Abstract
Bacterial artificial chromosomes (BACs) are capable of propagating large fragments of DNA and have become an invaluable tool for studying genome biology. To fill a phylogenetic gap in available genomic sequence and to complement ongoing molecular and genetic studies, we have generated a BAC library for the marine amphipod crustacean, Parhyale hawaiensis. The library was generated from genomic DNA isolated from whole adult animals and comprises 129,024 individual clones. The median insert size is ~140 kb and the genomic coverage is approximately five genome equivalents. We have screened the Parhyale BAC library for developmentally relevant genes and characterized the genomic organization of four genes: a hedgehog ortholog, and three Pax3/7 paralogs. Preliminary analysis suggests that introns are larger and more prevalent in Parhyale than in other arthropods whose genomes have been sequenced, which may partly account for the large genome size in Parhyale.
Keywords: Keywords: Evolution, Ecdysozoa, Crustacean, Arthropod, Amphipod, BAC library
Introduction
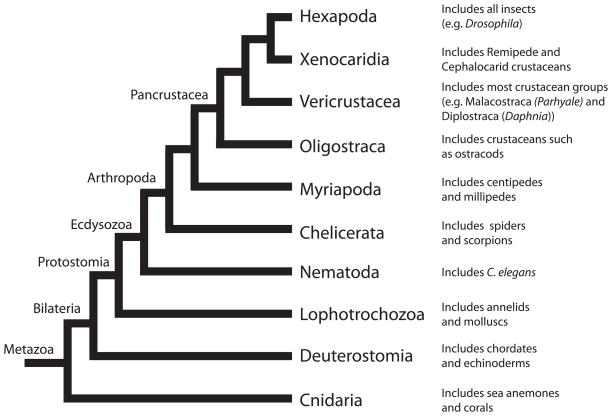
Bilaterian animals are separated into two main clades, the deuterostomes and the protostomes. The deuterostomes include the chordates (e.g. humans) and echinoderms (e.g sea urchins), while the protostomes are divided into two large clades, the lophotrochozoans and the ecdysozoans. Molluscs (e.g. snails) and annelids (e.g. earthworms) are part of the lophotochozoans, while nematodes (e.g. C. elegans) and arthropods (e.g. insects, crustaceans) are ecdysozoans. Of these groups, the ecdysozoans contain the vast majority of described animal species, due in large part to the diversity of arthropods. Our current understanding of the phylogenetic relationship of some of the major groups within the Ecdysozoa is shown in Fig 1.
Figure 1.
Evolutionary relationships. Phylogenetic relationships of the major groups of animals within Ecdysozoa. Crustaceans are paraphyletic and Parhyale is a member of the Vericrustacea lineage [21]. Tree topology and nomenclature based upon Regier et al, 2010 [21].
Within the Ecdysozoa lie two powerful genetic model systems, Drosophila melanogaster and C. elegans. These two animals were among the first to have their genome completely sequenced [1; 2], and the genomes of several closely related species have been sequenced in order to complement and leverage the large body of work involving these two model species [3; 4]. While Drosophila and C. elegans remain premier genetic systems for studies in many fields of biology, our initial reliance upon these two organisms may have biased some of our views of animal evolution and development.
For example, the genomes of Drosophila and C. elegans are somewhat unusual in that they are relatively small, which undoubtedly contributed to the decision to sequence their genomes. However, many studies suggest that these two species may not be representative of typical extant bilaterians. For instance, WNT genes are a family of highly conserved cell-cell signaling molecules whose founding member is wingless. In Drosophila there are seven (7), and in C. elegans there are five (5) WNT family members [5]. In contrast, the human genome contains nineteen (19) WNT genes, which at first glance might suggest an expansion of this family in the vertebrate lineage. However, more recent analyses of WNT family members in lophotrochozoans and the phylogenetically basal cnidarian Nematostella vectensis has revealed thirteen (13) ancestral WNT subfamilies [6; 7; 8]. This strongly suggests that the small number of WNTs in Drosophila and C. elegans is due to gene loss. More extensive evidence of gene loss for these two model species was provided by the finding of several important genetic pathways in Cnidaria that are absent in Drosophila and C. elegans [9]. The question of when these losses occurred in the evolution of Ecdysozoa remains largely unanswered, and it is quite possible that at least some of these losses represent cases of independent loss within the nematode and insect lineages. In addition to gene loss, the genomes of Drosophila and C. elegans are peculiar in that they appear to have undergone compaction, as evidenced by the loss and shortening of introns, and a general decrease in intergenic distance [10; 11].
Understanding when these genomic changes occurred and what role they played in the evolution of extant animals requires genomic sequence from a broader set of species. Recent advancements in sequencing and bioinformatics have enabled genomic studies of a more diverse set of animals, without having to focus exclusively on established model species with reduced genome size. Indeed, while the genomes of several ecdysozoans have been sequenced, the vast majority belong to two clades: Insecta, with a heavy bias toward holometabolous insects, especially dipterans [3; 12; 13; 14; 15; 16; 17; 18], and Nematoda [2; 4; 19]. Therefore, our understanding of genome evolution within Ecdysozoa will benefit greatly from obtaining genomic sequence from additional species representing a broader taxonomic sampling [20]. As a case in point, the phylogenomic analysis of 2.6 Mb of sequence from 62 single-copy genes of 75 arthropods was required to resolve the deep phylogenetic history of the major arthropod lineages [21].
Since arthropods are not only species-rich, but also morphologically and developmentally diverse, they are critically important for the study of evolution and development (evo-devo). A great deal is known about Drosophila development due to its powerful genetic tools and large research community. In comparison we know far less about the development of other arthropods, thus more taxonomic sampling is necessary to help us understand the forces underlying the radiation of this group [21]. To this end, the marine amphipod, Parhyale hawaiensis, has been developed as a new model system for evo-devo studies [22; 23; 24]. Part of a morphologically diverse group of crustaceans termed malacostracans, Parhyale is closely related to economically important animals such as shrimps, crabs, and lobsters, and its phylogenetic position allows important comparisons to reconstruct ancestral features within insects and Pancrustacea [21; 25].
