Summary
This study evaluates the influence of Lachancea thermotolerans on low-acidity Airén grape must from the south of Spain. For this purpose, combined fermentations with Lachancea thermotolerans and Saccharomyces cerevisiae were compared to a single fermentation by S. cerevisiae. Results of all developed analyses showed significant differences in several parameters including acidity, population growth kinetics, concentration of amino acids, volatile and non-volatile compounds, and sensorial parameters. The Airén wine quality increased mainly due to the acidification by L. thermotolerans. The acidification process caused a lactic acid increment of 3.18 g/L and a reduction of 0.22 in pH compared to the control fermentation, performed by S. cerevisiae.
Key words: Airén wine, Lachancea thermotolerans, Saccharomyces cerevisiae, l-lactic acid, pyruvic acid, glycerol, ethanol, amino acids, biogenic amines, combined fermentation
Introduction
In recent years, global climate change has set a trend towards an increase in sugar content and a decrease in the acidity of grape juices. Microbiological acidification can play an essential role in satisfying the growing wine market demand for quality wines.
Traditionally, Saccharomyces cerevisiae is the yeast used widely for winemaking. However, grapes are not sterile media and there are many other yeast species with plenty of potential to solve new oenology challenges that must be studied. Several research groups have studied non- -Saccharomyces yeast applications (1, 2) in different grape varieties such as Sauvignon blanc (3, 4), Chenin blanc (4), Chardonnay (4–6), Amarone (7), Muscat (8), Muscat d’Alexandrie (9), Debina (10), Macabeo (11, 12), Folle blanche (13), Bobal (14), Alvarinho, Loureiro, Trajadura, Pedernă, Azal Branco, Avesso (15), Airén (16, 17), Pedro Ximenez (18), Sangiovese (19), Pinot noir (20), Emir (21, 22), Syrah (23–26), Tempranillo (27, 28) and Riesling (29). In most cases improvements in wine quality were reported.
The presence of wild non-Saccharomyces yeasts in fermentations was traditionally associated with high levels of acetic acid and other off-flavours. Nevertheless, nowadays researchers and winemakers are aware of the positive influence of non-Saccharomyces yeasts on wine aroma complexity (1, 2, 29–40). The development of multistarter fermentation with Saccharomyces cerevisiae as a binding partner has been proposed to overcome the shortcomings of alcoholic fermentation with non-Saccharomyces yeasts. Mixed fermentations are of interest because of some enzymatic properties (glycosidases, β-lyase, etc.), ethanol reduction and the release of some interesting metabolites such as glycerol, pyruvic acid and mannoproteins, among others (41–44).
Some studies have analysed the use and influence of different non-Saccharomyces species on wine quality. In most cases sequential fermentation was reported to be the best option. These yeast species include Kloeckera apiculata (45), Hanseniaspora uvarum (46), Hanseniaspora vineae (6, 27), Torulospora delbrueckii (7, 28, 47), Metschnikowia pulcherrima (3, 47, 48), Candida zemplinina (49), Zygosaccharomyces bailii (50, 51), Schizosaccharomyces pombe (44, 52), Hansenula anomala (53), Pichia guillermondii (54) and Lachancea thermotolerans (19, 29, 55–59). Among these species, L. thermotolerans, previously called Kluyveromyces thermotolerans, has been used specifically to increase the acidity of wines, causing increases of l-lactic acid concentration from 0.23 to 9.6 g/L depending on the different trial conditions (19, 29, 55–59).
This study aims to enhance Airén wine quality. This Spanish variety is considered as a neutral and very productive grape but is usually associated with low-quality wine. However, this variety is the most planted in Spain. Most Airén vineyards are located in the centre of the southern Spain. This area is considered to be a warm semi-desert region, where high sugar contents and lack of acidity in wine are the main problems. Therefore, Lachancea thermotolerans 617 was selected among other non-Saccharomyces yeasts in this study to perform combined fermentations with S. cerevisiae in order to increase the acidity and quality of Spanish Airén wine.
Materials and Methods
Microorganisms
The following yeast strains were used for the experimental fermentation of the studied Airén must: Saccharomyces cerevisiae 87 (CECT 12512; Spanish Type Culture Collection, Valencia, Spain) and Lachancea thermotolerans 617 (CECT 12672; Spanish Type Culture Collection).
Vinification
Grapes of Airén cultivar (Vitis vinifera L.), grown in El Socorro experimental vineyard (Madrid, Spain) were used in the fermentations. Using a microvinification method similar to that described in scientific literature (60), 3.9 L of sterilised must (115 °C, 15 min) were placed in 4.9-litre glass fermentation vessels, leaving enough space for the emission of carbon dioxide. No sulphur dioxide was added to any vessel. Sugar concentration was 244.51 g/L, pH=3.68, primary amino nitrogen (PAN; Biosystems S.A., Barcelona, Spain) 177 mg/L, and lactic and acetic acids below 0.1 g/L. A concentration of 0.4 g/L of Actimax Natura (Agrovin S.A., Alcázar de San Juan, Spain), inactivated autolyzed yeasts naturally rich in amino acids, was added to provide nutrition for the media.
Three assays were performed (all in triplicate): (i) inoculation of the must with S. cerevisiae 87 alone (SC; 100 mL containing 1.18·107 CFU/mL), (ii) inoculation with S. cerevisiae 87 (1.18·107 CFU/mL) and L. thermotolerans 617 (100 mL containing 2.95·107 CFU/mL) together (mixed fermentation: LT×SC), and (iii) inoculation with L. thermotolerans 617 (100 mL containing 2.27·107 CFU/mL) followed by S. cerevisiae 87 (100 mL containing 107 CFU/mL) 96 h later (sequential fermentation: LT...SC). Yeast inocula were produced using 100 mL of sterilised must with 1 mL of yeast extract peptone dextrose (YEPD; Pronadisa, Madrid, Spain) liquid medium (61), in the concentration of 106 CFU/mL (determined using a counting chamber). To reach this population, 100 µL of each yeast suspension were cultivated in 10 mL of YEPD at 25 °C for 24 h. This procedure was repeated successively three times before the final inoculation of 1 mL of the suspension. All inocula were prepared in 250-mL flasks filled with 98% H2SO4 (Panreac, Barcelona, Spain), which allowed the release of CO2 while avoiding microbial contamination (62), and sealed with a 14-cm Muller valve (Alamo, Madrid, Spain). The temperature was maintained at 25 °C for 48 h. The development of inocula proceeded without aeration, oxygen injection or agitation. All fermentation processes, which were done in triplicate, were carried out at 25 °C. When the sugar concentration fell below 3 g/L, the wines were racked and stabilised for 7 days at 4 °C. The wine was then bottled, and a concentration of 40 mg/L of sulphur dioxide in the form of potassium disulfite was added. Sealed bottles were placed horizontally in a climate chamber at 4 °C for three weeks until the sensory evaluation.
Analytical determinations of non-volatile compounds
Glucose and fructose, l-lactic acid, acetic acid, glycerol, pyruvic acid, acetaldehyde, l-malic acid and primary amino nitrogen were all determined using a Y15 enzymatic autoanalyzer (Biosystems S.A.) with corresponding kits. Ethanol, pH, free SO2 and total SO2 profile were determined following the methods described in the Compendium of International Methods of Analysis of Wines and Musts (63).
Growth kinetics during microvinification
During fermentations, aliquots were taken periodically under aseptic conditions and further tenfold dilutions were made serially. Growth kinetics was monitored by plating 100 µL of the appropriate dilution on lysine medium (Oxoid, Basingstoke, UK) for counting non-Saccharomyces yeasts (64) and YEPD medium (Pronadisa) for total yeast counts (61). Colonies were counted after growth at 30 °C for 48–72 h.
