Summary
Fermented grains of buckwheat, oat, embryo rice and wheat, which were prepared by solid-state fermentation with Antrodia salmonea, and the mycelium was used to substitute 7% of wheat flour to make bread. No difference in proximate composition, texture profile and contents of non-volatile taste components was observed among bread samples. White bread and bread supplemented with mycelium and fermented grains looked different. Bread supplemented with fermented grains had similar thermal properties, which differed from those of white bread and bread supplemented with mycelium. Bread supplemented with fermented grains contained substantial mass fractions (on dry mass basis) of adenosine (0.92–1.96 µg/g), ergosterol (24.53–30.12 µg/g), ergothioneine (2.16–3.18 µg/g) and γ-aminobutyric acid (2.20–2.45 µg/g). In addition, bread supplemented with mycelium contained lovastatin (0.43 µg/g). White bread and bread supplemented with fermented grains had similar sensory results. Overall, fermented grains could be incorporated into bread to provide beneficial effects.
Key words: Antrodia salmonea, mycelium, fermented grain, bread, colour, sensory evaluation
Introduction
Antrodia salmonea T.T. Chang et W.N. Chou (Taiwanofungus salmoneus, Polyporaceae, Agaricomycotina), also called shiang-shan-chih, which usually grows on the empty rotten trunks of Cunninghamia konishii Hayata (Luanta fir or shiang-shan), is a medicinal fungus indigenous to Taiwan and used as a traditional Chinese medicine (1). The fruiting bodies have been used to remedy diarrhoea, abdominal pain, hypertension, itchy skin and liver cancer, and are also used as detoxicants in Taiwanese folk medicine (2). Several compounds isolated from A. salmonea exhibit antioxidant activities in human leukocytes and anti- -inflammatory activities in activated inflammatory cells (2, 3). Moreover, the fruiting bodies have shown to decrease inflammatory responses via inhibition of inducible nitric oxide oxidase and cyclooxygenase-2, reducing inflammatory cytokine production and enhancing antioxidant enzyme activities (4). In addition, its mycelium contains various bioactive components including lovastatin, γ-aminobutyric acid (GABA) and ergothioneine (5).
Solid-state fermentation is particularly suitable for propagating fungi used for food, medicine or health purposes. It generally runs at low water content, which reduces the risk of contamination and also offers favourable conditions for fungal growth, because it resembles the natural habitat of fungi. Solid-state fermentation of grains by A. salmonea has been studied and the proximate composition and non-volatile components of fermented products has been evaluated (6).
Bread made with wheat flour, water, salt and yeast is a staple food consumed globally. In addition to these ingredients, buckwheat (7) or Monascus-fermented rice (anka) (8) have been incorporated into bread formulation to increase the diversity, nutritional value and the appeal to the consumer. Besides that, fruiting bodies, including shiitake stipe (9), silver ear (10), mycelia (11) and fungal chitin (12) have been added into bread.
Accordingly, the objective of this study is to substitute 7% of wheat flour with A. salmonea-fermented buckwheat, oat, embryo rice or wheat during bread preparation. White bread and bread supplemented with A. salmonea mycelium were also made for comparison. The bread quality evaluation included analysis of specific volume, colour characteristics, proximate composition, texture profile, thermal properties and sensory evaluation. The mass fractions of non-volatile taste components and functional compounds were also determined.
Materials and Methods
Materials
High-gluten wheat flour, milk powder, sugar, salt, egg and yeast were obtained from Tzong-Hsin food ingredient company, Taichung, Taiwan. High-gluten wheat flour contained 13.63% of protein and 0.43% of ash at the moisture content of 14%. Wheat, buckwheat, oat and embryo rice were purchased at a local market in Taichung City, Taiwan. Mycelium of Antrodia salmonea was supplied by the Biotechnology Center, Grape King Inc., Chungli City, Taiwan. The mycelium and fermented grains were prepared in accordance with the recommended procedures (5). The pure mycelium culture was inoculated into basal medium and incubated at 25 °C for 14 days. The basal medium contained the following (in g/L): glucose 20, yeast extract 5, (NH4)2SO4 0.5, MgSO4·7H2O 0.5, KH2PO4 0.875 and K2HPO4 0.125. The culture was then homogenised in a Waring blender (51BL31; Torrington, CT, USA) and inoculated into autoclaved wheat, buckwheat, oat or embryo rice supplemented with 1% fructose and 2% soybean meal. New corresponding products were then produced after the colonization of fungal mycelium at 25 °C for 28 days. The four fermented products (buckwheat, oat, embryo rice and wheat fermented with A. salmonea) were freeze-dried, ground in a mill (RT-30HS; Rong Tsong Precision Technology Co., Taichung, Taiwan), and screened through a 0.5-mm sieve.
The ingredients for white bread making were as follows (in g): wheat flour 100, milk powder 4, sugar 10, salt 1.5, egg 8, yeast 1.5, shortening 10, and water 52 mL, with a total mass of 187 g. The ingredients for bread incorporated with mycelium or fermented grains contained the same as above, except for wheat flour 93 g, mycelium powder or fermented grains 7 g and water 57 mL, with a total mass of 192 g.
According to the requirements of Chinese National Standards, for bread to have a value-added health claim, the substitution of wheat flour with functional ingredients must be 5% (13). Accordingly, before bread making, the dough fermentation was studied to determine the maximal substitution with mycelium and fermented grains to maintain the same dough volume as that of white bread. The maximum substitution was found to be 7% and the volume of water added to make the dough was 5 mL higher than that in white bread.
Bread making and processing
Straight dough was made according to the previously described method (9). First, yeast powder was dispersed in water at 28 °C and then dry ingredients were added and mixed to form a paste. Shortening was melted and added into the paste. A SP-7MX mixer (SPAR Food Machinery Co., Taichung, Taiwan) was used to mix the paste at low speed for 2 min, then at high speed for 6 min. After the dough had been completely mixed, it was placed in the incubator (HT-16GP; Huang Ta Industrial Co., Taichung, Taiwan) at 28 °C and 75% relative humidity (RH) to ferment. The total fermentation time was 125 min.
After 60 min, the dough was removed from the incubator, punched and put back into the incubator. After 15 min, the dough was taken out again to punch as described above. The dough was divided into equal pieces of approx. 560 g. Each dough piece was shaped, placed into a pan and taken back to the incubator for the last 50-minute fermentation.