In an effort to develop a more powerful genetic system for evo-devo studies, and to begin to address issues of genome evolution within arthropods and, more broadly, in Ecdysozoa, we have generated a bacterial artificial chromosomes (BAC) library for Parhyale. BACs are a plasmid vector-based system capable of accommodating large inserts of DNA (> 100 kb) [26; 27; 28]. Using an F-factor origin of replication, these bacterial plasmids are maintained as a single copy within E. coli and allow for faithful propagation of large DNA fragments. These libraries can then be used for molecular and genomic studies such as positional cloning [29] or comparing gene structures and synteny across different species [30]. Moreover, BACs are a convenient tool for the initial physical and genetic mapping of genomic regions without whole genome sequencing. Thus, BAC libraries are an excellent means of studying genome level questions, particularly for those interested in comparative studies of specific genomic regions. Highlighting their usefulness, BAC libraries have now been constructed for hundreds of species ranging from bacteria to plants to animals. Here we report the building and initial characterization of a Parhyale hawaiensis BAC library.
Results and Discussion
We have constructed a BAC library for the amphipod crustacean, Parhyale hawaiensis, that consists of 129,024 clones with an average insert size of 140 kb. The genome size of P. hawaiensis is estimated to be 3.6 Gb (N.H.P.and Aziz Aboobaker, unpublished data), and therefore the coverage of our library is estimated to be close to five genome equivalents. The Parhyale BAC clones were robotically picked, grown, and stored in 384-well plates and they were arrayed onto several sets of nylon filters for screening. To assess the quality of the library, we screened the filters with gene-specific probes derived from cDNAs we cloned from Parhyale embryos for developmental and evolutionary studies. Probes were designed outside of conserved domains to minimize their potential for cross-hybridization. The identity of BAC clones identified by screening was confirmed by PCR and/or Southern analysis. Table 1 summarizes the results of screening the Parhyale BAC library with probes for twenty (20) single-copy genes. This initial study confirmed the anticipated 5X coverage of the library. Based on the success of our initial screening, we identified an additional 50 BACs and analyzed their insert size by pulse-field gel electrophoresis. This analysis revealed the average insert size to be 140 kb (Fig. 2), with inserts ranging in size from 95 kb to 170 kb. The majority of clones (46 of 70) have inserts of 130 to 150 kb (Fig. 3). Importantly, our results demonstrate the quality of this BAC library, which contains inserts of expected size, and possesses enough genomic coverage to identify several BACs spanning a genomic region of interest.
Table 1.
| Gene | Number of Clones |
|---|---|
|
| |
| Ph-pax3/7-1 | 5 |
| Ph-pax3/7-2 | 6 |
| Ph-pax3/7-3 | 5 |
| Ph-sloppy-paired-1 | 8 |
| Ph-hes-1 | 7 |
| Ph-hes-2 | 3 |
| Ph-hes-3 | 7 |
| Ph-hes-4 | 5 |
| Ph-runt-1 | 3 |
| Ph-runt-2 | 1 |
| Ph-engrailed-1 | 8 |
| Ph-engrailed-2 | 5 |
| Ph-odd-skipped-1 | 4 |
| Ph-odd-skipped-2 | 2 |
| Ph-odd-skipped-3 | 4 |
| Ph-odd-skipped-4 | 4 |
| Ph-odd-skipped-5 | 2 |
| Ph-odd-paired-1 | 5 |
| Ph-odd-paired-2 | 4 |
| Ph-hedgehog* | 12* |
|
| |
| mean = 5 | |
The probe for hedgehog spanned three introns (> 100kb of genomic sequence). This may have caused the number of clones to be higher than the other genes which were screened for using probes made against putative conserved exons.
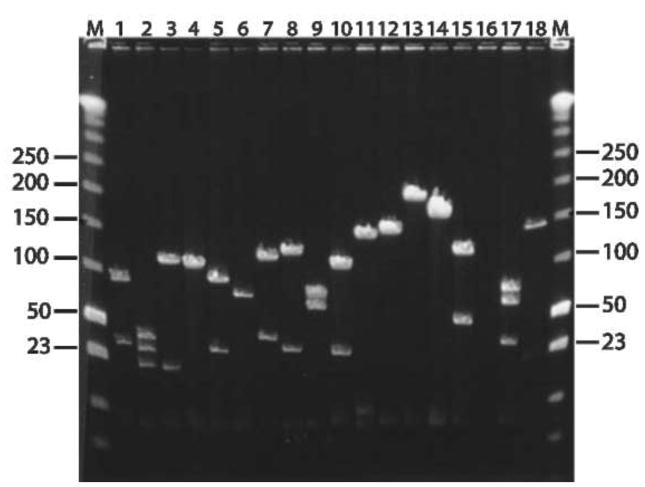
Figure 2.
Sizing and partial characterization of randomly selected recombinants from the P. hawaiensis BAC library. Eighteen clones were selected at random from the library before arraying. DNA was isolated using standard miniprep procedure, subjected to NotI digestion and analyzed by PFGE as described in Methods. Low-range PFGE molecular weight markers (New England Biolabs) are denoted by M and their sizes are given in kb. Miniprep for the BAC clone analyzed in lane 16 did not yield any DNA.
Figure 3.
Size distribution of Parhyale BAC clone inserts. A total of 70 clones selected for sequencing were prepared and digested with the restriction enzyme NotI. Samples were run on a CHEF gel and insert sizes were calculated manually by comparison with standard size markers.
One reason for investigating the genome of Parhyale is that it is larger than that of the classic ecdysozoan models, Drosophila and C. elegans. These organisms, potentially as a consequence of their rapid life cycle, appear to have undergone a reduction of their genome size resulting in intron loss and smaller gene size. To address this issue specifically, we have used BAC mapping and sequencing to verify whether genes are larger and introns more prevalent in Parhyale. Sequence analysis of four different BACs provides preliminary evidence that introns have both greater size and number in Parhyale when compared to their ortholog(s) in other species.
