Summary
The objective of this study is to investigate the effects of irradiation from light-emitting diodes (LEDs) on several fruits during storage. To improve storage and increase the contents of some bioactive compounds, apple, tomato and red bell pepper fruits were exposed to yellow light emitted from the diodes at 590 nm. The contents of ascorbic acid, total phenolics, total flavonoids and several pigments were investigated, along with the antioxidant potential. The colour parameters (L*, a* and b*) and firmness of the fruit were also determined. After 7 days of LED light irradiation, there was significantly higher total phenolic content and antioxidant potential in apple peel extracts. The irradiated fruit of tomato had significantly higher levels of total phenolic compounds, and the fruit of red bell pepper had significantly higher antioxidant potential. LED light had no effects on the colour parameters, although there was a tendency to accelerate colour development. Apple fruit irradiated with LED light was significantly less firm. Among twelve analysed pigments, significantly more β-carotene was detected in LED light-irradiated apple and bell pepper fruit, more α-tocopherol and γ-tocopherol in bell pepper fruit, and more lutein in apple peel and bell pepper fruit. The applied LED light slightly accelerated the ripening of the studied fruit, and affected the synthesis of some of the secondary metabolites.
Key words: LED light, antioxidant potential, phenolics, flavonoids, pigments, ascorbic acid, tocopherols
Introduction
The human body produces reactive oxygen species (ROS), such as superoxide anion radicals, hydroxyl radicals and hydrogen peroxide, which can be beneficial in small amounts, but can lead to oxidative stress in larger amounts. To balance the ROS, there is the need for antioxidants in our diet (1). As a strong scavenger activity against free radicals is found in many plants worldwide, the intake of fruit and vegetables is associated with a lower risk of cancer and cardiovascular disease (2). Consumption of natural exogenous antioxidants, such as polyphenols, has protective effects against these diseases, which can be partly attributed to several specific components: vitamins, flavonoids, anthocyanins and other phenolic compounds (1).
Polyphenols are secondary metabolites that have aromatic rings and hydroxyl groups, and these compounds can be divided into phenolic acids, stilbenes, flavonoids and lignans. The levels of polyphenols in plants vary depending on different cultivars, soil composition, growth conditions, maturity state and postharvest treatments (3). Flavonoids are a group of polyphenolic antioxidants that have the capacity to transfer electrons to free radicals, to activate antioxidant enzymes, and to reduce α-tocopherol radicals. Flavonoids occur in foods of plant origin in different variations: flavonols, flavones, catechins, flavanones, anthocyanidins and isoflavonoids (2, 4). Previous studies have confirmed that there are high levels of phenols and flavonoids in the fruit of apple (Malus domestica ‘Granny Smith’) (5, 6), tomato (Solanum lycopersicum L.) (7, 8) and sweet pepper (Capsicum annuum) (9, 10).
Light is one of the most important environmental factors for life, and its intensity and spectra can affect the physiological response of plants, including the range of accumulated phytochemicals, and their levels (11, 12). Irradiation in visible and UV range can induce stress that evokes antioxidant defence system responses (13).
Exposure of fruit to light in orchard plays an important role in the development of fruits, accumulation of secondary metabolites and consequently the postharvest behaviour (14). Solar irradiation together with climatic conditions (15), harvest date, genotype and postharvest factors, as storage temperature, O2 and CO2 contents, significantly influence postharvest behaviour of fruits and vegetables (16).
Nowadays more and more vegetables and fruits are produced in controlled environment with artificial light sources such as light-emitting diodes (LEDs) (17). Their usage in growth chambers and greenhouse is increasing due to numerous benefits, such as high energy-conversion efficiency, small mass and volume, and relatively cool surfaces with minimum heating and long life expectancy. Furthermore, a major advantage is the ability to control their spectral composition, and therefore their wavelengths can be matched to plant photoreceptors (17–19).
Wider application of LEDs in agriculture has led to several studies where the effects of the light spectrum and quality on the preharvest (6) and postharvest physiology (11, 20) were evaluated, often showing inconsistent results. Responses to different light spectra can vary across plant species or even varieties (21) and are dependent on the maturity stage. It has been shown that white and red LEDs enhance the tomato yield (22), whereas blue LED light had positive effects on their growth and development (23, 24). Similar effects have been observed in pepper plants, where supplemental irradiation with blue LED light resulted in better development of the stems, leaves and plant biomass (25). Light is not only the crucial factor for plant development during growth phase but can also influence the postharvest behaviour when fruits and vegetables are irradiated during storage. Some recent studies have shown that the application of LEDs during storage can preserve and even improve the nutritional quality of fruits (26) and vegetables (20, 27, 28). The results are nevertheless inconclusive and detailed analysis of LED light spectra on postharvest physiology is still lacking.