Conventional baking was performed at 200 °C for 40 min in an oven (PRO-300S3-4AP-D; Chung Pu Baking Machinery Co., Taichung, Taiwan). After baking, the finished product was cooled to room temperature for 2 h and weighed. The volume of bread loaves was measured according to the rapeseed displacement method (14) and expressed as specific volume (cm3/g). Three loaves of each type of bread were freeze-dried, ground into powder (particle size φ=0.5 mm) and stored at 20 °C for further analysis.
Colour measurement
The colour of bread crumbs was measured with a Sigma 80 colour difference meter (Nippon Denshoku Industries Co., Tokyo, Japan). Lightness (L), which ranges from 0 (darkness) to 100 (whiteness), positive and negative a values, which represent redness and greenness, respectively, and positive and negative b values, which represent blueness and yellowness, respectively, were recorded. Three samples of each type of bread were measured and colour difference (ΔE) was calculated using the following equation (15):
where L0, a0 and b0 are the values of white bread, and L, a and b are the values of bread with added mycelium or fermented grains.
Texture profile analysis
Within 2 to 6 h after baking, bread samples were cut into cubes of 2.5 cm×2.5 cm×2.5 cm and texture measurements were immediately carried out using a TA.XT2 texture analyser (Stable Micro Systems Ltd., Godalming, UK) with a 25-kg load cell. Six samples of each type of bread were analysed using a P30C cylinder probe (30 mm diameter; Stable Micro Systems) with a pre-test speed of 2 mm/s, test speed of 2 mm/s, post speed of 2 mm/s, and compression of 50%.
Thermal analysis
Thermal analysis was carried out using a DSC121 differential scanning calorimeter (Setaram Instrumentation, Caluire, France). Freeze-dried samples (100–140 mg) were weighed into a stainless crucible with an aluminium O- -ring. The crucible was hermetically sealed using a sample encapsulation press and heated from 30 to 350 °C at the rate of 5 °C/min. As a reference, an empty stainless crucible was used and indium was used for calibrating the instrument. For each sample, temperatures of onset (to), peak (tp) and completion (tc) and peak enthalpy (ΔH) were recorded.
Proximate analysis
The mass fractions of water (moisture content), crude ash, crude fat, crude fibre and crude protein in the bread samples were determined based on the methods of AOAC 14.091 (16), 14.103 (17), 14.093 (18), 14.111 (19) and 14.108 (20). For crude protein determination, a nitrogen conversion factor of 5.70 was used. The carbohydrate mass fraction was calculated by subtracting the mass fractions of crude ash, fat, fibre and protein from total dry matter (100%). Total reducing sugars were determined using the 3,5-dinitrosalicylic acid method (21). The absorbance of each sample solution was measured by a U-2900 spectrophotometer (Hitachi, Tokyo, Japan) at 540 nm and the mass fraction of total reducing sugars was calculated based on the calibration curve of glucose.
Analysis of non-volatile taste components
Soluble sugars, and amino acids and 5’-nucleotides that give umami taste were extracted and analysed according to the methods of Mau et al. (22) and Taylor et al. (23). For sugar assay, each bread powder was extracted with 80% ethanol by shaking for 45 min at room temperature and filtered. The filtrate was then evaporated on a rotary evaporator at 40 °C and redissolved in deionised water. The aqueous extract was filtered using a 0.45-µm PVDF membrane filter (Merck Millipore, Billerica, MA, USA) prior to injection into high-performance liquid chromatography (HPLC) system equipped with a RID- -10A refractive index detector (Shimadzu, Tokyo, Japan) and a Luna® NH2 100 Ĺ LC column (4.6 mm×250 mm, i.d. 5 µm; Phenomenex, Torrance, CA, USA). The mobile phase was acetonitrile (Tedia, Fairfield, OH, USA) and deionised water in a volume ratio of 85:15 and flow rate was 1.0 mL/min.
The synergistic effect of disodium 5’-inosinate (5’- -IMP) and monosodium glutamate (MSG), which provide umami taste, can be elucidated according to the following equation (24):
where Y is the mass fraction of MSG that gives the same intensity of the umami taste as the mixture of 5’-IMP and MSG (in g of MSG per 100 g), w(MSG) is the mass fraction of MSG in the mixture (in %), w(5’-IMP) is the mass fraction of 5′-IMP in the mixture (in %), and r is a synergistic constant (1218).
For determination of amino acid content, each bread powder was treated with 0.1 mol/L of HCl for 45 min at ambient temperature and filtered using a 0.45-µm PVDF membrane filter (Merck Millipore). The purified filtrate was mixed with o-phthalaldehyde reagent (Sigma-Aldrich, St. Louis, MO, USA) in an Eppendorf® tube (Eppendorf, Hauppauge, NY, USA), shaken to facilitate derivatisation, and then immediately injected into HPLC. The HPLC system consisted of an L-7485 fluorescence detector (Hitachi) working at an excitation wavelength of 340 nm and emission wavelength of 450 nm, and a LiChrospher® 100 RP-18 column (4.6 mm×250 mm, i.d. 5 µm; Merck Millipore, Darmstadt, Germany). The mobile phase consisted of 50 mmol/L of sodium acetate at pH=5.7, containing 0.5% tetrahydrofuran (solvent A), deionised water (solvent B) and methanol (solvent C). The linear gradient elution was performed using A:B:C in a volume ratio from 80:0:20 to 33:0:67 at 0–38 min, then to 0:33:67 at 38–40 min, and to 0:100:0 at 40–43 min, with the flow rate of 1.2 mL/min.
For the analysis of 5’-nucleotide content, each bread powder was dissolved in deionised water. This suspension was boiled for 1 min, cooled, and then centrifuged at 11 800×g for 15 min. The filtrate was then evaporated, and filtered prior to HPLC injection. The HPLC system included a RID-10A refractive index detector (Shimadzu) and a LiChrospher® 100 RP-18 column (Merck Millipore). The mobile phase was 0.5 mol/L of KH2PO4 and H3PO4 (pH=4.3; Wako Pure Chemical Industries, Osaka, Japan), and the analysis was performed at a flow rate of 1 mL/min and UV detection at 254 nm. Each sugar, amino acid and 5’-nucleotide content was calculated based on the calibration curve of the respective standard (Sigma-Aldrich).