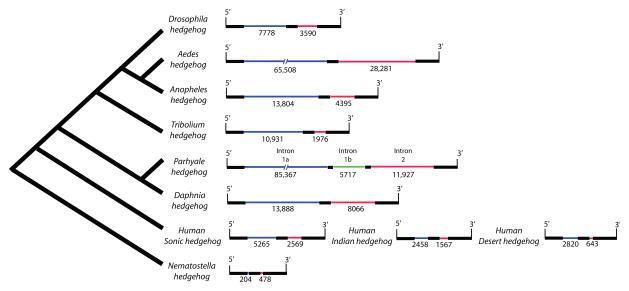
For example, comparative analysis of the hedgehog locus illustrates the differences between Parhyale and other metazoans (Fig. 4). In the case of Drosophila, the hedgehog gene contains two introns that flank a highly conserved 262 bp exon. The first intron in Drosophila is 7,778 bp whereas its homolog in Parhyale is ten times larger at 77,848 bp. The second intron is 3,590 bp in Drosophila, while its homolog is 11,927 bp in Parhyale. Interestingly, the 262 bp exon, which is conserved in all metazoans we analyzed, is disrupted by an additional phase 0 intron (5,717 bp) in Parhyale (Fig. 4). Therefore, at the hedgehog locus, Parhyale has approximately ten times the number of intronic base pairs when compared to Drosophila. For perspective, this means that at least 71% of our 146 kb BAC insert is intronic. The yellow fever mosquito, Aedes aegypti, which has undergone genome expansion due to an increased mobilization of transposable elements, has a slightly smaller hedgehog locus than Parhyale and no additional intron when compared to other metazoans (Fig. 4). Vertebrates, which in general have expanded genomes with large genes and increased intron number, also have much smaller hedgehog introns (Fig. 4).
Figure 4.
Comparison of hedgehog genomic organization in metazoans. These schematics correspond to the protein-coding region of the hedgehog locus. Black bars represent exons (not to scale), the green bar represents an intron in Parhyale hedgehog with no corresponding intron in other metazoans in this analysis. Blue and red bars represent homologous introns between species. The large size of intron one in Parhyale and Aedes hedgehog is denoted by a broken line (and thus are not to scale).
Analysis of other Parhyale BAC sequences suggests similar differences in intron size and number, for example within the Pax group III genes. In arthropods, Pax group III genes are part of the larger Pax family of transcription factors and are homologous to vertebrate Pax3 and Pax7 genes [31; 32; 33]. Like other Pax genes, Pax group III are defined by the presence of three domains: the “paired” domain, an octapeptide, and a “paired” class homeodomain [34; 35]. In Drosophila, they consist of three paralogs; paired (prd), gooseberry (gsb), and gooseberry-neuro (gsb-n), and are important in several developmental processes, namely segmentation and neural development [32; 33]. In Drosophila, gsb and gsb-n are syntenic and reside on opposite strands of a 23.5 kb locus. This organization is conserved in Anopheles and Tribolium, where orthologous genes are found in a 51 kb and 39 kb locus, respectively. The Pax group III genes are not annotated in the Aedes aegypti genome, but BLAST searches of the assembly indicate that two homologous genes are possibly syntenic as they are both located in supercontig 1.800, but with the caveat that sequence for the homeodomain is missing from one these genes (http://aaegypti.vectorbase.org). Outside of insects, the branchiopod crustacean, Daphnia pulex has undergone an expansion of this family, as there are 6 putative Pax group III genes organized in three clusters of two genes each (F.P. and John Colbourne, unpublished data). The first cluster is 27 kb, the second 63 kb and the third 48 kb, with the six genes ranging from 4 to 18 kb in size. However, not all six Daphnia genes are predicted to be functional as two of them have a frameshift or a deletion in the homeodomain sequence.
To better understand the evolution of Pax group III genes in Pancrustacea, we cloned three paralogs from Parhyale embryonic cDNA and characterized three BACs containing one gene each (Table 2). The large size of these genes (primary transcript lengths greater than 33 kb, 46 kb, and 18 kb) limits the amount of flanking genomic sequence contained within our BACs, and was not sufficient to uncover any synteny, if it exists, between the Parhyale Pax group III genes we cloned. These findings further illustrate the large size of Parhyale introns and suggest that intergenic distance may also be greater relative to that in most other sequenced arthropods. Additionally, a comparison of intron number within Pax group III genes across several phylogenetically distant species suggests that Parhyale has more introns per gene than currently sequenced insects and shares similarities in this regard to Daphnia (Table 2). For example, the three Pax group III genes in Parhyale have either five or six introns, whereas insects such as Drosophila, Tribolium and Anopheles possess far fewer (mode: 1, range: 1–5). The similarity in intron number between Parhyale and Daphnia supports the idea that some insects, especially dipterans, may have undergone genome compaction, possibly due to generalized intron loss. Exploring metazoan genomes outside Ecdysozoa also indicates that the Pax3/7 genes have more introns in the vertebrate lineage. For instance, in Nematostella there are four paralogous PaxD genes which have three introns each and relatively small size (4 to 8 kb; Table 2). This is in contrast to humans, where Pax3 and Pax7 have seven introns, and much larger gene sizes (95 and 103kb, respectively). To what degree greater intron number in this gene family represents parallel intron gain will require additional taxonomic sampling of genes from the Pax3/7 family.
Table 2.