Analytical determination of volatile compounds
The concentration of volatile compounds, all of which influence wine quality, was measured at the end of alcoholic fermentation by gas chromatography using an Agilent Technologies 6850 gas chromatograph with a flame ionisation detector (Hewlett Packard, Palo Alto, CA, USA) (65), calibrated with 4-methyl-2-pentanol (Fluka, Sigma- -Aldrich Corp., Buchs, Switzerland) as an internal standard. Gas chromatography standards (Fluka, Sigma–Aldrich Corp.) were used to provide standard patterns. Higher alcohols were separated according to the Compendium of International Methods of Analysis of Wines and Musts (63), with the detection limit of 0.1 mg/L. Minor compounds were quantified using gas chromatography–mass spectrometry as described by Lopez et al. (66) with the modifications introduced by Loscos et al. (67).
Analytical determination of amino acids
The amino acids were analysed using a Jasco (Tokyo, Japan) ultra-high-performance liquid chromatograph (UHPLC) series X-LCTM, equipped with a fluorescence detector 3120-FP. Gradients of solvent A (methanol/acetonitrile 50:50, by volume) and B (sodium acetate/tetrahydrofuran 99:1, by volume) were used in a C18 (HALO®, Wilmington, DE, USA) column (100 mm×2.1 mm; particle size 2.7 µm) as follows: 90% B at 0.25 mL/min, from 0 to 6 min; 90–78% linear gradient B at 0.2 mL/min, from 6 to 7.5 min; 78% B from 7.5 to 8 min, 78–74% linear gradient B at 0.2 mL/min, from 8 to 8.5 min, 74% B at 0.2 mL/min, from 8.5 to 11 min, 74–50% linear gradient B at 0.2 mL/min, from 11 to 15 min, 50% B at 0.2 mL/min, from 15 to 17 min, 50–20% linear gradient B at 0.2 mL/min, from 17 to 21 min, 20–90% linear gradient B at 0.2 mL/min, from 21 to 25 min and re-equilibration of the column from 25 to 26 min to the initial gradient conditions. The scanning range for the detection of amino acids was 340–455 nm. Amino acids were quantified by comparison against their external standards, and different acids were identified by their retention times.
Analytical determination of biogenic amines
The biogenic amines were analysed using a Jasco UHPLC chromatograph series X-LCTM, equipped with a fluorescence detector 3120-FP. Gradients of solvent A (methanol/acetonitrile, 50:50, by volume) and B (sodium acetate/tetrahydrofuran, 99:1, by volume) were used in a C18 (HALO®) column (100 mm×2.1 mm; particle size 2.7 µm) as follows: 60% B at 0.25 mL/min, from 0 to 5 min; 60–50% linear gradient B at 0.25 mL/min, from 5 to 8 min; 50% B from 8 to 9 min, 50–20% linear gradient B at 0.2 mL/min, from 9 to 12 min, 20% B at 0.2 mL/min, from 12 to 13 min, 20–60% linear gradient B at 0.2 mL/min, from 13 to 14.5 min, and re-equilibration of the column from 14.5 to 17 min to the initial gradient conditions. The scanning range for the detection of biogenic amines was 340–420 nm. Biogenic amines were quantified by comparison against their external standards, and different amines were identified by their retention times.
Sensory analysis
The obtained wines were assessed using a blind test by a panel of 15 experienced wine tasters, all staff members of the Chemistry and Food Technology Department of the Polytechnic University of Madrid (Madrid, Spain) and the Accredited Oenology Laboratory of Haro (Haro, Spain). Following consistent terminology by consensus, five aromas and five taste attributes were chosen to describe the wines. The panellists used an unstructured scale, with scores ranging from 0 (no character) to 6 (very strong character), to rate the intensity of the 11 attributes.
Statistical analysis
PC Statgraphics v. 5 software (Graphics Software Systems, Rockville, MD, USA) was used for statistical analyses. The significance was set to p<0.05 for the ANOVA matrix F value. The mean values were compared using multiple range test.
Results and Discussion
Fermentation kinetics of the yeast population
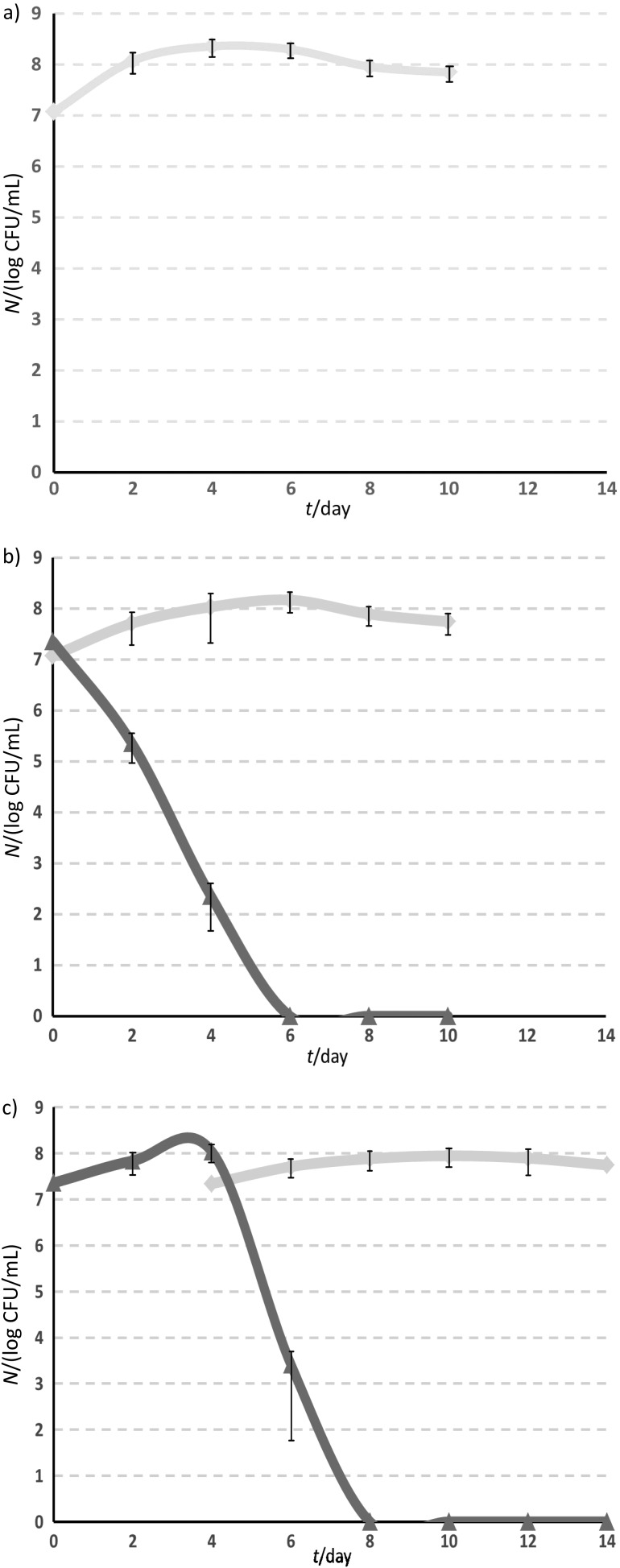
Fig. 1 shows the development of different yeast strains during fermentation. Fermentation time varied from 10 to 14 days. In all mixed fermentations (LT×SC or LT...SC) when Saccharomyces cerevisiae 87 was inoculated, the number of L. thermotolerans 617 cells started to decline fast. Other authors reported previously fermentation kinetics of other non-Saccharomyces strains, in which the presence of non-Saccharomyces strains was also observed during the early stages of fermentation. In this trial L. thermotolerans 617 strain disappeared on day 8 in the sequential (LT...SC) fermentation (Fig. 1). This can be explained by the higher fermentation activity of this species compared to other low-fermenting non-Saccharomyces strains. Some S. cerevisiae strains were also reported to secrete antimicrobial peptides that inhibit non-Saccharomyes yeast growth (68). This could explain the early disappearance of L. thermotolerans once S. cerevisiae was inoculated, even though it has been reported to tolerate up to 9% (by volume) of ethanol when it ferments on its own (55). In this trial, the LT...SC fermentation was the best option. In the case of the LT×SC fermentation, L. thermotolerans disappeared fast so acidification was not completed. Cell flocculation or loss of viability can explain the observed reduction in cell numbers during fermentation.