Determination of mass fractions of functional compounds
Mass fraction of γ-aminobutyric acid (GABA) was determined following the procedure described above for the analysis of amino acid content.
Lovastatin and ergothioneine were extracted and analysed according to the method used by Yang et al. (6). Lovastatin was extracted from each bread powder by stirring with 30 mL of acetonitrile at 25 °C and 150 rpm for 24 h. The extract was then evaporated using a rotary evaporator at 40 °C, filtered and injected into HPLC. The HPLC system included an L-2455 diode array detector (Hitachi), and a LiChrospher® 100 RP-18 column (Merck Millipore). The mobile phase was acetonitrile and deionised water in a volume ratio of 85:15, and for the analysis a flow rate of 1.0 mL/min and UV detection at 254 nm were used. For ergothioneine assay, each bread powder was added to the mixture of 10 mmol/L of 1,4-dithiothreitol, 100 mmol/L of betaine and 100 mmol/L of 2-mercapto-1-methylimidazole in 70% ethanol, and vortexed for 90 s. After 1% sodium dodecyl sulphate solution was added, the mixture was centrifuged at 25 °C and 3000×g for 10 min. The supernatant was then evaporated on a rotary evaporator at 40 °C and filtered. The HPLC system included a diode array detector, and a Luna® PFP(2) 100 Ĺ column (4.6 mm×250 mm, 5 µm i.d.; Phenomenex). The mobile phase was 500 mmol/L of sodium phosphate in water with 3% acetonitrile and 0.1% triethylamine, adjusted to the pH= 7.3, and the analysis was performed at a flow rate of 1 mL/min and UV detection at 254 nm.
Adenosine and ergosterol were analysed following the methods of Liu et al. (25) and Liang et al. (26), respectively. For determining adenosine content, each bread powder was mixed with deionised water, then sonicated at 40 °C for 30 min and centrifuged at 3000×g for 10 min. The filtrate was evaporated on a rotary evaporator at 40 °C, redissolved in deionised water, and filtered prior to HPLC injection. The HPLC system included a UV detector and a LiChrospher® 100 RP-18e column (Merck Millipore). The mobile phase was methanol and 0.02 mol/L of KH2PO4 in a volume ratio of 18:85, the flow rate was 1.0 mL/min, and UV detection was done at 254 nm.
For ergosterol content estimation, each bread powder was mixed with n-hexane in a vortex mixer for 1.5 min and then centrifuged at 4000×g for 5 min. The filtrate was evaporated and then redissolved in methanol prior to HPLC injection. The HPLC system included a UV detector and a LiChrospher® 100 RP-18 column (Merck Millipore). The isocratic mobile phase was methanol, the flow rate was 1.2 mL/min, and UV detection was performed at 282 nm. GABA, lovastatin, ergothioneine, adenosine and ergosterol were quantified by the calibration curve of the respective standard (Sigma-Aldrich).
Sensory evaluation
Within 3 to 6 h after baking, the bread samples were sliced (1.5 cm thick) with a bread slicer (TBS31; Omega, Hebei, China) for sensory evaluation at the University campus, Taichung, Taiwan. A total of 59 untrained consumers at the age from 19 to 30 had completed the questionnaire. Five sensory attributes including appearance, colour, flavour, mouthfeel and overall acceptability were measured for each type of bread. The values of 1, 4 and 7 on the seven-point hedonic scale represented extremely dislike, neither like nor dislike and extremely like, respectively.
Statistical analysis
For each type of bread, quality measurements were conducted in triplicate, except for texture profile analysis (N=6) and sensory evaluation (N=59). The experimental data were expressed as mean value±standard error and subjected to an analysis of variance for a completely random design using a Statistical Analysis System v. 9.2 (SAS Institute, Inc., Cary, NC, USA). Duncan’s multiple range test was used to determine the differences among the mean values at the level of p=0.05.
Results and Discussion
Specific volume and colour characteristics of bread samples
Mushroom mycelium and fermented grains have been found to hold more water than wheat flour. It seems that more water was needed for the hydration of mycelium and fermented grains. Therefore, more water was added to the dough in order not to influence the hydration of wheat flour, and to maintain the final volume of the bread. The observation is consistent with the findings in previous works (9–11) where fruiting body and mycelium were used. Therefore, the bread supplemented with 7% mycelium and fermented grain required additional 5% of water. Specific volumes of bread containing mycelium and fermented grain ranged from 5.7 to 6.3 cm3/g; significantly smaller than that of white bread (Table 1). The range of 3.5–6 cm3/g is the standard specific volume of bread (13). Nevertheless, all bread samples supplemented with mycelium and fermented grains had acceptable specific volumes.
Table 1. Specific volume, colour characteristics, texture profile and thermal properties of bread samples.