| Species | Gene | Transcript length (bp) | Number of introns | Intron sizes (bp) | Intergenic distance (bp) |
|---|---|---|---|---|---|
| Drosophila | prd | 2,216 | 1 | 374 | |
| gsb | 2,315 | 1 | 1,031 | 10,138 | |
| gsb-n | 11,062 | 4 | 9,712 | ||
| Aedes | pax3/7-1 | >138000 | >2 | >137000 | ND |
| pax3/7-2 | ND | 1? | ND | ND | |
| pax3/7-3 | >69500 | >3 | >69000 | ND | |
| Anopheles | gsb | 680 | 1 | 4,110 | 32,254 |
| gsb-n | 18,130 | 3 | 17,388 | ||
| Tribolium | prd | 28,747 | 1 | 27,503 | |
| gsb | 11,004 | 5 | 9,771 | 6,121 | |
| gsb-n | 22,316 | 4 | 21,299 | ||
| Parhyale | pax3/7-1 | 33,721 | 5 | 32,257 | ND |
| pax3/7-2 | 46,023 | 6 | 44,568 | ND | |
| pax3/7-3 | 18,680 | 5 | 17,391 | ND | |
| Daphnia | pax3/7-C | 7,488 | 6 | 6,153 | |
| pax3/7 D | 4,231 | 4 | 3,433 | 51,752 | |
| pax3/7 A | 4,830 | 3? | 3,052 | ||
| pax3/7 B | 11,652 | 4 | 10,350 | 10,604 | |
| pax3/7 E | 5,905 | 5 | 4,321 | 24,319 | |
| pax3/7 F | 17,997 | 5 | 16,506 | ||
| Human | Pax3 | 97,268 | 7 | 95,251 | |
| Pax7 | 105,132 | 7 | 102,861 | ||
| Nematostella | PaxD2 | 7,650 | 3 | 6,555 | ~10,000 |
| PaxD1 | 8,608 | 3 | 7,612 | ||
| PaxD3 | ND | ND | ND | ND | |
| PaxD4 | 5,059 | 3 | 4,372 | ND |
ND = Not determined
Light grey highlights two potential pseudogenes in Daphnia.
Dark grey boxes indicate that no synteny was observed after the analysis of completed genomic sequences
Interestingly, the large genome size and gene structure in Parhyale are reminiscent of the mosquito, Aedes aegypti. Analysis of Aedes genome indicates that it has increased in size relative to other dipterans due to higher transposable element (TE) content [17]. Consistent with TE mobilization, intron size and intergenic distance have increased, but not intron number [17]. In contrast, our analysis of four BACs suggests that Parhyale not only has larger introns and greater intergenic distance, but may also have a global increase in the number of introns per gene (Fig. 4 and Table 2). Unfortunately, the phylogenetic distance between Parhyale and other well-characterized genomes, and the limited amount of genomic sequence obtained thus far, did not allow us to characterize the repeat content of Parhyale. However, similitudes with Aedes suggest that the large genome size of Parhyale may be attributable, at least in part, to an increase in the number of repetitive elements. This question is critical to our understanding of the forces that shaped the crustacean genome and can be investigated further when greater sequence data are available. Although preliminary, our survey of Parhyale BAC sequences suggests that it may have undergone a unique form of genome expansion that could include an increase in intron number, a phenomenon yet to be fully characterized. It is intriguing that a similar phenomenon of intron gain has recently been described in extant populations of another crustacean, Daphnia pulex [36] A similar process occurring in Parhyale could explain the variation in intron position that is observed in Pax group III genes relative to that in other arthropods (Table 2). As an alternative to intron gain, Parhyale could represent an arthropod genome possessing ancestral introns that were lost in other arthropod lineages with sequenced genomes. These two alternatives are not mutually exclusive, although intron loss is expected to be more prevalent and less deleterious to organismal fitness relative to intron gain. Further analysis of arthropod genomes, especially those from crustaceans of varied lineages, and with genomes comparable in size to Parhyale, may shed light on this issue.
Practically speaking, the presence of large and frequent introns in Parhyale will necessitate careful mapping of BAC inserts and/or the use of multiple BACs to study intron-containing loci. For example, the coding region of Parhyale hedgehog occupies a genomic region of 104,631 bp, whereas Drosophila only occupies 12,782 bp. If one considers that the hedgehog BAC chosen for sequencing was 146 kb, of which 104 kb spanned the coding region, it is apparent that our ability to identify a BAC containing both coding and large upstream or downstream regions is limited. Nevertheless, by “walking” through the BAC library we have been able to analyze very large loci, such as the Hox gene complex of Parhyale (N.H.P. and Julia Serano, unpublished data).
In summary, we have generated a BAC library for the emerging model system, Parhyale hawaiensis. Our initial characterization of this library indicates that its genomic coverage is 5X and that the average insert size is 140 kb, thereby providing a valuable resource for genomic studies in Parhyale. Additionally, we have isolated 70 BAC clones for developmentally regulated genes and have undertaken their complete sequencing. Interestingly, the data presented here suggests significant differences in genome structure, particularly in intron size and frequency, in Parhyale relative to the other arthropods that have been sequenced. Importantly, these preliminary findings allow us to generate initial hypotheses regarding genome size evolution. These hypotheses can be tested by both further analyzing genome organization in Parhyale and by increasing the overall phylogenetic diversity of characterized genomes, making certain to avoid a bias towards only small genomes. Since Parhyale is phylogenetically distant from current model systems and analysis of its genome may in part help understand ecdysozoan genome evolution, we believe this BAC library represents an important tool for comparative evolutionary studies.
Materials and Methods
High molecular weight DNA extraction
We isolated high molecular weight (HMW) DNA from forty adult Parhyale hawaiensis (20 males and 20 females) collected from an isofemale population established in 2001 (Iso2). The animals were starved for 6 days, rinsed several times in 0.22 μm filter sterilized sea water (FSW), and treated for 48 hours with antibiotics (Tetracycline 30 μg/mL; 1% PenStrep (Invitrogen)). The specimens were rinsed several times with ice-cold FSW, rinsed twice with ice-cold Homogenization Buffer (HB: 0.35 M sucrose, 0.1 M EDTA, 50 mM Tris pH 8.0), minced grossly with needles, and added to 15 mL of ice cold HB. Nuclei were released with a dounce homogenizer on ice, 30 cycles with the loose pestle and 15 cycles with the tight pestle. The homogenate was filtered through three 100 μm cell strainers (BD Falcon), and then two 70 μm cell strainers (BD Falcon). The suspension was pelleted at 3200 x g and 4°C for 15 min. The nuclei pellet was resuspended in 20 mL ice cold HB, filtered through three successive 70 μm cell strainers (BD Falcon), and pelleted at 3200 x g and 4°C for 15 min. The nuclei were resuspended in 500 μL ice cold HB and incubated at 37°C for 10 min before mixing with 500 μL of 2% Incert agarose (Lonza) prepared in HB and kept at 37°C. The suspension was dispensed in 80 μL block molds (Bio-Rad) and placed at 4°C for 10 min. The blocks were incubated at 37° C in cell lysis solution (CLS: 1% LDS, 10mM Tris pH 8.0, 150mM EDTA pH 8.0) for 4 days, with daily changes of CLS. The CLS was substituted with Block Storage Solution (BSS: 0.2% N-laurylsarcosine, 2mM Tris pH 8.0, 140mM EDTA pH 8.0) by rinsing four times in 2 h. The blocks were stored in BSS at 4°C until processing.