Fig. 1.
Yeast cell counts during: a) fermentation with Saccharomyces cerevisiae 87 alone (SC; light gray), b) mixed fermentation with Lachancea thermotolerans 617 (dark gray) and S. cerevisiae 87 (LT×SC), and c) sequential fermentation with L. thermotolerans 617 followed by S. cerevisiae 87 (LT…SC)
Sugar consumption kinetics and alcohol production
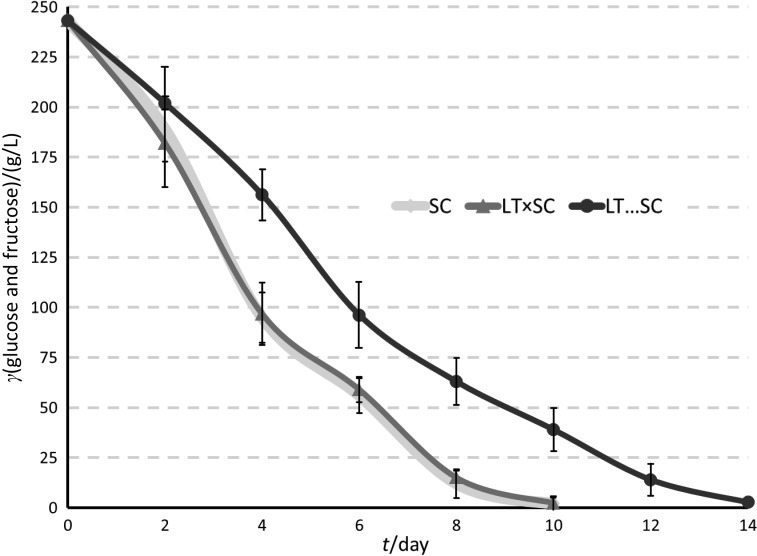
The Saccharomyces cerevisiae 87 fermenting on its own (SC) and in the LT×SC fermentation consumed the sugar the fastest (Fig. 2). Fermentation time varied from 10 to 14 days and final alcohol content varied from 13.91 to 14.36% (by volume). The ethanol content was lower in the LT...SC fermentation (Table 1). The sugar consumption results analysed in this work (Fig. 2) are in agreement with the lower fermentation activity of Lachancea spp. compared with S. cerevisiae (55), due to the fact that in the last stages of fermentation only S. cerevisiae was detected. Several authors question the usefulness of non-Saccharomyces yeast in the production of lower volume fractions of alcohol in wines (43, 69). These previous results are in agreement with the lower final alcohol content of the wines produced in the sequential fermentations involving Lachancea thermotolerans 617 (Table 1). However, in our case the alcohol reduction was about 0.4% (Table 1).
Fig. 2.
Change in the glucose and fructose concentration in the studied Airén wines during fermentation with Saccharomyces cerevisiae 87 alone (SC), mixed fermentation with Lachancea thermotolerans 617 and S. cerevisiae 87 (LT×SC), and sequential fermentation with L. thermotolerans 617 followed by S. cerevisiae 87 (LT…SC)
Table 1. Analytical results for the wines produced by different fermentations.
| Compound | SC | LT×SC | LT…SC |
|---|---|---|---|
| γ(l-lactic acid)/(g/L) | (0.02±0.01)a | (0.24±0.04)b | (3.18±0.19)c |
| γ(l-malic acid)/(g/L) | (0.98±0.02)a | (1.02±0.03)ab | (1.04±0.03)b |
| γ(acetic acid)/(g/L) | (0.38±0.02)a | (0.39±0.02)a | (0.31±0.03)b |
| γ(glucose+fructose)/(g/L) | (1.88±0.42)a | (2.32±0.48)a | (2.77±0.56)a |
| γ(glycerol)/(g/L) | (7.11±0.05)a | (7.18±0.08)a | (7.55±0.16)b |
| γ(free SO2)/(mg/L) | (21.12±2.72)a | (19.99±3.26)a | (17.82±3.42)a |
| γ(total SO2)/(mg/L) | (48.11±1.12)a | (46.28±2.46)a | (41.32±2.21)b |
| ϕ(alcohol)% | (14.36±0.02)a | (14.29±0.04)a | (13.91±0.08)b |
| γ(acetaldehyde)/(mg/L) | (39.00±3.02)a | (35.00±2.01)b | (27.00±4.02)c |
| pH | (3.74±0.01)a | (3.71±0.02)a | (3.52±0.06)b |
Results represent the mean value±S.D. of three replicates. Mean values in the same row with the same letter are not significantly different (p<0.05) SC=fermentation with Saccharomyces cerevisiae 87 alone, LT×SC= mixed fermentation with Lachancea thermotolerans 617 and S. cerevisiae 87, LT…SC=sequential fermentation with L. thermotolerans 617 followed by S. cerevisiae 87
Acetic acid metabolism
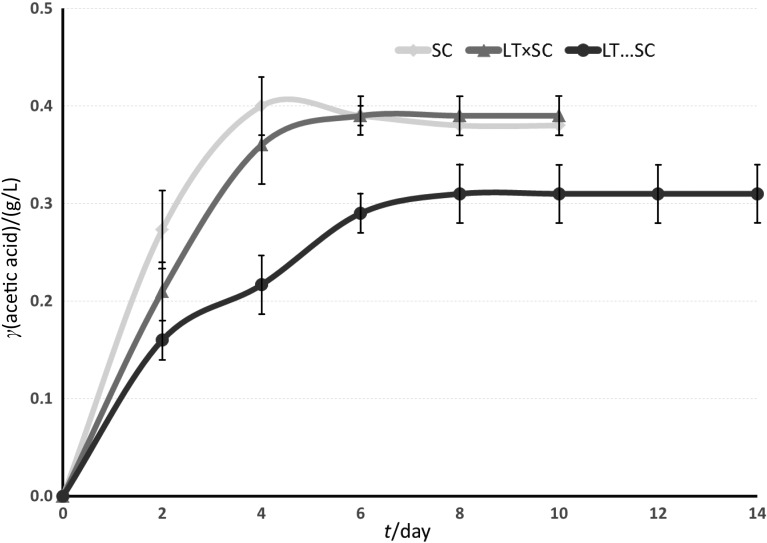
Fig. 3 shows the kinetics of acetic acid release. Acetic acid concentration varied from 0.31 to 0.39 g/L (Table 1). LT...SC fermentation produced the lowest final acetic acid concentration. SC and LT×SC fermentations had similar final acetic acid content of about 0.38 g/L (Fig. 3). One of the problems raised by winemakers is the excessive increase of acetic acid in wines with high presence of non- -Saccharomyces yeasts (1). However, previous experiments with L. thermotolerans reported significant reduction in final volatile acidity in sequential fermentations of 0.25 (19), 0.06 (56), 0.2 (42) and 0.08 g/L (70). Our results confirm an additional decrease in this compound related to the presence of L. thermotolerans (Fig. 3; Table 1). Nevertheless, acetic acid concentration in all fermentations was not excessive and it did not affect wine quality negatively. The results show that a controlled use of L. thermotolerans in sequential fermentations can cause a decrease of acetic acid production.
Fig. 3.