| Bread sample | ||||||
|---|---|---|---|---|---|---|
| White | A | B | C | D | E | |
| Specific volume/(cm3/g) | (6.6±0.3)a | (6.1±0.1)b | (5.7±0.3)c | (6.1±0.2)b | (6.2±0.3)b | (6.3±0.2)b |
| L | (60.5±1.7)a | (30.2±1.4)d | (37.8±1.5)c | (46.3±1.2)b | (43.1±1.7)b | (39.5±2.1)bc |
| a | (0.5±0.2)e | (5.5±0.3)a | (3.0±0.2)b | (1.1±0.2)cd | (0.9±0.2)d | (1.3±0.1)c |
| b | (6.8±0.2)d | (12.3±0.6)a | (9.2±0.3)b | (7.8±0.1)c | (7.2±0.3)c | (8.9±0.4)b |
| ΔE | – | (31.3±0.5)a | (23.0±0.2)b | (14.3±0.5)d | (17.5±0.1)c | (21.2±0.5)b |
| Hardness/g | (36.1±0.3)a | (37.6±0.7)a | (36.5±0.3)a | (36.1±0.5)a | (36.3±0.4)a | (36.1±0.4)a |
| Springiness | (0.89±0.02)a | (0.88±0.01)a | (0.87±0.02)a | (0.88±0.01)a | (0.89±0.01)a | (0.88±0.02)a |
| Cohesiveness | (0.64±0.01)a | (0.62±0.01)a | (0.63±0.02)a | (0.63±0.01)a | (0.63±0.02)a | (0.64±0.02)a |
| Chewiness/g | (20.6±0.1)a | (20.5±0.1)a | (20.0±0.1)a | (20.0±0.1)a | (20.4±0.1)a | (20.3±0.1)a |
| Resilience | (0.35±0.02)a | (0.33±0.01)b | (0.34±0.01)a | (0.34±0.01)a | (0.34±0.02)a | (0.34±0.02)a |
| to/°C | (50.8±0.3)d | (63.1±0.4)a | (57.9±0.3)c | (60.7±0.5)b | (58.1±0.2)c | (58.4±0.4)c |
| tp/°C | (103.0±0.8)a | (66.3±0.3)d | (89.3±0.4)c | (88.6±0.6)c | (87.2±0.2)c | (96.0±0.3)b |
| tf/°C | (120.5±1.5)e | (128.0±1.4)d | (115.7±2.3)f | (139.7±2.0)c | (165.4±0.9)a | (145.8±1.6)b |
| ΔH/(J/g) | (140.5±3.0)a | (94.3±1.1)c | (123.0±1.1)b | (126.0±0.9)b | (127.1±1.5)b | (125.1±1.7)b |
Bread supplemented with: A=Antrodia salmonea mycelium, B=fermented buckwheat, C=fermented oat, D=fermented embryo rice, E=fermented wheat. Values are expressed as mean±standard error (N=3 or N=6 for texture profile analysis). Mean values with different superscript letters within a row differ significantly (p<0.05) to=onset, tp=peak and tf=final temperature; ΔH=peak enthalpy expressed on dry mass basis
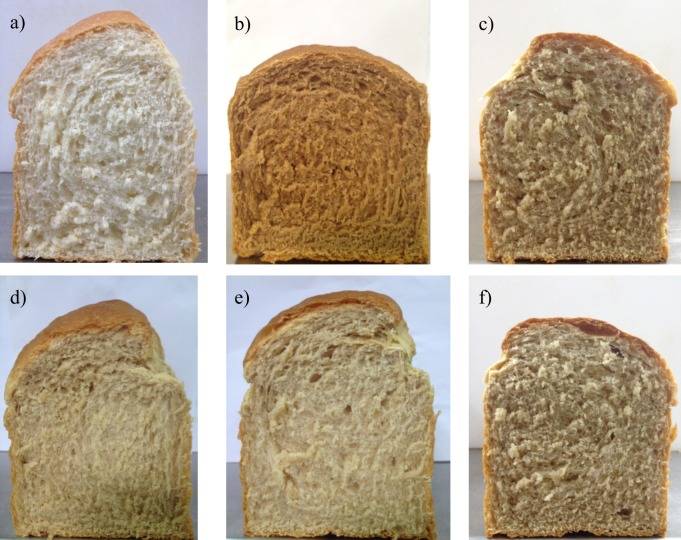
White bread had higher L and lower a and b values than all bread samples supplemented with mycelium and fermented grains (Table 1). The calculated ΔE values were 31.3, 23.0, 14.3, 17.5 and 21.2 for bread samples supplemented with mycelium only, or mycelium and fermented buckwheat, oat, embryo rice or wheat, respectively. Hsu (15) found that ΔE values of 3–6 were acceptable since only professionals could distinguish the difference. The ΔE value of >6.5 was high enough for average consumer to notice the colour difference (15). Therefore, bread supplemented with mycelium and fermented grains looked different from the white bread, as shown in Fig. 1. In addition, the bread supplemented with A. salmonea looked different from the bread samples supplemented with fermented grains. However, the bread samples containing fermented buckwheat, embryo rice and wheat grains were indistinguishable among themselves, whereas those with oat, embryo rice and wheat grains looked similar due to their low ΔE differences of <6.9. Proper colour change in bread might attract consumer’s attention and affect their preference and acceptability. It seems that bread supplemented with mycelium and fermented grains had a distinctive colour quality.
Fig. 1.
Finished bread products: a) white bread, and bread supplemented with: b) Antrodia salmonea mycelium, c) A. salmonea-fermented buckwheat, d) A. salmonea-fermented oat, e) A. salmonea-fermented embryo rice, and f) A. salmonea-fermented wheat
Texture of bread samples
Texture profile analysis showed that all bread samples had similar properties of hardness, springiness, cohesiveness, chewiness and resilience (Table 1). It seems that 7% supplementation with A. salmonea mycelium and fermented grains had no influence on the physical quality attributes of the bread samples. Ulziijargal et al. (11) found that the 5% substitution with mycelium showed no difference among bread samples containing Agaricus blazei mycelium, Antrodia camphorata mycelium and white bread. However, the bread containing Hericium erinaceus mycelium was harder than the bread containing Phellinus linteus mycelium. Lin et al. (27) found that 15% substitution with buckwheat resulted in the bread with greater hardness. In this research, 7% supplementation with fermented wheat grain did not change the hardness of the bread. This might be due to the fact that buckwheat was degraded during fermentation.
Thermal properties of bread samples
Thermal properties of all bread samples were analysed at temperatures from 30 to 350 °C using differential scanning calorimetry. The onset and final temperatures of white bread were 50.8 and 120.5 °C, those of bread supplemented with mycelium 63.07 and 127.99 °C, and those of bread supplemented with fermented grains 57.9–60.7 and 115.7–165.4 °C, respectively (Table 1). However, the thermal decomposition enthalpies (ΔH) of bread samples were in the range of 94.3–140.5 J/g (on dry mass basis) and were in the descending order: white bread>bread supplemented with fermented grains>>bread supplemented with mycelium. It seems that the bread supplemented with mycelium had the highest onset, lowest peak and highest final temperatures. However, bread supplemented with fermented grains had comparable onset, peak and final temperatures.
Yen and Mau (28) noted that the higher the peak enthalpy, the denser the crystallinity. Therefore, white bread should show the densest crystallinity as evidenced by its highest ΔH, whereas bread supplemented with mycelium should show the least dense crystallinity. The discrepancy in the thermal profiles of these bread samples could partially be attributed to their distinct inter-sheet or intra- -sheet hydrogen-bonding systems. Nevertheless, the higher ΔH of white bread could explain why it was less subject to collapsing during the thermal decomposition process.
Proximate composition of bread samples
The moisture contents of all bread samples were comparable and were 39.1–40.1% of fresh mass (Table 2). On dry mass basis, carbohydrate content in all bread samples was similar but the reducing sugar content was lower in the bread supplemented with mycelium. Although the supplementation with mycelium and fermented grains more or less affected the contents of ash, fibre and protein, all bread samples had a similar proximate composition.