BAC library construction
For size fragmentation, genomic DNA (1/2 block per reaction) was partially digested at 37° C for 2.5 hours in 500 μL reaction volumes containing 1 unit of the restriction enzyme EcoRI (New England Biolabs) and 100 units EcoRI methylase. The quantity of EcoRI and EcoRI methylase was derived from a titration using various amounts of enzymes on 1/6th block per 500 μL reaction. The reaction also contained 2.6mM spermidine, 0.5mg/ml BSA (New England Biolabs) and 1x EcoRI reaction buffer (0.08mM S-adenosylmethionine, 2mM MgCl2, 1mM DTT). The reaction was stopped by adding 300 μg Proteinase K, 2.9 % N-laurylsarcosine, and 0.29 M EDTA, followed by an incubation at room temperature for 1 h. Proteinase K was inactivated by transferring blocks in 15mL of X TE with 15 μL PMSF (100mM), incubating for 20 min at 4°C, and repeating 3 times with fresh solution. Blocks were equilibrated in 50 mL ½X TE for 1 hour at 4°C. DNA fragments were separated on a 1% agarose gel (Pulse-Field certified Bio Rad) using pulse-field gel electrophoresis (BioRad CHEF XA Mapper) in ½X TBE buffer as previously described in Lang et al [37]. Gel fragments were excised from the preparative lane that contained HWM DNA, and the HMW DNA was electroeluted at 4 V/cm for 4 hours at 4°C and then dialyzed at 4°C for 16 h in 4L of ½X TE. The sample volume was reduced to ~180 μL by dialyzing at 4°C against 30% PEG8000 in ½X TE. The CopyControl BAC Cloning Kit (Epicentre) was used for ligation according to the manufacturer s instructions. Approximately 100 ng HMW DNA and 25 ng of the CopyControl pCC1BAC (EcoRI) vector were ligated in 50 μL reactions that were incubated overnight at 16°C. After ligation, reactions were stopped by a brief Proteinase K treatment, deslated by drop dialysis against ddH2O for 1.5 h using 0.025 μm nitrocellulose filters (Millipore) and the sample volume was reduced to ~20 μL by drop dialysis against PEG8000 (30% in X TE) at 4°C. For transformation, 8 μL of ligation reaction was added to 100 μL electrocompetent cells DH10BT1 (Invitrogen). Electroporations were performed using 2mm cuvettes and a BTXECM 630 (Harvard Apparatus Inc.) set at 2.5kV, 225 Ohms, and 25 μF. Transformed cells were recovered in 50 ml conical tubes containing 10 mL SOC (2 transformations per tube), incubated with shaking (250 rpm) for 1 h at 37°C, and glycerol was added to the transformation mix to a final concentration of 10%. Small aliquots were removed for titration and the remaining transformants were flash-frozen in liquid nitrogen and stored at −80°C.
Insert size estimation
The EpiLyse Solution (Epicentre) was used to analyze BAC DNA from 36 random white colonies (12 per size fraction), and estimate the frequency and size of inserts. For further analysis, BAC DNA from 18 clones was isolated using a standard alkaline lysis miniprep protocol and a third of each BAC clone preparation was digested using NotI (New England Biolabs) and the size of each clone was determined using pulse-field gel electrophoresis (15 h, 1 sec initial time, 20 sec final time, 14° C, field angle120°, 6 V/cm) and low range PFG marker (New England Biolabs).
Library arrays and screening
To array the library, transformed cells were grown overnight at 37°C on LB agar plates (12.5 μg/mL chloramphenicol, 0.4 mM IPTG and 40 μg/mL X-GAL) and single white colonies were transfered into 336 x 384-well microtiter plates (Genetix) using a colony-picking robot (Norgren Systems). A Total Array System (BioRobotics) was used to replicate the library and prepare high-density nylon filter sets (22cm x 22cm) containing BAC colony DNA. One filter set was hybridized with α32P-dCTP labeled DNA probes amplified from twenty different cDNAs (Table 1) as previously described [37]. Restriction digestion of positive clones was performed with NotI, followed by pulse-field gel electrophoresis for insert sizing. The Parhyale BAC library is stored in the Patel Lab and filters are available upon request.