Yellow light (wavelength around 580 nm) is one of the least studied when the influence on plant physiology is evaluated. There are nevertheless some promising reports that irradiation with light in the range of 500–600 nm results in an increase of ascorbic acid and anthocyanin content (29) and that yellow light with wavelengths in the range of 580–600 nm may influence gene expression in plants during growth (30). However, there are no literature data available about the influence of irradiation with yellow LED light during storage on secondary metabolites of fruits and vegetables. The challenge to horticulturists and postharvest operators is to manipulate and manage light application to maximize yield and storability of fruits and vegetables. Therefore, the present study was carried out to investigate the contents of ascorbic acid (AAC), total phenolic compounds (TPC), total flavonoids (TFC), chlorophylls, carotenoids and tocopherols, and the antioxidant potential (AOP) of the apple, tomato and red pepper fruit during 7 days of storage under LED light at a wavelength of 590 nm.
Materials and Methods
Plant material
Apple, tomato and bell pepper fruit were subjected to LED light irradiation at the wavelength of 590 nm. Commercial varieties of apple (Malus domestica ‘Granny Smith’), red tomato (Solanum lycopersicum L.) and bell pepper (Capsicum annuum) fruit were studied. The selected fruit were purchased from a supermarket in Ljubljana, Slovenia, in January 2014. The fruit were of commercial size, adequate colour, and at a physiologically mature stage. The purchased samples were immediately prepared for storage in LED light irradiation box, or in darkness as the control. All of the analyses were carried out before the LED light irradiation and after 7 days, i.e. at the end of the fruit storage.
Storage conditions
Half of the fruit used for storage (three fruits per group) were put into a LED light irradiation cardboard box at 10 °C for 7 days under constant lighting provided by yellow LEDs at a wavelength of 590 nm. The other half of the fruit (three fruits per group) represented the controls, which were stored in the dark under the same conditions. Each fruit group for the experimental samples was irradiated with six LEDs providing a total radian flux of 0.14 W on the irradiated surface. The LEDs were positioned approx. 15 cm from the fruit surfaces and the average irradiance on the fruit surface was 1.81 W/m2.
Sample preparation
For the analysis of apple fruit, only the skin of the fruit was used. Strips of apple skin approx. 15 mm wide were removed using a commercial hand peeler. Tomato and bell pepper samples (three fruits of each) included fruit skin and flesh, without seeds approx. 1 cm thick, taken using a cork borer. Sampling procedure included only irradiated parts of the fruit, i.e. upper part of the fruit.
For the sample extraction, 6 g of fruit sample were homogenised with 18 g of metaphosphoric acid dissolved in distilled water (2% by mass), using a T 25 Ultra-Turrax® homogenizer (IKA®-Werke GmbH&Co. KG, Staufen, Germany) at 13 500 rpm. The homogenised samples were filtered first through a filter paper, and then through 0.45--µm filters (17-mm cellulose acetate (CA) syringe filter; Sartorius AG, Goettingen, Germany). The samples were then stored at –80 °C until further analysis.
Ascorbic acid
Ascorbic acid content (AAC) determination was performed on an HPLC system (model 1260 Infinity; Agilent Technologies, Santa Clara, CA, USA) using a diode array detector, with the wavelength set at 254 nm. The separation of ascorbic acid was carried out on a 100 mm×2 mm, i.d. 3 µm, Scherzo SM-C18 column (Imtakt, Kyoto, Japan), at a flow rate of 0.3 mL/min. The mobile phase consisted of water (A) and acetonitrile (B), both of which contained 0.3% (by volume) formic acid. The following elution gradient was used for solvent B: 0–3 min, 0–10%; 3–4 min, 10–100% and 4–6 min, 100%. The temperature of the column was maintained at 30 °C, and the temperature of the automatic sample feeder at 4 °C. AAC was calculated using an external standard, and expressed in mg of ascorbic acid per 100 g of fresh mass (FM).
DPPH radical-scavenging activity
Antioxidant potential (AOP) of the samples was determined spectrophotometrically, as the 2,2-diphenyl-1- -picrylhydrazyl (DPPH; Sigma-Aldrich, Darmstadt, Germany) free-radical-scavenging capacity, based on the modified method of Brand-Williams et al. (31). For this analysis, a 560-µM DPPH solution was prepared in methanol. A volume of 400 µL of the solution was mixed with 1580 µL (for apple peel and bell pepper samples) or 1550 µL of methanol (for tomato samples), and 20 µL of the individual samples (or 50 µL of tomato samples) were added, followed by vortexing for 10 s (VWR VM-3000 mini vortexer; Henry Troemner LLC, Thorofare, NJ, USA). The absorbance was measured after 40 min of incubation at room temperature by spectrophotometer (Cecil Aurius Series CE 2021 UV/Vis; Cecil Instruments Limited, Cambridge, UK) at 520 nm, against methanol as the blank. Calibration was done through seven-point standard curves of Trolox (Sigma-Aldrich) (R2=0.9985), which ranged from 1.56 to 10.94 mg per L, and the AOP of the samples was determined in triplicate and expressed in µmol per L of Trolox equivalents (TE) per 1 g of FM.