Table 2. Proximate composition of bread samples.
| Component | Bread sample | |||||
|---|---|---|---|---|---|---|
| White | A | B | C | D | E | |
| w/% | ||||||
| Moisture | (40.1±0.3)a | (39.9±0.3)a | (39.1±0.2)a | (39.9±0.2)a | (39.8±0.1)a | (39.1±0.1)a |
| Carbohydrate | (79.2±0.2)a | (78.0±0.5)a | (77.59±0.03)a | (77.1±0.4)a | (78.6±0.1)a | (77.99±0.04)a |
| Reducing sugar | (60.6±2.3)a | (52.6±1.6)b | (62.2±1.3)a | (63.6±2.0)a | (60.0±2.1)a | (61.2±1.7)a |
| Crude ash | (1.23±0.03)b | (1.29±0.04)a | (1.27±0.01)ab | (1.28±0.02)a | (1.30±0.02)a | (1.29±0.02)a |
| Crude fat | (4.9±0.4)b | (5.4±0.6)ab | (5.2±0.4)ab | (5.7±0.5)a | (5.30±0.1)ab | (5.3±0.3)ab |
| Crude fibre | (1.3±0.2)b | (1.7±0.4)a | (1.48±0.03)ab | (1.9±0.5)a | (1.4±0.1)ab | (1.55±0.04)ab |
| Crude protein | (13.4±0.3)b | (13.7±0.2)ab | (14.5±0.5)a | (14.1±0.3)ab | (13.4±0.2)b | (13.91±0.01)ab |
Bread supplemented with: A=Antrodia salmonea mycelium, B=fermented buckwheat, C=fermented oat, D=fermented embryo rice, E=fermented wheat. Each value is expressed as mean±standard error (N=3). Mean values with different superscript letters within a row differ significantly (p<0.05). Moisture is presented based on normal bread mass basis; other values are presented on dry mass basis
Non-volatile taste components of bread samples
Fructose, glucose and lactose were present in all bread samples and the mass fraction (on dry mass basis) of each sugar ranged from 7.3 to 19.5 mg/g (Table 3). Arabinose (0.3–1.0 mg/g) was detected in the bread supplemented with mycelium and fermented grains, whereas sucrose (0.02 mg/g) and trehalose (0.1 mg/g) were only found in bread supplemented with mycelium. Soluble sugars and polyols usually contribute to sweet taste (29). However, only 35.7–37.2 mg/g of sugars would give the bread a relatively weak sweet perception (29).
Table 3. Mass fractions (on dry mass basis) of soluble sugars, amino acids and 5’-nucleotides that give umami taste to bread samples.
| Compound | Bread sample | |||||
|---|---|---|---|---|---|---|
| White | A | B | C | D | E | |
| w/(mg/g) | ||||||
| Arabinose | n.d. | (1.0±0.1)c | (0.6±0.2)b | (0.4±0.3)c | (0.4±0.2)c | (0.3±0.1)c |
| Fructose | (18.3±0.2)b | (17.1±0.2)a | (19.5±0.2)a | (19.1±0.3)a | (19.1±0.1)a | (19.0±0.2)a |
| Glucose | (9.1±0.1)b | (10.5±0.1)a | (10.0±0.2)a | (10.0±0.1)a | (9.9±0.1)a | (10.1±0.1)a |
| Lactose | (8.3±0.2)a | (8.4±0.2)b | (8.0±0.2)ab | (8.0±0.2)ab | (7.3±0.1)b | (8.1±0.2)a |
| Sucrose | n.d. | 0.02±0.01 | n.d. | n.d. | n.d. | n.d. |
| Trehalose | n.d. | 0.1±0.1 | n.d. | n.d. | n.d. | n.d. |
| Total | (35.7±0.3)b | (36.1±0.4)ab | (37.5±0.3)a | (37.1±0.2)a | (36.3±0.4)ab | (37.2±0.5)a |
| w/(mg/g) | ||||||
| l-aspartic acid | (2.12±0.02)a | (2.18±0.07)a | (2.73±0.03)a | (2.15±0.04)a | (2.13±0.03)a | (2.15±0.04)a |
| l-glutamic acid | (4.06±0.04)a | (4.12±0.05)a | (4.11±0.04)a | (4.02±0.03)a | (3.97±0.02)a | (4.03±0.03)a |
| Total | (6.18±0.02)a | (6.30±0.06)a | (6.84±0.04)a | (6.17±0.03)a | (6.10±0.02)a | (6.18±0.04)a |
| w/(µg/g) | ||||||
| 5’-AMP | (14.1±0.3)c | (17.0±0.2)a | (16.5±0.3)a | (18.1±0.5)a | (15.2±0.4)b | (17.4±0.9)a |
| 5’-GMP | (25.6±0.5)c | (29.5±0.7)a | (26.0±0.6)c | (27.1±0.5)b | (25.8±0.2)c | (27.1±0.4)b |
| 5’-IMP | (11.3±0.1)b | (14.0±0.1)a | (11.1±0.1)b | (15.4±0.2)a | (11.8±0.2)b | (14.5±0.3)a |
| 5’-XMP | (9.6±0.1)c | (16.1±0.2)a | (15.6±0.2)a | (14.1±0.2)b | (15.6±0.1)a | (13.5±0.1)b |
| Total | (60.6±0.4)d | (76.6±0.5)a | (69.2±0.3)c | (74.7±0.4)ab | (68.4±0.3)c | (72.5±0.5)ab |
Bread supplemented with: A=Antrodia salmonea mycelium, B=fermented buckwheat, C=fermented oat, D=fermented embryo rice, E=fermented wheat. Each value is expressed as mean±standard error (N=3). Mean values with different superscript letters within a row differ significantly (p<0.05). 5’-AMP=5’-adenosine monophosphate, 5’-GMP=5’-guanosine monophosphate, 5’-IMP=5’-inosine monophosphate, 5’-XMP=5’-xanthosine monophosphate; n.d.=not detected
Aspartic and glutamic acids were classified into MSG-like group based on their taste characteristics as described by Komata (30). Chen (31) performed a series of sensory evaluations on synthetic mushroom extracts prepared by omitting and adding soluble components and found that aspartic and glutamic acids were taste-active amino acids in common mushrooms. Mass fractions of aspartic and glutamic acids of all bread samples were similar and total mass fraction (on dry mass basis) of free amino acids that give umami taste were 6.10–6.84 mg/g (Table 3). Furthermore, mass fraction of each 5’-nucleotide that gives umami taste was comparable and their total mass fraction was 60.6–76.6 µg/g. 5’-Guanosine monophosphate (5’-GMP) was found to have meaty flavour and is also a flavour enhancer. Its umami taste is much stronger than that of MSG (32). The synergistic effect of 5’-nucleotides with amino acids might greatly increase the umami taste of soups (32).