Change in acetic acid concentration in the studied Airén wines during fermentation with S. cerevisiae 87 alone (SC), simultaneous fermentation with Lachancea thermotolerans 617 and S. cerevisiae 87 (LT×SC), and sequential fermentation with L. thermotolerans 617 followed by S. cerevisiae 87 (LT…SC)
l-lactic acid metabolism
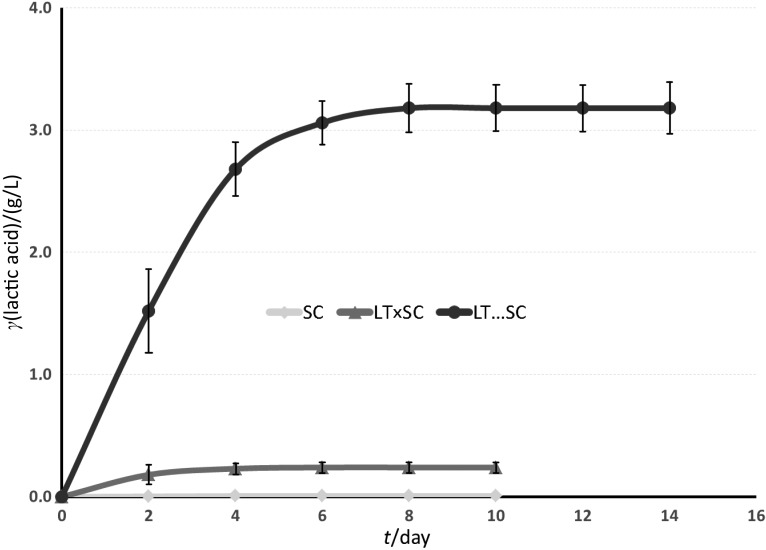
Fig. 4 reports that only the fermentations involving Lachancea thermotolerans 617 produced l-lactic acid. The results varied from 0.24 g/L in LT×SC to 3.18 g/L in LT...SC (Table 1). Other authors obtained significant acidifications using combined microbiological cultures of L. thermotolerans and S. cerevisiae with the main objective of acidifying musts that were low in titratable acidity. Previously obtained values such as 3.42 g/L (19) were similar to the ones reported in this work. In other cases, acidification was higher; up to 5.13 g/L (56) has been reported depending on different trial conditions. The production of l-lactic acid is linked to the viable cell content (70). LT...SC fermentation proved to be the best option for acidifying wine in this study (Fig. 4; Table 1). In the case of LT×SC fermentation, the acidification was significantly lower due to the fast Saccharomyces growth, which impeded a higher acidification by L. thermotolerans.
Fig. 4.
Change in l-lactic acid concentration in the studied Airén wines during fermentation with Saccharomyces cerevisiae 87 alone (SC), mixed fermentation with Lachancea thermotolerans 617 and S. cerevisiae 87 (LT×SC), and sequential fermentation with L. thermotolerans 617 followed by S. cerevisiae 87 (LT…SC)
l-malic acid metabolism
Only the final malic acid content in the SC fermentation was lower than in the other fermentations (Table 1); the maximum malic acid reduction rates of 17.65% in SC, 14.29% in LT×SC and 9.25% in LT...SC fermentation from the initial concentration of 1.19 g/L were detected. The slight decrease in malic acid content observed in the fermentations (Table 1) is in agreement with other authors who confirmed that malic acid can be metabolised by several yeast species (44, 52, 57) at levels lower than 20%, unless Schizosaccharomyces genus is involved.
Glycerol production
The glycerol content in LT...SC fermentation was higher than those observed in SC and LT×SC fermentations (Table 1). Final levels of glycerol varied from 7.11 to 7.55 g/L (Table 1). Increased glycerol content is described as one of the main contributions of non-Saccharomyces strains to wine quality (71) because it contributes positively to the mouthfeel. L. thermotolerans has been described before in literature as a higher glycerol producer than S. cerevisiae, reporting increases of about 0.69 (19) and 0.93 g/L (56). However, some authors have reported that an increase in glycerol production is usually related to an increase in acetic acid production (72), which can be detrimental to wine quality. Our results confirm that this fact seems to be irrelevant in the case of LT…SC fermentation.
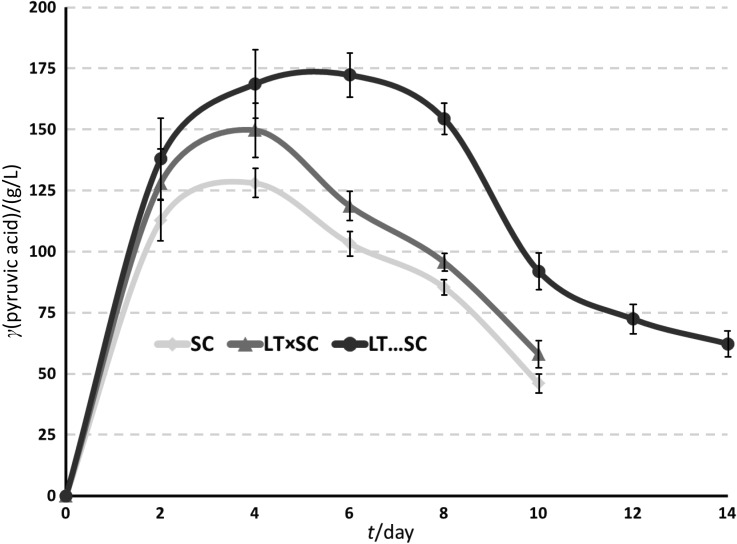
Pyruvic acid production
Maximum pyruvic acid production was observed between the second and fourth day, reaching 128 and 149 mg/L, respectively (Fig. 5) during the fermentation of Saccharomyces cerevisiae 87 alone (SC) or LT×SC fermentation. LT...SC fermentation had higher values with a maximum concentration of pyruvic acid of 172.36 mg/L on day 6. Previous studies on the production of pyruvic acid by S. cerevisiae strains reported maximum values of 60–132 mg/L after 4 days of fermentation (52). Similar values were obtained in the present study in SC and slightly higher in LT×SC fermentation (Fig. 5). Nevertheless, the LT...SC fermentation obtained significantly higher levels, but not as high as those described for the genus Schizosaccharomyces (52). The concentrations of pyruvic acid and glycerol could indicate that L. thermotolerans possesses a highly active glyceropyruvic pathway (73).
Fig. 5.
Change in pyruvic acid concentration in the studied Airén wines during fermentation with S. cerevisiae 87 alone (SC), mixed fermentation with Lachancea thermotolerans 617 and S. cerevisiae 87 (LT×SC), and sequential fermentation with L. thermotolerans 617 followed by S. cerevisiae 87 (LT…SC)
Acetaldehyde production
The fermentations involving L. thermotolerans 617 produced less acetaldehyde, with values that varied from 27 in LT…SC to 35 mg/L in LTx×SC (Table 1). SC fermentation produced more acetaldehyde than the others, with a final concentration of 39.00 mg/L (Table 1). Acetaldehyde is produced from the yeast metabolism of sugars and it is partly re-utilized (74). Although SC fermentation produced more acetaldehyde than the others (Table 1), all final values were under the sensory threshold of 100–125 mg/L (75).
Volatile aroma
Isoamyl alcohol, ethyl octanoate and isoamyl acetate were formed in higher total concentrations during SC and LT×SC fermentations (Table 2). On the other hand, final concentrations of ethyl lactate, 2-phenylethanol and 2-phenylethyl acetate (Table 2) up to 5.98, 3.92 and 0.16 mg/L higher, respectively, were reported in LT…SC than in SC fermentation. Other authors have described non- -Saccharomyces yeasts as weaker producers of higher alcohols than Saccharomyces cerevisiae (10, 11, 19, 46, 76). LT…DC fermentation produced the most 2-phenylethanol (Table 2). Other authors have reported higher production of 2-phenylethanol and ethyl lactate by L. thermotolerans than by S. cerevisiae (19), by up to 7.92 and 14.34 mg/L, respectively. L. thermotolerans has also been reported as a weaker ethyl acetate producer than S. cerevisiae (19).