Acknowledgments
We would like to thank Lisa Chuong for her assistance with BAC data collection and analysis, William Browne for establishing the isogenic Parhyale population while in the Patel lab. The U.S. Department of Energy Joint Genome Institute provided sequencing and analyses under the Community Sequencing Program supported by the Office of Science of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231, and we thank Jane Grimwood for helping to coordinate the sequencing project. F.P. is a Research Fellow of The Terry Fox Foundation through an award from the National Cancer Institute of Canada. N.H.P. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, George RA, Lewis SE, Richards S, Ashburner M, Henderson SN, Sutton GG, Wortman JR, Yandell MD, Zhang Q, Chen LX, Brandon RC, Rogers YH, Blazej RG, Champe M, Pfeiffer BD, Wan KH, Doyle C, Baxter EG, Helt G, Nelson CR, Gabor GL, Abril JF, Agbayani A, An HJ, Andrews-Pfannkoch C, Baldwin D, Ballew RM, Basu A, Baxendale J, Bayraktaroglu L, Beasley EM, Beeson KY, Benos PV, Berman BP, Bhandari D, Bolshakov S, Borkova D, Botchan MR, Bouck J, Brokstein P, Brottier P, Burtis KC, Busam DA, Butler H, Cadieu E, Center A, Chandra I, Cherry JM, Cawley S, Dahlke C, Davenport LB, Davies P, de Pablos B, Delcher A, Deng Z, Mays AD, Dew I, Dietz SM, Dodson K, Doup LE, Downes M, Dugan-Rocha S, Dunkov BC, Dunn P, Durbin KJ, Evangelista CC, Ferraz C, Ferriera S, Fleischmann W, Fosler C, Gabrielian AE, Garg NS, Gelbart WM, Glasser K, Glodek A, Gong F, Gorrell JH, Gu Z, Guan P, Harris M, Harris NL, Harvey D, Heiman TJ, Hernandez JR, Houck J, Hostin D, Houston KA, Howland TJ, Wei MH, Ibegwam C, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–95. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- 2.Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 1998;282:2012–8. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- 3.Clark AG, Eisen MB, Smith DR, Bergman CM, Oliver B, Markow TA, Kaufman TC, Kellis M, Gelbart W, Iyer VN, Pollard DA, Sackton TB, Larracuente AM, Singh ND, Abad JP, Abt DN, Adryan B, Aguade M, Akashi H, Anderson WW, Aquadro CF, Ardell DH, Arguello R, Artieri CG, Barbash DA, Barker D, Barsanti P, Batterham P, Batzoglou S, Begun D, Bhutkar A, Blanco E, Bosak SA, Bradley RK, Brand AD, Brent MR, Brooks AN, Brown RH, Butlin RK, Caggese C, Calvi BR, Bernardo de Carvalho A, Caspi A, Castrezana S, Celniker SE, Chang JL, Chapple C, Chatterji S, Chinwalla A, Civetta A, Clifton SW, Comeron JM, Costello JC, Coyne JA, Daub J, David RG, Delcher AL, Delehaunty K, Do CB, Ebling H, Edwards K, Eickbush T, Evans JD, Filipski A, Findeiss S, Freyhult E, Fulton L, Fulton R, Garcia AC, Gardiner A, Garfield DA, Garvin BE, Gibson G, Gilbert D, Gnerre S, Godfrey J, Good R, Gotea V, Gravely B, Greenberg AJ, Griffiths-Jones S, Gross S, Guigo R, Gustafson EA, Haerty W, Hahn MW, Halligan DL, Halpern AL, Halter GM, Han MV, Heger A, Hillier L, Hinrichs AS, Holmes I, Hoskins RA, Hubisz MJ, Hultmark D, Huntley MA, Jaffe DB, Jagadeeshan S, et al. Evolution of genes and genomes on the Drosophila phylogeny. Nature. 2007;450:203–18. doi: 10.1038/nature06341. [DOI] [PubMed] [Google Scholar]
- 4.Stein LD, Bao Z, Blasiar D, Blumenthal T, Brent MR, Chen N, Chinwalla A, Clarke L, Clee C, Coghlan A, Coulson A, D'Eustachio P, Fitch DH, Fulton LA, Fulton RE, Griffiths-Jones S, Harris TW, Hillier LW, Kamath R, Kuwabara PE, Mardis ER, Marra MA, Miner TL, Minx P, Mullikin JC, Plumb RW, Rogers J, Schein JE, Sohrmann M, Spieth J, Stajich JE, Wei C, Willey D, Wilson RK, Durbin R, Waterston RH. The genome sequence of Caenorhabditis briggsae: a platform for comparative genomics. PLoS Biol. 2003;1:E45. doi: 10.1371/journal.pbio.0000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 6.Kusserow A, Pang K, Sturm C, Hrouda M, Lentfer J, Schmidt HA, Technau U, von Haeseler A, Hobmayer B, Martindale MQ, Holstein TW. Unexpected complexity of the Wnt gene family in a sea anemone. Nature. 2005;433:156–60. doi: 10.1038/nature03158. [DOI] [PubMed] [Google Scholar]
- 7.Prud'homme B, Lartillot N, Balavoine G, Adoutte A, Vervoort M. Phylogenetic analysis of the Wnt gene family. Insights from lophotrochozoan members. Curr Biol. 2002;12:1395. doi: 10.1016/s0960-9822(02)01068-0. [DOI] [PubMed] [Google Scholar]
- 8.Cho SJ, Valles Y, Giani VC, Jr, Seaver EC, Weisblat DA. Evolutionary dynamics of the Wnt gene family: a lophotrochozoan perspective. Mol Biol Evol. doi: 10.1093/molbev/msq052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Putnam NH, Srivastava M, Hellsten U, Dirks B, Chapman J, Salamov A, Terry A, Shapiro H, Lindquist E, Kapitonov VV, Jurka J, Genikhovich G, Grigoriev IV, Lucas SM, Steele RE, Finnerty JR, Technau U, Martindale MQ, Rokhsar DS. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science. 2007;317:86–94. doi: 10.1126/science.1139158. [DOI] [PubMed] [Google Scholar]
- 10.Jeffares DC, Mourier T, Penny D. The biology of intron gain and loss. Trends Genet. 2006;22:16–22. doi: 10.1016/j.tig.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Loh YH, Brenner S, Venkatesh B. Investigation of loss and gain of introns in the compact genomes of pufferfishes (Fugu and Tetraodon) Mol Biol Evol. 2008;25:526–35. doi: 10.1093/molbev/msm278. [DOI] [PubMed] [Google Scholar]