Besides, the mass fraction (on dry mass basis) of MSG equivalent to the umami intensity of the mixture of MSG and 5’-nucleotide was calculated to be 4.46–5.37 g per 100 g in all bread samples.
Functional compounds of bread samples
GABA was present in all bread samples and its mass fraction (on dry mass basis) was 2.20–2.45 µg/g (Table 4). Bread supplemented with A. salmonea mycelium contained five functional compounds, all of which originate from the mycelium. Based on ergosterol content, the mycelial biomass in fermented grains was estimated to be 26.6–32.7%. Therefore, adenosine and ergothioneine mass fractions in the bread supplemented with fermented grains were much lower than those in the bread supplemented with mycelium. Lovastatin was only found in the bread supplemented with the mycelium. However, the bread supplemented with fermented grains contained (on dry mass basis) adenosine 0.9–2.0 µg/g, ergosterol 24.5–30.1 µg/g and ergothioneine 2.2–3.2 µg/g, besides GABA. It seems that the bread supplemented with mycelium and fermented grains could provide substantial amounts of functional compounds.
Table 4. Mass fractions (on dry mass basis) of functional compounds in bread samples.
| Compound | Bread sample | |||||
|---|---|---|---|---|---|---|
| White | A | B | C | D | E | |
| w/(µg/g) | ||||||
| Adenosine | n.d. | (14.1±0.2)a | (0.9±0.1)c | (2.0±0.1)b | (1.2±0.2)c | (1.1±0.1)c |
| Ergosterol | n.d. | (92.0±7.2)a | (29.9±1.2)b | (28.3±1.1)b | (24.5±1.2)c | (30.1±1.1)b |
| Ergothioneine | n.d. | (8.6±2.2)a | (3.2±0.3)b | (2.9±0.4)b | (2.3±0.4)c | (2.2±0.2)c |
| GABA | (2.23±0.03)b | (2.27±0.04)b | (2.45±0.01)a | (2.39±0.02)a | (2.20±0.04)b | (2.21±0.02)b |
| Lovastatin | n.d. | 0.4±0.2 | n.d. | n.d. | n.d. | n.d. |
Bread supplemented with: A=Antrodia salmonea mycelium, B=fermented buckwheat, C=fermented oat, D=fermented embryo rice, E=fermented wheat. Each value is expressed as mean±standard error (N=3). Mean values with different superscript letters within a row differ significantly (p<0.05). GABA=γ-aminobutyric acid; n.d.=not detected
GABA is a hypotensive agent (33), and ergothioneine is an excellent antioxidant in vivo (34) and a cellular protector against oxidative damage (35). Adenosine inhibits platelet aggregation (36). Lovastatin is capable of inhibiting cholesterol production and lowering the amounts of total and low-density lipoprotein (LDL) cholesterol. Besides, it is effective in preventing coronary heart disease due to the anti-inflammatory, antioxidant and profibrinolytic activities (37).
A dose of 2–23 mg of adenosine was capable of terminating supraventricular tachycardia (38). An anecdotal event of the successful treatment by Braverman and Pfeiffer (39) showed that 800 mg of GABA per day could cure a 40-year-old woman suffering from anxiety. Moreover, 20 mg of lovastatin per day has satisfactory effect on reducing LDL cholesterol and increasing high-density lipoprotein (HDL) cholesterol (40). However, the therapeutic dose of ergosterol and ergothioneine is not available.
Four slices of bread (100 g with 40% moisture, by mass) would provide 850 and 55–118 µg of adenosine, 5.52 and 1.47–1.81 mg of ergosterol, and 513 and 130–191 µg of ergothioneine in bread samples supplemented with mycelium and fermented grains, respectively. Moreover, the mass of lovastatin ingested from the bread supplemented with mycelium was 128 µg, whereas that of GABA in all bread samples was 133–147 µg. Altogether, the amounts of functional compounds in consumed bread were relatively low as compared to their therapeutic doses. Although these bread samples are food and not medicine, substantial amounts of the compounds in the mycelium and fermented grain would still contribute to human health.
Sensory properties of bread samples
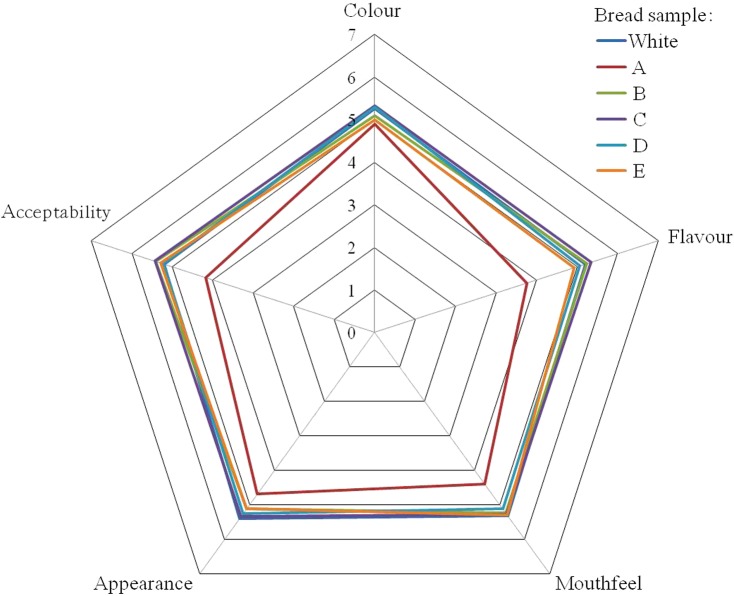
On a 7-point hedonic scale, all sensory results of white bread were the highest (5.20–5.41) and were moderately acceptable considering the fact that rice is dominant starch food in Taiwan instead of bread (Fig. 2). All sensory results of bread supplemented with mycelium were 3.76–4.89 and were lower than those of other bread samples. Similarly, Ulziijargal et al. (11) found that the 5% substitution with mycelium in bread decreased all sensory results of the bread samples, especially flavour and overall scores. All sensory results of the bread supplemented with fermented grains were more or less similar to those of white bread.