Table 2. Concentrations of volatile compounds detected during different fermentations.
| γ/(mg/L) | SC | LT×SC | LT…SC |
|---|---|---|---|
| Hexanol | (0.96±0.03)a | (1.02±0.04)a | (0.98±0.06)a |
| Isoamyl alcohol | (132.82±6.06)a | (126.92±9.11)a | (102.43±10.64)b |
| Isobutanol | (11.36±1.28)a | (12.56±1.86)a | (14.42±2.76)a |
| Ethyl acetate | (54.42±3.33)a | (53.61±3.42)a | (50.36±5.56)a |
| Ethyl decanoate | (0.11±0.02)a | (0.13±0.03)a | (0.15±0.06)a |
| Ethyl hexanoate | (0.32±0.02)a | (0.34±0.04)a | (0.29±0.06)a |
| Ethyl lactate | (7.16±0.21)a | (8.89±0.48)b | (13.14±2.18)c |
| Ethyl octanoate | (0.36±0.06)a | (0.39±0.09)a | (0.25±0.07)b |
| Isoamyl acetate | (1.62±0.03)a | (1.48±0.06)a | (0.98±0.11)b |
| Hexanoic acid | (8.24±0.54)a | (8.32±0.72)a | (8.18±1.16)a |
| Octanoic acid | (4.32±0.12)a | (4.44±0.18)a | (3.16±0.22)a |
| 2-Phenylethanol | (18.16±0.15)a | (19.32±0.82)a | (22.08±0.93)b |
| 2-Phenylethyl acetate | (0.36±0.01)a | (0.39±0.03)a | (0.52±0.06)b |
Results represent the mean value±S.D. of three replicates. Mean values in the same row with the same letter are not significantly different (p<0.05) SC=fermentation with Saccharomyces cerevisiae 87 alone, LT×SC= mixed fermentation with Lachancea thermotolerans 617 and S. cerevisiae 87, LT…SC=sequential fermentation with L. thermotolerans 617 followed by S. cerevisiae 87
Amino acids and biogenic amines
Higher final levels of histidine, glycine and leucine were obtained in SC and LT×SC fermentations than in LT…SC fermentation (Table 3). LT…SC fermentation had higher final levels of alanine, lysine and serine (Table 3). The final concentrations of each biogenic amine were always lower than 1 mg/L (Table 4). Differences in the amino acid patterns among the different fermentations were found, but they could not be related to the aroma of the Airén wines. Different autolysis behaviour might be the reason for this. A histamine value of 2 mg/L is considered a limiting factor (77) in some countries due to food safety legislation. Our results prove that L. thermotolerans does not produce higher levels of biogenic amines than S. cerevisiae. However, most biogenic amines are produced during malolactic fermentation and wine ageing (78). Nevertheless, the lower concentration of histidine (precursor of histamine) found during LT…SC fermentation (Table 3) contributes to reducing the potential risk of histamine formation by bacterial metabolism. Even though no significant differences were found in final biogenic amine contents, other authors have reported reductions of histamine of up to 2.2 mg/L during alcoholic fermentation with the non-Saccharomyces species Hanseniaspora vineae (6).
Table 3. Concentrations of amino acids determined after different fermentations.
| γ/(mg/L) | SC | LT×SC | LT…SC |
|---|---|---|---|
| Histidine | (6.42±0.87)a | (6.79±1.06)a | (4.15±1.21)b |
| Aspartic acid | (8.62±1.25)a | (9.13±1.60)a | (10.21±2.12)a |
| Alanine | (50.12±2.58)a | (52.27±2.89)ab | (58.14±3.12)b |
| Arginine | (26.06±1.86)a | (27.16±2.52)a | (29.42±3.06)a |
| Asparagine | (29.18±2.13)a | (28.42±2.82)a | (25.22±3.16)a |
| Phenylalanine | (8.52±0.63)a | (8.62±0.89)a | (8.76±1.62)a |
| Glycine | (28.43±1.08)a | (27.12±1.78)ab | (23.56±2.22)b |
| Tryptophan | (0.00±0.00)a | (0.00±0.00)a | (0.00±0.00)a |
| Isoleucine | (2.06±0.22)a | (2.18±0.42)a | (2.36±1.11)a |
| Lysine | (2.42±0.62)a | (2.82±0.86)a | (6.13±1.88)b |
| Leucine | (5.14±0.42)b | (4.92±0.91)a | (3.11±0.89)b |
| Ornithine | (25.17±0.16)a | (25.19±1.06)a | (23.18±1.18)a |
| Serine | (2.28±0.26)a | (2.36±0.76)a | (4.13±0.85)b |
| Tyrosine | (5.36±0.46)a | (5.39±0.68)a | (6.28±0.72)a |
| Threonine | (36.42±0.18)a | (35.43±0.68)a | (34.21±1.13)a |
Results represent the mean value±S.D. of three replicates. Mean value in the same row with the same letter are not significantly different (p<0.05) SC=fermentation with Saccharomyces cerevisiae 87 alone, LT×SC= mixed fermentation with Lachancea thermotolerans 617 and S. cerevisiae 87, LT…SC=sequential fermentation with L. thermotolerans 617 followed by S. cerevisiae 87
Table 4. Biogenic amine concentration in the studied fermentations.
| γ/(mg/L) | SC | LT×SC | LT…SC |
|---|---|---|---|
| Histamine | (0.37±0.02)a | (0.38±0.03)a | (0.40±0.04)a |
| Tyramine | (0.04±0.01)a | (0.03±0.02)a | (0.03±0.02)a |
| Phenylethylamine | n.d. | n.d. | n.d. |
| Putrescine | (0.76±0.03)a | (0.79±0.04)a | (0.75±0.05)a |
| Cadaverine | (0.22±0.01)a | (0.23±0.02)a | (0.21±0.04)a |
Results represent the mean value±S.D. of three replicates. Mean values in the same row with the same letter are not significantly different (p<0.05). n.d.=not detected SC=fermentation with Saccharomyces cerevisiae 87 alone, LT×SC= mixed fermentation with Lachancea thermotolerans 617 and S. cerevisiae 87, LT…SC=sequential fermentation with L. thermotolerans 617 followed by S. cerevisiae 87
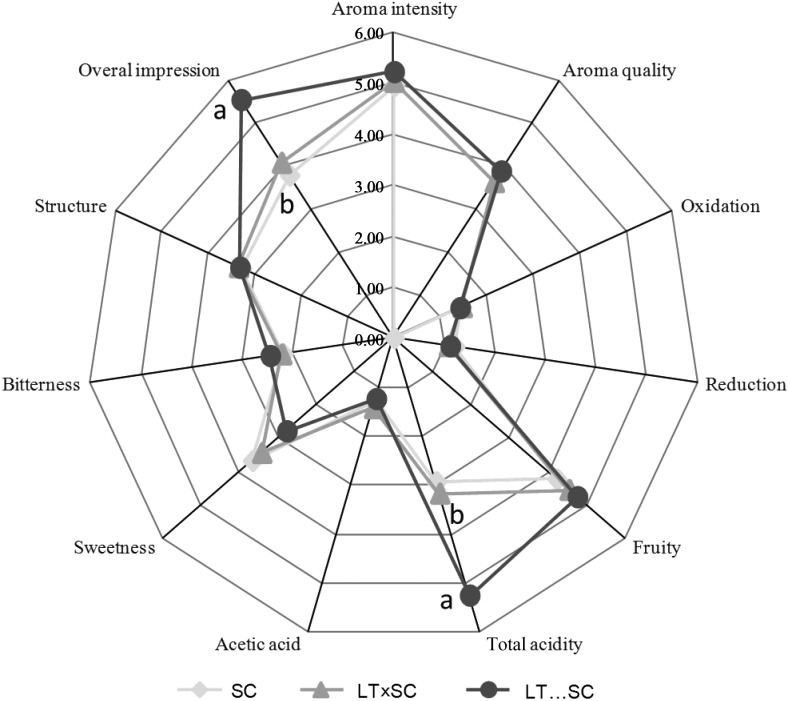
Sensory evaluation
Wines produced in LT…SC fermentation trials had better sensorial properties and general acidity (Fig. 6). However, SC and LT...SC fermentations scored highest in sweetness (Fig. 6). This can be easily explained by the elevated l-lactic acid production by L. thermotolerans. Lack of acidity is a common fault described for Spanish Airén grape variety when compared to other European varieties. Although the wines obtained in SC and LT×SC fermentations were evaluated as sweeter than those in the LT...SC fermentation, all final wines were considered dry from a chemical point of view (Table 1). This perception could be explained due to the different balance between the acidity and sweetness.