- 12.Richards S, Gibbs RA, Weinstock GM, Brown SJ, Denell R, Beeman RW, Gibbs R, Bucher G, Friedrich M, Grimmelikhuijzen CJ, Klingler M, Lorenzen M, Roth S, Schroder R, Tautz D, Zdobnov EM, Muzny D, Attaway T, Bell S, Buhay CJ, Chandrabose MN, Chavez D, Clerk-Blankenburg KP, Cree A, Dao M, Davis C, Chacko J, Dinh H, Dugan-Rocha S, Fowler G, Garner TT, Garnes J, Gnirke A, Hawes A, Hernandez J, Hines S, Holder M, Hume J, Jhangiani SN, Joshi V, Khan ZM, Jackson L, Kovar C, Kowis A, Lee S, Lewis LR, Margolis J, Morgan M, Nazareth LV, Nguyen N, Okwuonu G, Parker D, Ruiz SJ, Santibanez J, Savard J, Scherer SE, Schneider B, Sodergren E, Vattahil S, Villasana D, White CS, Wright R, Park Y, Lord J, Oppert B, Brown S, Wang L, Weinstock G, Liu Y, Worley K, Elsik CG, Reese JT, Elhaik E, Landan G, Graur D, Arensburger P, Atkinson P, Beidler J, Demuth JP, Drury DW, Du YZ, Fujiwara H, Maselli V, Osanai M, Robertson HM, Tu Z, Wang JJ, Wang S, Song H, Zhang L, Werner D, Stanke M, Morgenstern B, Solovyev V, Kosarev P, Brown G, Chen HC, Ermolaeva O, Hlavina W, Kapustin Y, et al. The genome of the model beetle and pest Tribolium castaneum. Nature. 2008;452:949–55. doi: 10.1038/nature06784. [DOI] [PubMed] [Google Scholar]
- 13.Werren JH, Richards S, Desjardins CA, Niehuis O, Gadau J, Colbourne JK, Beukeboom LW, Desplan C, Elsik CG, Grimmelikhuijzen CJ, Kitts P, Lynch JA, Murphy T, Oliveira DC, Smith CD, van de Zande L, Worley KC, Zdobnov EM, Aerts M, Albert S, Anaya VH, Anzola JM, Barchuk AR, Behura SK, Bera AN, Berenbaum MR, Bertossa RC, Bitondi MM, Bordenstein SR, Bork P, Bornberg-Bauer E, Brunain M, Cazzamali G, Chaboub L, Chacko J, Chavez D, Childers CP, Choi JH, Clark ME, Claudianos C, Clinton RA, Cree AG, Cristino AS, Dang PM, Darby AC, de Graaf DC, Devreese B, Dinh HH, Edwards R, Elango N, Elhaik E, Ermolaeva O, Evans JD, Foret S, Fowler GR, Gerlach D, Gibson JD, Gilbert DG, Graur D, Grunder S, Hagen DE, Han Y, Hauser F, Hultmark D, Hunter HCt, Hurst GD, Jhangian SN, Jiang H, Johnson RM, Jones AK, Junier T, Kadowaki T, Kamping A, Kapustin Y, Kechavarzi B, Kim J, Kiryutin B, Koevoets T, Kovar CL, Kriventseva EV, Kucharski R, Lee H, Lee SL, Lees K, Lewis LR, Loehlin DW, Logsdon JM, Jr, Lopez JA, Lozado RJ, Maglott D, Maleszka R, Mayampurath A, Mazur DJ, McClure MA, Moore AD, Morgan MB, Muller J, Munoz-Torres MC, Muzny DM, Nazareth LV, et al. Functional and evolutionary insights from the genomes of three parasitoid Nasonia species. Science. 2010;327:343–8. doi: 10.1126/science.1178028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Insights into social insects from the genome of the honeybee Apis mellifera. Nature. 2006;443:931–49. doi: 10.1038/nature05260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR, Wincker P, Clark AG, Ribeiro JM, Wides R, Salzberg SL, Loftus B, Yandell M, Majoros WH, Rusch DB, Lai Z, Kraft CL, Abril JF, Anthouard V, Arensburger P, Atkinson PW, Baden H, de Berardinis V, Baldwin D, Benes V, Biedler J, Blass C, Bolanos R, Boscus D, Barnstead M, Cai S, Center A, Chaturverdi K, Christophides GK, Chrystal MA, Clamp M, Cravchik A, Curwen V, Dana A, Delcher A, Dew I, Evans CA, Flanigan M, Grundschober-Freimoser A, Friedli L, Gu Z, Guan P, Guigo R, Hillenmeyer ME, Hladun SL, Hogan JR, Hong YS, Hoover J, Jaillon O, Ke Z, Kodira C, Kokoza E, Koutsos A, Letunic I, Levitsky A, Liang Y, Lin JJ, Lobo NF, Lopez JR, Malek JA, McIntosh TC, Meister S, Miller J, Mobarry C, Mongin E, Murphy SD, O'Brochta DA, Pfannkoch C, Qi R, Regier MA, Remington K, Shao H, Sharakhova MV, Sitter CD, Shetty J, Smith TJ, Strong R, Sun J, Thomasova D, Ton LQ, Topalis P, Tu Z, Unger MF, Walenz B, Wang A, Wang J, Wang M, Wang X, Woodford KJ, Wortman JR, Wu M, Yao A, Zdobnov EM, Zhang H, Zhao Q, et al. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298:129–49. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- 16.Xia Q, Zhou Z, Lu C, Cheng D, Dai F, Li B. A draft sequence for the genome of the domesticated silkworm (Bombyx mori) Science. 2004 doi: 10.1126/science.1102210. [DOI] [PubMed] [Google Scholar]
- 17.Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi Z, Megy K, Grabherr M, Ren Q, Zdobnov EM, Lobo NF, Campbell KS, Brown SE, Bonaldo MF, Zhu J, Sinkins SP, Hogenkamp DG, Amedeo P, Arensburger P, Atkinson PW, Bidwell S, Biedler J, Birney E, Bruggner RV, Costas J, Coy MR, Crabtree J, Crawford M, Debruyn B, Decaprio D, Eiglmeier K, Eisenstadt E, El-Dorry H, Gelbart WM, Gomes SL, Hammond M, Hannick LI, Hogan JR, Holmes MH, Jaffe D, Johnston JS, Kennedy RC, Koo H, Kravitz S, Kriventseva EV, Kulp D, Labutti K, Lee E, Li S, Lovin DD, Mao C, Mauceli E, Menck CF, Miller JR, Montgomery P, Mori A, Nascimento AL, Naveira HF, Nusbaum C, O'Leary S, Orvis J, Pertea M, Quesneville H, Reidenbach KR, Rogers YH, Roth CW, Schneider JR, Schatz M, Shumway M, Stanke M, Stinson EO, Tubio JM, Vanzee JP, Verjovski-Almeida S, Werner D, White O, Wyder S, Zeng Q, Zhao Q, Zhao Y, Hill CA, Raikhel AS, Soares MB, Knudson DL, Lee NH, Galagan J, Salzberg SL, Paulsen IT, Dimopoulos G, Collins FH, Birren B, Fraser-Liggett CM, Severson DW. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;316:1718–23. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genome Sequence of the Pea Aphid Acyrthosiphon pisum. PLoS Biol. 2010;8:e1000313. doi: 10.1371/journal.pbio.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris TW, Chen N, Cunningham F, Tello-Ruiz M, Antoshechkin I, Bastiani C, Bieri T, Blasiar D, Bradnam K, Chan J, Chen CK, Chen WJ, Davis P, Kenny E, Kishore R, Lawson D, Lee R, Muller HM, Nakamura C, Ozersky P, Petcherski A, Rogers A, Sabo A, Schwarz EM, Van Auken K, Wang Q, Durbin R, Spieth J, Sternberg PW, Stein LD. WormBase: a multi-species resource for nematode biology and genomics. Nucleic Acids Res. 2004;32:D411–7. doi: 10.1093/nar/gkh066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budd GE, Telford MJ. The origin and evolution of arthropods. Nature. 2009;457:812–7. doi: 10.1038/nature07890. [DOI] [PubMed] [Google Scholar]
- 21.Regier JC, Shultz JW, Zwick A, Hussey A, Ball B, Wetzer R, Martin JW, Cunningham CW. Arthropod relationships revealed by phylogenomic analysis of nuclear protein-coding sequences. Nature. 2010;463:1079–83. doi: 10.1038/nature08742. [DOI] [PubMed] [Google Scholar]
- 22.Browne WE, Price AL, Gerberding M, Patel NH. Stages of embryonic development in the amphipod crustacean, Parhyale hawaiensis. Genesis. 2005;42:124–49. doi: 10.1002/gene.20145. [DOI] [PubMed] [Google Scholar]
- 23.Pavlopoulos A, Averof M. Establishing genetic transformation for comparative developmental studies in the crustacean Parhyale hawaiensis. Proc Natl Acad Sci U S A. 2005;102:7888–93. doi: 10.1073/pnas.0501101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rehm EJ, Hannibal RL, Chaw RC, Vargas-Vila MA, Patel NH. The crustacean Parhyale hawaiensis: a new model for arthropod development. In: Gann A, Crotty D, editors. Emerging Model Organisms, A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2009. pp. 373–404. [DOI] [PubMed] [Google Scholar]
- 25.Hwang UW, Friedrich M, Tautz D, Park CJ, Kim W. Mitochondrial protein phylogeny joins myriapods with chelicerates. Nature. 2001;413:154–7. doi: 10.1038/35093090. [DOI] [PubMed] [Google Scholar]
- 26.Osoegawa K, Woon PY, Zhao B, Frengen E. An improved approach for construction of bacterial artificial chromosome libraries. Genomics. 1998 doi: 10.1006/geno.1998.5423. [DOI] [PubMed] [Google Scholar]
- 27.Amemiya CT, Zhong TP, Silverman GA, Fishman MC, Zon LI. Zebrafish YAC, BAC, and PAC genomic libraries. Methods Cell Biol. 1999;60:235–58. doi: 10.1016/s0091-679x(08)61904-4. [DOI] [PubMed] [Google Scholar]
- 28.Shizuya H, Birren B, Kim UJ, Mancino V, Slepak T, Tachiiri Y, Simon M. Cloning and stable maintenance of 300-kilobase-pair fragments of human DNA in Escherichia coli using an F-factor-based vector. Proc Natl Acad Sci U S A. 1992;89:8794–7. doi: 10.1073/pnas.89.18.8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito K, Kidokoro K, Sezutsu H, Nohata J, Yamamoto K, Kobayashi I, Uchino K, Kalyebi A, Eguchi R, Hara W, Tamura T, Katsuma S, Shimada T, Mita K, Kadono-Okuda K. Deletion of a gene encoding an amino acid transporter in the midgut membrane causes resistance to a Bombyx parvo-like virus. Proc Natl Acad Sci USA. 2008;105:7523–7. doi: 10.1073/pnas.0711841105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahara K, Yoshido A, Marec F, Fuková I, Zhang H, Wu CC, Goldsmith M, Yasukochi Y. Conserved synteny of genes between chromosome 15 of Bombyx mori and a chromosome of Manduca sexta shown by five-color BAC-FISH. Genome. 2007;50:1061–5. doi: 10.1139/g07-082. [DOI] [PubMed] [Google Scholar]
- 31.Buckingham M, Relaix F. The role of Pax genes in the development of tissues and organs: Pax3 and Pax7 regulate muscle progenitor cell functions. Annu Rev Cell Dev Biol. 2007;23:645–73. doi: 10.1146/annurev.cellbio.23.090506.123438. [DOI] [PubMed] [Google Scholar]
- 32.Davis GK, D'Alessio JA, Patel NH. Pax3/7 genes reveal conservation and divergence in the arthropod segmentation hierarchy. Dev Biol. 2005;285:169–84. doi: 10.1016/j.ydbio.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Davis GK, Jaramillo CA, Patel NH. Pax group III genes and the evolution of insect pair-rule patterning. Development. 2001;128:3445–58. doi: 10.1242/dev.128.18.3445. [DOI] [PubMed] [Google Scholar]
- 34.Breitling R, Gerber JK. Origin of the paired domain. Dev Genes Evol. 2000;210:644–50. doi: 10.1007/s004270000106. [DOI] [PubMed] [Google Scholar]
- 35.Matus DQ, Pang K, Daly M, Martindale MQ. Expression of Pax gene family members in the anthozoan cnidarian, Nematostella vectensis. Evol Dev. 2007;9:25–38. doi: 10.1111/j.1525-142X.2006.00135.x. [DOI] [PubMed] [Google Scholar]
- 36.Li W, Tucker AE, Sung W, Thomas WK, Lynch M. Extensive, recent intron gains in Daphnia populations. Science. 2009;326:1260–2. doi: 10.1126/science.1179302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lang M, Miyake T, Braasch I, Tinnemore D, Siegel N, Salzburger W, Amemiya C, Meyer A. A BAC library of the East African haplochromine cichlid fish Astatotilapia burtoni. J Exp Zool B Mol Dev Evol. 2006;306:35–44. doi: 10.1002/jez.b.21068. [DOI] [PubMed] [Google Scholar]