Fig. 2.
Sensory results of bread samples. Bread supplemented with: A=Antrodia salmonea mycelium, B=A. salmonea-fermented buckwheat, C=A. salmonea-fermented oat, D=A. salmonea-fermented embryo rice, E=A. salmonea-fermented wheat. Each value in the scale is expressed as mean value±standard error (N=59). Colour version at www.ftb.com.hr
Apart from white bread, the colour and appearance scores of bread samples were consistent with their ΔE values. The flavour scores were in the descending order: white bread, bread samples supplemented with fermented buckwheat or oat>bread supplemented with embryo rice or wheat>bread supplemented with A. salmonea mycelium. The lowest flavour score of the bread with mycelium might be due to its unique mycelial flavour. The mouthfeel scores of bread samples were consistent with their hardness except for the bread with mycelium. The lowest mouthfeel score of the same bread might relate to its lowest enthalpy, which implied less dense crystallinity and it collapsed easily when chewing.
However, the results show that the substitution of 7% of wheat flour in the bread formula with mycelium significantly lowers the acceptability of the bread, whereas the substitution with fermented grains could maintain its acceptability. Therefore, bread supplemented with fermented grains could be a new functional product.
Conclusion
Antrodia salmonea-fermented buckwheat, oat, embryo rice or wheat was used to substitute 7% of wheat flour to make bread. White bread and bread samples supplemented with mycelium and fermented grains looked different but were comparable in proximate composition, texture profile analysis results, mass fractions of non-volatile taste components and MSG that gives umami taste equivalent to the mixture of MSG and 5’-IMP. Bread samples supplemented with fermented grains had similar differential scanning calorimetry results, which differed from those of white bread and bread supplemented with mycelium. Bread supplemented with fermented grains contained substantial amounts of adenosine, ergosterol, ergothioneine and GABA. It seems that the bread supplemented with mycelium and fermented grains could provide functional compounds. In addition, bread supplemented with mycelium contained lovastatin. All sensory results showed that the substitution of 7% wheat flour in the formula with fermented grains would not affect negatively the acceptability of bread. Overall, fermented grains could be incorporated into bread to provide their beneficial effects.
Acknowledgements
This study was supported by the Ministry of Science and Technology, Taiwan, Republic of China (NSC-104- -2911-I-005-301, NSC-103-2911-I-005-301) and the Ministry of Education, Taiwan, R.O.C. under the Aiming for the Top University and Elite Research Center Development Plan.
References
- 1.Chang TT, Chou WN. Antrodia cinnamomea reconsidered and A. salmonea sp. nov. on Cunninghamia konishii in Taiwan. Bot Bull Acad Sin. 2004;45:347–52. [Google Scholar]
- 2.Shen CC, Shen YC, Wang YH, Lin LC, Don MJ, Liou KT, et al. New lanostanes and naphthoquinones isolated from Antrodia salmonea and their antioxidative burst activity in human leukocytes. Planta Med. 2006;72:199–203. 10.1055/s-2005-916175 [DOI] [PubMed] [Google Scholar]
- 3.Shen CC, Wang YH, Chang TT, Lin LC, Don MJ, Hou YC, et al. Anti-inflammatory ergostanes from the basidiomata of Antrodia salmonea. Planta Med. 2007;73:1208–13. 10.1055/s-2007-981591 [DOI] [PubMed] [Google Scholar]
- 4.Huang GJ, Pan CH, Liu FC, Wu TS, Wu CH. Anti-inflammatory effects of ethanolic extract of Antrodia salmonea in the lipopolysaccharide-stimulated RAW246.7 macrophages and the λ-carrageenan-induced paw edema model. Food Chem Toxicol. 2012;50:1485–93. 10.1016/j.fct.2012.01.041 [DOI] [PubMed] [Google Scholar]
- 5.Chen SY, Ho KJ, Hsieh YJ, Wang LT, Mau JL. Contents of lovastatin, γ-aminobutyric acid and ergothioneine in mushroom fruiting bodies and mycelia. LWT –. Food Sci Technol (Campinas). 2012;47:274–8. 10.1016/j.lwt.2012.01.019 [DOI] [Google Scholar]
- 6.Yang JH, Tseng YH, Chang HL, Lee YL, Mau JL. Storage stability of monascal adlay. Food Chem. 2005;90:303–9. 10.1016/j.foodchem.2004.03.053 [DOI] [Google Scholar]
- 7.Lin LY, Hsieh YJ, Liu HM, Lee CC, Mau JL. Flavor components in buckwheat bread. J Food Process Preserv. 2009;33:814–26. 10.1111/j.1745-4549.2008.00313.x [DOI] [Google Scholar]
- 8.Tseng YH, Yang JH, Chen CH, Mau JL. Quality and antioxidant properties of anka enriched bread. J Food Process Preserv. 2011;35:518–23. 10.1111/j.1745-4549.2010.00497.x [DOI] [Google Scholar]
- 9.Lin LY, Tseng YH, Li RC, Mau JL. Quality of shiitake stipe bread. J Food Process Preserv. 2008;32:1002–15. 10.1111/j.1745-4549.2008.00229.x [DOI] [Google Scholar]
- 10.Tseng YH, Yang JH, Li RC, Mau JL. Quality of bread supplemented with silver ear. J Food Qual. 2010;33:59–71. 10.1111/j.1745-4557.2009.00288.x [DOI] [Google Scholar]
- 11.Ulziijargal E, Yang JH, Lin LY, Chen CP, Mau JL. Quality of bread supplemented with mushroom mycelia. Food Chem. 2013;138:70–6. 10.1016/j.foodchem.2012.10.051 [DOI] [PubMed] [Google Scholar]
- 12.Yen MT, Yang JH, Tseng YH, Li RC, Mau JL. Quality of fungal chitin bread. J Food Process Preserv. 2011;35:708–13. 10.1111/j.1745-4549.2011.00521.x [DOI] [Google Scholar]
- 13.Bread making. Taipei, Taiwan: China Grain Products Research & Development Institute (CGPRDI); 1983.
