar]
- 11.Marwah RK, Chopra S, Gopalakrishnan S, Sharma B, Kanwar RS, Sastry S, et al. Establishment of reference range for thyroid hormones in normal pregnant Indian women. Int J Obstet Gynecol. 2008;115:602–6. doi: 10.1111/j.1471-0528.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- 12.Maji R, Nath S, Lahiri S, Saha Das M, Bhattacharyya AR, Das HN. Establishment of trimester-specific reference intervals of serum TSH and fT4 in a pregnant Indian population at North Kolkata. Indian J Clin Biochem. 2014;29:167–73. doi: 10.1007/s12291-013-0332-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, et al. Management of thyroid dysfunction during pregnancy and postpartum: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2543–65. doi: 10.1210/jc.2011-2803. [DOI] [PubMed] [Google Scholar]
- 14.Stricker R, Echenard M, Eberhart R, Chevailler MC, Perez V, Quinn FA, et al. Evaluation of maternal thyroid function during pregnancy: The importance of using gestational age-specific reference intervals. Eur J Endocrinol. 2007;157:509–14. doi: 10.1530/EJE-07-0249. [DOI] [PubMed] [Google Scholar]
- 15.Soldin OP, Tractenberg RE, Hollowell JG, Jonklaas J, Janicic N, Soldin SJ. Trimester-specific changes in maternal thyroid hormone, thyrotropin, and thyroglobulin concentrations during gestation: Trends and associations across trimesters in iodine sufficiency. Thyroid. 2004;14:1084–90. doi: 10.1089/thy.2004.14.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thevarajah M, Chew YY, Lim SC, Sabir N, Sickan J. Determination of trimester specific reference intervals for thyroid hormones during pregnancy in Malaysian women. Malays J Pathol. 2009;31:23–7. [PubMed] [Google Scholar]
- 17.Panesar NS, Li CY, Rogers MS. Reference intervals for thyroid hormones in pregnant Chinese women. Ann Clin Biochem. 2001;38(Pt 4):329–32. doi: 10.1258/0004563011900830. [DOI] [PubMed] [Google Scholar]
- 18.Cotzias C, Wong SJ, Taylor E, Seed P, Girling J. A study to establish gestation-specific reference intervals for thyroid function tests in normal singleton pregnancy. Eur J Obstet Gynecol Reprod Biol. 2008;137:61–6. doi: 10.1016/j.ejogrb.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Boas M, Forman JL, Juul A, Feldt-Rasmussen U, Skakkebaek NE, Hilsted L, et al. Narrow intra-individual variation of maternal thyroid function in pregnancy based on a longitudinal study on 132 women. Eur J Endocrinol. 2009;161:903–10. doi: 10.1530/EJE-09-0579. [DOI] [PubMed] [Google Scholar]
- 20.Azizi F, Mehran L, Amouzegar A, Delshad H, Tohidi M, Askari S, et al. Establishment of the trimester-specific reference range for free thyroxine index. Thyroid. 2013;23:354–9. doi: 10.1089/thy.2012.0407. [DOI] [PubMed] [Google Scholar]
- 21.Yan YQ, Dong ZL, Dong L, Wang FR, Yang XM, Jin XY, et al. Trimester-and method-specific reference intervals for thyroid tests in pregnant Chinese women: Methodology, euthyroid definition and iodine status can influence the setting of reference intervals. Clin Endocrinol (Oxf) 2011;74:262–9. doi: 10.1111/j.1365-2265.2010.03910.x. [DOI] [PubMed] [Google Scholar]
- 22.Price A, Obel O, Cresswell J, Catch I, Rutter S, Barik S, et al. Comparison of thyroid function in pregnant and non-pregnant Asian and Western Caucasian women. Clin Chim Acta. 2001;308:91–8. doi: 10.1016/s0009-8981(01)00470-3. [DOI] [PubMed] [Google Scholar]
- 23.Zarghami N, Rohbani-Noubar M, Khosrowbeygi A. Thyroid hormones status during pregnancy in normal Iranian women. Indian J Clin Biochem. 2005;20:182–5. doi: 10.1007/BF02867424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moon HW, Chung HJ, Park CM, Hur M, Yun YM. Establishment of trimester-specific reference intervals for thyroid hormones in Korean pregnant women. Ann Lab Med. 2015;35:198–204. doi: 10.3343/alm.2015.35.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehran L, Amouzegar A, Delshad H, Askari S, Hedayati M, Amirshekari G, et al. Trimester-specific reference ranges for thyroid hormones in Iranian pregnant women. J Thyroid Res. 2013;2013:651517. doi: 10.1155/2013/651517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988–1028. doi: 10.4158/EP12280.GL. [DOI] [PubMed] [Google Scholar]
- 27.Reh A, Grifo J, Danoff A. What is a normal thyroid-stimulating hormone (TSH) level? Effects of stricter TSH thresholds on pregnancy outcomes after in vitro fertilization. Fertil Steril. 2010;94:2920–2. doi: 10.1016/j.fertnstert.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 28.Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in serum T(4) and T(3) in normal subjects: A clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab. 2002;87:1068–72. doi: 10.1210/jcem.87.3.8165. [DOI] [PubMed] [Google Scholar]
- 29.La’ulu SL, Roberts WL. First-trimester reference intervals for thyroid tests: The role of ethnicity. Clin Chem. 2011;57:913–26. doi: 10.1373/clinchem.2007.089680. [DOI] [PubMed] [Google Scholar]
- 30.Peeters RP, van Toor H, Klootwijk W, de Rijke YB, Kuiper GG, Uitterlinden AG, et al. Polymorphisms in thyroid hormone pathway genes are associated with plasma TSH and iodothyronine levels in healthy subjects. J Clin Endocrinol Metab. 2003;88:2880–8. doi: 10.1210/jc.2002-021592. [DOI] [PubMed] [Google Scholar]