Fig. 6.
Taste and olfactory attribute scores for the final wines
Conclusions
The comparison of the results between the fermentation trials showed differences in several analysed parameters and the positive influence of the studied Lachancea thermotolerans yeast strain on Airén wine quality. Finally, sequential fermentation with L. thermotolerans and Saccharomyces cerevisiae remains the best option, as it considerably increased acidity and complexity of the studied neutral grape variety.
Acknowledgements
The authors are very grateful to EUITA Agricola oenology students for their help in performing the trials during the wine microbiology lessons and to the accredited laboratory Estación Enológica de Haro directors, Montserrat Ińiguez and Elena Meléndez (Haro, Spain) for performing the analyses of volatile aromas, amino acids and biogenic amines.
References
- 1.Jolly NP, Varela C, Pretorius IS. Not your ordinary yeast: non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014;14:215–37. 10.1111/1567-1364.12111 [DOI] [PubMed] [Google Scholar]
- 2.Ciani M, Comitini F, Mannazzu I, Domizio P. Controlled mixed culture fermentation: a new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010;10:123–33. 10.1111/j.1567-1364.2009.00579.x [DOI] [PubMed] [Google Scholar]
- 3.Sadoudi M, Tourdot-Marechal R, Rousseaux S, Steyer D, Gallardo-Chacon JJ, Ballester J, et al. Yeast-yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012;32:243–53. 10.1016/j.fm.2012.06.006 [DOI] [PubMed] [Google Scholar]
- 4.Jolly NP, Augustyn OPH, Pretorius IS. The effect of non-Saccharomyces yeasts on fermentation and wine quality. S Afr J Enol Vitic. 2003;24:55–62. [Google Scholar]
- 5.Soden A, Francis IL, Oakey H, Henschke PA. Effects of co- -fermentation with Candida stellata and Saccharomyces cerevisiae on the aroma and composition of Chardonnay wine. Aust J Grape Wine Res. 2000;6:21–30. 10.1111/j.1755-0238.2000.tb00158.x [DOI] [Google Scholar]
- 6.Medina K, Boido E, Farińa L, Gioia O, Gomez ME, Barquet M, et al. Increased flavour diversity of Chardonnay wines by spontaneous fermentation and co-fermentation with Hanseniaspora vineae. Food Chem. 2013;141:2513–21. 10.1016/j.foodchem.2013.04.056 [DOI] [PubMed] [Google Scholar]
- 7.Azzolini M, Fedrizzi B, Tosi E, Finato F, Vagnoli P, Scrinzi C, et al. Effects of Torulaspora delbrueckii and Saccharomyces cerevisiae mixed cultures on fermentation and aroma of Amarone wine. Eur Food Res Technol. 2012;235:303–13. 10.1007/s00217-012-1762-3 [DOI] [Google Scholar]
- 8.King A, Dickinson JR. Biotransformation of monoterpene alcohols by Saccharomyces cerevisiae, Torulaspora delbrueckii and Kluyveromyces lactis. Yeast. 2000;16:499–506. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez ME, Lopes CA, Barbagelata RJ, Barda NB, Caballero AC. Influence of Candida pulcherrima Patagonian strain on alcoholic fermentation behaviour and wine aroma. Int J Food Microbiol. 2010;138:19–25. 10.1016/j.ijfoodmicro.2009.12.025 [DOI] [PubMed] [Google Scholar]
- 10.Parapouli M, Hatziloukas E, Drainas C, Perisynakis A. The effect of Debina grapevine indigenous yeast strains of Metschnikowia and Saccharomyces on wine flavour. J Ind Microbiol Biotechnol. 2010;37:85–93. 10.1007/s10295-009-0651-7 [DOI] [PubMed] [Google Scholar]
- 11.Clemente-Jimenez JF, Mingorance-Cazorla L, Martínez-Rodríguez S, Las Heras-Vázquez FJ, Rodríguez-Vico F. Molecular characterization and oenological properties of wine yeast isolated during spontaneous fermentation of six varieties of grape must. Food Microbiol. 2004;21:149–55. 10.1016/S0740-0020(03)00063-7 [DOI] [Google Scholar]
- 12.Andorrŕ I, Berradre M, Rozčs N, Mas A, Guillamón JM, Esteve-Zarzoso B. Effect of pure and mixed cultures of the main wine yeast species on grape must fermentations. Eur Food Res Technol. 2010;231:215–24. 10.1007/s00217-010-1272-0 [DOI] [Google Scholar]
- 13.Rementeria A, Rodriguez JA, Cadaval A, Amenabar R, Muguruza JR, Hernando FL, et al. Yeast associated with spontaneous fermentations of white wines from the ‘Txakoli de Bizkaia’ region (Basque Country, North Spain). Int J Food Microbiol. 2003;86:201–7. 10.1016/S0168-1605(03)00289-7 [DOI] [PubMed] [Google Scholar]
- 14.Viana F, Gil JV, Vallčs S, Manzanares P. Increasing the levels of 2-phenylethyl acetate in wine through the use of a mixed culture of Hanseniaspora osmophila and Saccharomyces cerevisiae. Int J Food Microbiol. 2009;135:68–74. 10.1016/j.ijfoodmicro.2009.07.025 [DOI] [PubMed] [Google Scholar]
- 15.Moreira N, de Pinho PG, Santos C, Vasconcelos I. Volatile sulphur compounds composition of monovarietal white wines. Food Chem. 2010;123:1198–203. 10.1016/j.foodchem.2010.05.086 [DOI] [Google Scholar]
- 16.Izquierdo-Cańas PM, Palacios García AT, García Romero E. Enhancement of flavour properties in wines using sequential inoculations of non-Saccharomyces (Hansenula and Torulaspora) and Saccharomyces yeast starter. Vitis. 2011;50:177–82. [Google Scholar]
- 17.Benito S, Palomero F, Morata A, Calderón F, Palmero D, Suárez-Lepe JA. Physiological features of Schizosaccharomyces pombe of interest in making of white wines. Eur Food Res Technol. 2013;236:29–36. 10.1007/s00217-012-1836-2 [DOI] [Google Scholar]
- 18.Peinado RA, Moreno JJ, Maestre O, Mauricio JC. Removing gluconic acid by using different treatments with a Schizosaccharomyces pombe mutant: effect on fermentation byproducts. Food Chem. 2007;104:457–65. 10.1016/j.foodchem.2006.11.070 [DOI] [Google Scholar]
- 19.Gobbi M, Comitini F, Domizio P, Romani C, Lencioni L, Mannazzu I, et al. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: a strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013;33:271–81. 10.1016/j.fm.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 20.Takush DG, Osborne JP. Impact of yeast on the aroma and flavour of Oregon Pinot Noir wine. Aust J Grape Wine Res. 2012;18:131–7. 10.1111/j.1755-0238.2012.00181.x [DOI] [Google Scholar]
- 21.Erten H, Tanguler H. Influence of Williopsis saturnus yeasts in combination with Saccharomyces cerevisiae on wine fermentation. Lett Appl Microbiol. 2010;50:474–9. 10.1111/j.1472-765X.2010.02822.x [DOI] [PubMed] [Google Scholar]
- 22.Tanguler H. Evaluation of Williopsis saturnus inoculum level on fermentation and flavor compounds of white wines made from Emir (Vitis vinifera L.) grown in Anatolia. Food Biotechnol. 2012;26:351–68. 10.1080/08905436.2012.724038 [DOI] [Google Scholar]
- 23.Toro ME, Vazquez F. Fermentation behaviour of controlled mixed and sequential cultures of Candida cantarellii and Saccharomyces cerevisiae wine yeasts. World J Microbiol Biotechnol. 2002;18:347–54. 10.1023/A:1015242818473 [DOI] [Google Scholar]