- 14.Methods AACC. 10-11.01. Baking quality of bread flour – sponge-dough, pound-loaf method. St. Paul, MN, USA: American Association of Cereal Chemists (AACC) International; 1999. [Google Scholar]
- 15.Hsu PC. A fusing technique for high dynamic range imaging using a pixel based non-linear color compensation approach [Master Thesis]. Taoyuan, Taiwan: National Central University; 2011 (in Chinese). [Google Scholar]
- 16.Official Method AOAC. 14.091. Total solids in entire loaf of bread. Rockville, MD, USA: AOAC International; 1990. [Google Scholar]
- 17.Official Method AOAC. 14.103. Ash. Rockville, MD, USA: AOAC International; 1990. [Google Scholar]
- 18.Official Method AOAC. 14.093. Fat and fat number. Rockville, MD, USA: AOAC International; 1990. [Google Scholar]
- 19.Official Method AOAC. 14.111. Crude fiber. Rockville, MD, USA: AOAC International; 1990. [Google Scholar]
- 20.Official Method AOAC. 14.108. Protein. Rockville, MD, USA: AOAC International; 1990. [Google Scholar]
- 21.James CS. Analytical chemistry of foods. London, UK: Chapman & Hall; 1995. pp. 124–5. http://dx.doi.org/ 10.1007/978-1-4615-2165-5 [DOI] [Google Scholar]
- 22.Mau JL, Chyau CC, Li JY, Tseng YH. Flavor compounds in straw mushrooms Volvariella volvacea harvested at different stages of maturity. J Agric Food Chem. 1997;45:4726–9. 10.1021/jf9703314 [DOI] [Google Scholar]
- 23.Taylor MW, Hershey HV, Levine RA, Coy K, Olivelle S. Improved method of resolving nucleotides by reverse-phase high performance liquid chromatography. J Chromatogr. 1981;219:133–9. 10.1016/S0021-9673(00)80584-1 [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi S. The synergistic taste effect of monosodium glutamate and disodium 5′-inosinate. J Food Sci. 1967;32:473–8. 10.1111/j.1365-2621.1967.tb09715.x [DOI] [Google Scholar]
- 25.Liu ZM, Cao YS, Chang Q. Study on solid fermentation cultured Cordyceps sinensis mycelium. Henan Sci. 1994;12:335–8. [Google Scholar]
- 26.Liang CH, Huang SJ, Tsai SY, Lee YL, Kuo HC, Wu TP, et al. Preparation of novel culinary-medicinal mushroom products using solid-state fermentation and their taste quality. Int J Med Mushrooms. 2009;11:141–56. 10.1615/IntJMedMushr.v11.i2.40 [DOI] [Google Scholar]
- 27.Lin LY, Wang HE, Lin SD, Liu HM, Mau JL. Changes in buckwheat bread during storage. J Food Process Preserv. 2013;37:285–90. 10.1111/j.1745-4549.2011.00647.x [DOI] [Google Scholar]
- 28.Yen MT, Mau JL. Selected physical properties of chitin prepared from shiitake stipes. LWT –. Food Sci Technol (Campinas). 2007;40:558–63. 10.1016/j.lwt.2005.10.008 [DOI] [Google Scholar]
- 29.Litchfield JH. Morel mushroom mycelium as a food flavoring material. Biotechnol Bioeng. 1967;9:289–304. 10.1002/bit.260090303 [DOI] [Google Scholar]
- 30.Komata Y. The taste and constituents of foods. Nippon Shokuhin Kogyo Gakkaishi. 1969;3:26 [in Japanese] [Google Scholar]
- 31.Chen HK. Studies on the characteristics of taste-active components in mushroom concentrate and its powderization [Master Thesis]. Taichung, Taiwan: National Chung Hsing University; 1986 (in Chinese). [Google Scholar]
- 32.Yamaguchi S, Yoshikawa T, Ikeda S, Ninomiya T. Measurement of the relative taste intensity of some α-amino acid and 5′-nucleotides. J Food Sci. 1971;36:846–9. 10.1111/j.1365-2621.1971.tb15541.x [DOI] [Google Scholar]
- 33.Kohama Y, Matsumoto S, Mimura T, Tanabe N, Inada A, Nakanishi T. Isolation and identification of hypotensive principles in red-mold rice. Chem Pharm Bull (Tokyo). 1987;35:2484–9. 10.1248/cpb.35.2484 [DOI] [PubMed] [Google Scholar]
- 34.Hartman PE. Ergothioneine as an antioxidant. Methods Enzymol. 1990;186:310–8. 10.1016/0076-6879(90)86124-E [DOI] [PubMed] [Google Scholar]
- 35.Aruoma OI, Spencer JPE, Mahmood N. Protection against oxidative damage and cell death by the natural antioxidant ergothioneine. Food Chem Toxicol. 1999;37:1043–53. 10.1016/S0278-6915(99)00098-8 [DOI] [PubMed] [Google Scholar]
- 36.Ikumoto T, Sasaki S, Namba H, Toyama R, Horitoki H, Mouri T. Physiologically active compounds in the extracts from tochukaso and culture mycelia of Cordyceps and lsaria. Yakugaku Zasshi. 1991;111:504–9. [DOI] [PubMed] [Google Scholar]
- 37.Aarons CB, Cohen PA, Gower A, Reed KL, Leeman SE, Stucchi AF, et al. Statins (HMG-CoA reductase inhibitors) decrease postoperative adhesions by increasing peritoneal fibrinolytic activity. Ann Surg. 2007;245:176–84. 10.1097/01.sla.0000236627.07927.7c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.diMarco JP, Sellers TD, Lerman BB, Greenberg ML, Berne RM, Belardinelli L. Diagnostic and therapeutic use of adenosine in patients with supraventricular tachyarrhythmias. J Am Coll Cardiol. 1985;6:417–25. 10.1016/S0735-1097(85)80181-9 [DOI] [PubMed] [Google Scholar]
- 39.Braverman E, Pfeiffer C. The healing nutrients within. New Canaan, CT, USA: Keats Publishing; 1987. pp. 191–210. [Google Scholar]
- 40.Lambert M, Lupien PJ, Gagné C, Lévy E, Blaichman S, Langlois S, et al. Treatment of familial hypercholesterolemia in children and adolescents: effect of lovastatin. Pediatrics. 1996;97:619–8. [PubMed] [Google Scholar]