- 24.Morata A, Benito S, Loira I, Palomero F, González MC, Suárez-Lepe JA. Formation of pyranoanthocyanins by Schizosaccharomyces pombe during the fermentation of red must. Int J Food Microbiol. 2012;159:47–53. 10.1016/j.ijfoodmicro.2012.08.007 [DOI] [PubMed] [Google Scholar]
- 25.Benito S, Palomero P, Morata A, Calderón F, Suárez-Lépe JA. New applications for Schizosaccharomyces pombe in the alcoholic fermentation of red wines. Int J Food Sci Technol. 2012;47:2101–8. 10.1111/j.1365-2621.2012.03076.x [DOI] [Google Scholar]
- 26.Benito S, Palomero P, Gálvez L, Morata A, Calderón F, Palmero D, et al. Quality and composition of red wine fermented with Schizosaccharomyces pombe as sole fermentative yeast, and in mixed and sequential fermentations with Saccharomyces cerevisiae. Food Technol Biotechnol. 2014;52:376–82. [Google Scholar]
- 27.Viana F, Belloch C, Vallés S, Manzanares P. Monitoring a mixed starter of Hanseniaspora vineae-Saccharomyces cerevisiae in natural must: Impact on 2-phenylethyl acetate production. Int J Food Microbiol. 2011;151:235–40. 10.1016/j.ijfoodmicro.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 28.Belda I, Navascués E, Marquina D, Santos A, Calderon F, Benito S. Dynamic analysis of physiological properties of Torulaspora delbrueckii in wine fermentations and its incidence on wine quality. Appl Microbiol Biotechnol. 2015;99:1911–22. 10.1007/s00253-014-6197-2 [DOI] [PubMed] [Google Scholar]
- 29.Benito S, Hofmann T, Laier M, Lochbühler B, Schüttler A, Ebert K, et al. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur Food Res Technol. 2015;241:707–17. 10.1007/s00217-015-2497-8 [DOI] [Google Scholar]
- 30.Egli CM, Edinger WD, Mitrakul CM, Henick-Kling T. Dynamics of indigenous and inoculated yeast populations and their effect on the sensory character of Riesling and Chardonnay wines. J Appl Microbiol. 1998;85:779–89. 10.1046/j.1365-2672.1998.00521.x [DOI] [PubMed] [Google Scholar]
- 31.Esteve-Zarzoso B, Manzanares P, Ramón D, Querol A. The role of non-Saccharomyces yeasts in industrial winemaking. Int Microbiol. 1998;1:143–8. [PubMed] [Google Scholar]
- 32.Fleet GH, Heard GM. Yeast-growth during fermentation. In: Fleet GH, editor. Wine microbiology and biotechnology. Chur, Switzerland: Harwood Academic Publishers; 1993. pp. 27–54. [Google Scholar]
- 33.Fleet GH. Yeast interactions and wine flavour. Int J Food Microbiol. 2003;86:11–22. 10.1016/S0168-1605(03)00245-9 [DOI] [PubMed] [Google Scholar]
- 34.Fleet GH. Wine yeasts for the future. FEMS Yeast Res. 2008;8:979–95. 10.1111/j.1567-1364.2008.00427.x [DOI] [PubMed] [Google Scholar]
- 35.Gil JV, Mateo JJ, Jiménez M, Pastor A, Huerta T. Aroma compounds in wines as influenced by apiculate yeasts. J Food Sci. 1996;61:1247–50. 10.1111/j.1365-2621.1996.tb10971.x [DOI] [Google Scholar]
- 36.Henick-Kling T, Edinger W, Daniel P, Monk P. Selective effects of sulfur dioxide and yeast starter culture addition on indigenous yeast populations and sensory characteristics of wine. J Appl Microbiol. 1998;84:865–76. 10.1046/j.1365-2672.1998.00423.x [DOI] [Google Scholar]
- 37.Lambrechts MG, Pretorius IS. Yeast and its importance to wine aroma – a review. S Afr J Enol Vitic. 2000;21:97–129. [Google Scholar]
- 38.Romano P, Fiore C, Paraggio M, Caruso M, Capece A. Function of yeast species and strains in wine flavour. Int J Food Microbiol. 2003;86:169–80. 10.1016/S0168-1605(03)00290-3 [DOI] [PubMed] [Google Scholar]
- 39.Viana F, Gil JV, Genovés S, Vallés S, Manzanares P. Rational selection of non-Saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol. 2008;25:778–85. 10.1016/j.fm.2008.04.015 [DOI] [PubMed] [Google Scholar]
- 40.Carrau F, Gaggero C, Aguilar PS. Yeast diversity and native vigor for flavor phenotypes. Trends Biotechnol. 2015;33:148–54. 10.1016/j.tibtech.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 41.Rojas V, Gil JV, Pińaga F, Manzanares P. Studies on acetate ester production by non-Saccharomyces wine yeasts. Int J Food Microbiol. 2001;70:283–9. 10.1016/S0168-1605(01)00552-9 [DOI] [PubMed] [Google Scholar]
- 42.Ciani M, Beco L, Comitini F. Fermentation behaviour and metabolic interactions of multistarter wine yeast fermentations. Int J Food Microbiol. 2006;108:239–45. 10.1016/j.ijfoodmicro.2005.11.012 [DOI] [PubMed] [Google Scholar]
- 43.Contreras A, Hidalgo C, Henschke PA, Chambers PJ, Curtin C, Varela C. Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl Environ Microbiol. 2014;80:1670–8. 10.1128/AEM.03780-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benito S, Palomero P, Calderón F, Palmero D, Suárez-Lepe JA. Schizosaccharomyces. In: Batt CA, Tortorello ML, editors. Encyclopedia of food microbiology. Amsterdam, The Netherlands: Elsevier Ltd, Academic Press; 2014. pp. 365–70. [Google Scholar]
- 45.Herraiz T, Reglero G, Herraiz M, Martin-Alvarez PJ, Cabezudo MD. The influence of the yeast and type of culture on the volatile composition of wines fermented without sulfur dioxide. Am J Enol Vitic. 1990;41:313–8. [Google Scholar]
- 46.Zironi R, Romano P, Suzzi G, Battistutta F, Comi G. Volatile metabolites produced in wine by mixed and sequential cultures of Hanseniaspora guilliermondii or Kloeckera apiculata and Saccharomyces cerevisiae. Biotechnol Lett. 1993;15:235–8. 10.1007/BF00128311 [DOI] [Google Scholar]
- 47.González-Royo E, Pascual O, Kontoudakis N, Esteruelas M, Esteve-Zarzoso B, Mas A, et al. Oenological consequences of sequential inoculation with non-Saccharomyces yeasts (Torulaspora delbrueckii or Metschnikowia pulcherrima) and Saccharomyces cerevisiae in base wine for sparkling wine production. Eur Food Res Technol. 2015;240:999–1012. 10.1007/s00217-014-2404-8 [DOI] [Google Scholar]
- 48.Oro L, Ciani M, Comitini F. Antimicrobial activity of Metschnikowia pulcherrima on wine yeasts. J Appl Microbiol. 2014;116:1209–17. 10.1111/jam.12446 [DOI] [PubMed] [Google Scholar]
- 49.Di Maio S, Genna G, Gandolfo V, Amore G, Ciaccio M, Oliva D. Presence of Candida zemplinina in Sicilian musts and selection of a strain for wine mixed fermentations. S Afr J Enol Vitic. 2012;33:80–7. [Google Scholar]
- 50.Domizio P, Romani C, Lencioni L, Comitini F, Gobbi M, Mannazzu I, et al. Outlining a future for non-Saccharomyces yeasts: selection of putative spoilage wine strains to be used in association with Saccharomyces cerevisiae for grape juice fermentation. Int J Food Microbiol. 2011;147:170–80. 10.1016/j.ijfoodmicro.2011.03.020 [DOI] [PubMed] [Google Scholar]
- 51.Domizio P, Romani C, Comitini F, Gobbi M, Lencioni L, Mannazzu I, et al. Potential spoilage non-Saccharomyces yeasts in mixed cultures with Saccharomyces cerevisiae. Ann Microbiol. 2011;61:137–44. 10.1007/s13213-010-0125-1 [DOI] [Google Scholar]
- 52.Benito S, Palomero F, Calderón F, Palmero D, Suárez-Lepe JA. Selection of appropriate Schizosaccharomyces strains for winemaking. Food Microbiol. 2014;42:218–24. 10.1016/j.fm.2014.03.014 [DOI] [PubMed] [Google Scholar]
- 53.Izquierdo-Cańas PM, García-Romero E, Heras Manso JM, Fernández-González M. Influence of sequential inoculation of Wickerhamomyces anomalus and Saccharomyces cerevisiae in the quality of red wines. Eur Food Res Technol. 2014;239:279–86. 10.1007/s00217-014-2220-1 [DOI] [Google Scholar]
- 54.Benito S, Morata A, Palomero F, González MC, Suárez-Lepe JA. Formation of vinylphenolic pyranoanthocyanins by Saccharomyces cerevisiae and Pichia guillermondii in red wines produced following different fermentation strategies. Food Chem. 2011;124:15–23. 10.1016/j.foodchem.2010.05.096 [DOI] [Google Scholar]
- 55.Kapsopoulou K, Kapaklis A, Spyropoulos H. Growth and fermentation characteristics of a strain of the wine yeast Kluyveromyces thermotolerans isolated in Greece. World J Microbiol Biotechnol. 2005;21:1599–602. 10.1007/s11274-005-8220-3 [DOI] [Google Scholar]
- 56.Kapsopoulou K, Mourtzini A, Anthoulas M, Nerantzis E. Biological acidification during grape must fermentation using mixed cultures of Kluyveromyces thermotolerans and Saccharomyces cerevisiae. World J Microbiol Biotechnol. 2007;23:735–9. 10.1007/s11274-006-9283-5 [DOI] [Google Scholar]
- 57.Su J, Wang T, Wang Y, Li YY, Li H. The use of lactic acid-producing, malic acid-producing, or malic acid-degrading yeast strains for acidity adjustment in the wine industry. Appl Microbiol Biotechnol. 2014;98:2395–413. 10.1007/s00253-014-5508-y [DOI] [PubMed] [Google Scholar]
- 58.Benito Á, Calderón F, Palomero F, Benito S. Combine use of selected Schizosaccharomyces pombe and Lachancea thermotolerans yeast strains as an alternative to the traditional malolactic fermentation in red wine production. Molecules. 2015;20:9510–23. 10.3390/molecules20069510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mora J, Barbas JI, Mulet A. Growth of yeast species during the fermentation of musts inoculated with Kluyveromyces thermotolerans and Saccharomyces cerevisiae. Am J Enol Vitic. 1990;41:156–9. [Google Scholar]
- 60.Sampaio TL, Kennedy A, Vasconcelos MC. Use of microscale fermentations in grape and wine research. Am J Enol Vitic. 2007;58:534–9. [Google Scholar]
- 61.Yarrow D. Methods for the isolation, maintenance and identification of yeasts. In: Kurtzman CP, Fell JW, editors. The yeast. A taxonomic study. Amsterdam, The Netherlands: Elsevier Ltd, Academic Press; 1998. pp. 77–100. [Google Scholar]
- 62.Vaughnan-Martini A, Martini A. Determination of ethanol production. In: Kurtzman CP, Fell JW, editors. The yeast. A taxonomic study. Amsterdam, The Netherlands: Elsevier Ltd, Academic Press; 1999. p. 107. [Google Scholar]
- 63.Official Methods OIV. Compendium of international methods of analysis of wine and must. Paris, France: International Organisation of Vine and Wine, 2015. Available from: http://www.oiv.int. [Google Scholar]
- 64.Morris EO, Eddy AA. Method for the measurement of wild yeast infection in pitching yeast. J Inst Brew. 1957;63:34–5. 10.1002/j.2050-0416.1957.tb02902.x [DOI] [Google Scholar]
- 65.Ortega C, López R, Cacho J, Ferreira V. Fast analysis of important wine volatile compounds: development and validation of a new method based on gas chromatographic-flame ionisation detection analysis of dichloromethane microextracts. J Chromatogr A. 2001;923:205–14. 10.1016/S0021-9673(01)00972-4 [DOI] [PubMed] [Google Scholar]
- 66.López R, Aznar M, Cacho J, Ferreira V. Determination of minor and trace volatile compounds in wine by solid-phase extraction and gas chromatography with mass spectrometric detection. J Chromatogr A. 2002;966:167–77. 10.1016/S0021-9673(02)00696-9 [DOI] [PubMed] [Google Scholar]
- 67.Loscos N, Hernandez-Orte P, Cacho J, Ferreira V. Release and formation of varietal aroma compounds during alcoholic fermentation from nonfloral grape odorless flavor precursors fractions. J Agric Food Chem. 2007;55:6674–84. 10.1021/jf0702343 [DOI] [PubMed] [Google Scholar]
- 68.Albergaria H, Francisco D, Gori K, Arneborg N, Gírio F. Saccharomyces cerevisiae CCMI 885 secretes peptides that inhibit the growth of some non-Saccharomyces wine-related strains. Appl Microbiol Biotechnol. 2010;86:965–72. 10.1007/s00253-009-2409-6 [DOI] [PubMed] [Google Scholar]
- 69.Kutyna DR, Varela C, Henschke PA, Chambers PJ, Stanley GA. Microbiological approaches to lowering ethanol concentration in wine. Trends Food Sci Technol. 2010;21:293–302. 10.1016/j.tifs.2010.03.004 [DOI] [Google Scholar]
- 70.Comitini F, Gobbi M, Domizio P, Romani C, Lencioni L, Mannazzu I, et al. Selected non-Saccharomyces wine yeast in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011;28:873–82. 10.1016/j.fm.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 71.Jolly NP, Augustyn OPH, Pretorius IS. The role and use of non-Saccharomyces yeasts in wine production. S Afr J Enol Vitic. 2006;27:15–39. [Google Scholar]
- 72.Prior BA, Toh TH, Jolly N, Baccari C, Mortimer RK. Impact of yeast breeding for elevated glycerol production on fermentation activity and metabolite formation in Chardonnay. S Afr J Enol Vitic. 2000;21:92–9. [Google Scholar]
- 73.Ciani M, Maccarelli F. Oenological properties of non-Saccharomyces yeasts associated with winemaking. World J Microbiol Biotechnol. 1998;14:199–203. 10.1023/A:1008825928354 [DOI] [Google Scholar]
- 74.Jackowetz N, Dierschke S, Mira de Orduna R. Multifactorial analysis of acetaldehyde kinetics during alcoholic fermentation by Saccharomyces cerevisiae. Food Res Int. 2011;44:310–6. 10.1016/j.foodres.2010.10.014 [DOI] [Google Scholar]
- 75.Zoecklein B, Fugelsang K, Gump B, Nury F, editors. Wine analysis and production. New York, NY, USA: Harwood Academic Publishers; 1995. [Google Scholar]
- 76.Romano P, Suzzi G. Higher alcohol and acetoin production by Zygosaccharomyces wine yeasts. J Appl Bacteriol. 1993;75:541–5. 10.1111/j.1365-2672.1993.tb01592.x [DOI] [Google Scholar]
- 77.Lehtonen P. Determination of amines and amino acids in wine: A review. Am J Enol Vitic. 1996;47:127–33. [Google Scholar]
- 78.Alcaide-Hidalgo JM, Moreno-Arribas MV, Martín-Álvarez PJ, Polo MC. Influence of malolactic fermentation, postfermentative treatments and ageing with lees on nitrogen compounds of red wines. Food Chem. 2007;103:572–81. 10.1016/j.foodchem.2006.09.002 [DOI] [PubMed] [Google Scholar]