Total phenolic content
Total phenolic content (TPC) was determined according to the Folin-Ciocalteu method described by Singleton and Rossi (32), with minor modifications. Apple peel and red bell pepper samples (20 µL) or 100 µL of the tomato samples were incubated with 1380 or 1300 µL, respectively of deionised water, and 300 µL of diluted Folin-Ciocalteu reagent (10 mL of Folin-Ciocalteu reagent; Merck, Darmstad, Germany, in 20 mL of deionised water). These were mixed in 2-mL microcentrifuge tubes using a vortex (10 s). After 5 min of incubation, 300 µL of 20% Na2CO3 (Merck) were added to the mixtures, which were vortexed again (10 s). After a further incubation at room temperature for 30 min, the absorbances of the mixtures were measured on a spectrophotometer at 765 nm, with deionised water as the blank. All of the samples were processed in triplicate. TPC was quantified through calibration using gallic acid (Fluka, Buchs, Switzerland) as standard. The eight-point calibration curves ranged from 1.7 to 13.6 mg/L of gallic acid (R2=0.9988). TPC was expressed in mg of gallic acid equivalents (GAE) per 100 g of FM.
Total flavonoid content
Total flavonoid content (TFC) was determined according to the method described by Lin and Tang (9). A volume of 250 µL of the samples was mixed with 750 µL of 95% ethanol, 50 µL of AlCl3 (Fluka), 50 µL of 1 mol/L CH3COOK (Sigma-Aldrich), and 1400 µL of deionised water. After incubation at room temperature for 40 min, the absorbance of the reaction mixture was measured by a spectrophotometer at 415 nm, against deionised water as the blank. For the calibration, five-point standard curves for quercetin were used (R2=0.9998), which ranged between 0.3 and 15.0 mg/L. TFC of the samples was determined in triplicate and expressed in mg of quercetin equivalents (QE) per 100 g of FM.
Pigments
The analysis of the pigments was carried out according to a method described previously (33). Pigments were extracted from approx. 200 mg of frozen fruits with 5 mL of ice-cold acetone in an ice bath, using T 25 Ultra-Turrax homogenizer (Ika-Labortechnik) for 30 s. All extraction procedures were performed in dim light. Acetone extracts were filtered through 0.2-µm Minisart SRP 15 filter (Sartorius Stedim Biotech GmbH, Goettingen, Germany). Levels of the pigments lycopene, neoxanthin, violaxanthin, antheraxanthin, lutein, chlorophyll a, chlorophyll b, α-carotene and β-carotene were determined using an HPLC system (a Spherisorb ODS2 column, 250 mm×4.6 mm, i.d. 5 µm, with a Spherisorb ODS2 precolumn, 50 mm×4.6 mm, i.d. 5 µm; Alltech Associaties, Inc., Deerfield, IL, USA), with elution using acetonitrile/water/methanol (100:10:5, by volume) as mobile phase A, and acetone/ethyl acetate (2:1, by volume) as mobile phase B, at a flow rate of 1 mL/min. The following linear gradient was used for solvent B: 0–18 min, 10–70%, with a run time 30 min. The injection volume was 20 µL. The pigment compounds were monitored at a wavelength of 440 nm. The analysis was performed on a Surveyor HPLC system with a diode array detector (Thermo Finnigan, San Jose, CA, USA). Identification of compounds was achieved by comparing the retention times and the spectra as well as by the addition of standards. The mass fractions (in mg per g of FM) of the pigments were calculated by the external standard method, using the following standards: α-carotene, β-carotene, neoxanthin, violaxanthin, lutein, antheraxanthin and chlorophylls (DHI Water & Environment, Hřrsholm, Denmark), and lycopene (Sigma-Aldrich). All standards were highly purified (at least 95% pure). The solvents acetone, ethylacetate, methanol and acetonitrile were from Merck, all of HPLC grade. Water used for preparation of solutions and eluents was double-distilled and purified with a Milli-Q water purification system by Millipore (Bedford, MA, USA).
Tocopherol content
The method used for the analysis of tocopherols was described by Wildi and Lütz (34). Tocopherols were extracted from frozen samples with ice-cold acetone exactly as described for chloroplast pigments. Spectra-Physics HPLC system with a P4000 SpectraSYSTEM™ pump and an AS1000 SpectraSYSTEM™ automatic sample feeder (Thermo Fisher Scientific Inc.) was used. The tocopherols were passed through a Spherisorb ODS2 guard column (50 mm×4.6 mm, 5 µm), and separated on a Spherisorb ODS2 column (250 mm×4.6 mm, 5 µm). The temperature of the automatic sample feeder was 4 °C, and the column was at room temperature. Methanol was used as the mobile phase, at flow rate of 1 mL/min. The injection volume of the samples was 20 µL, with an analysis duration of 30 min. Excitation was determined at 295 nm and emission at 325 nm, with the tocopherol levels calculated against an external standard (Sigma-Aldrich). The tocopherol levels were expressed in µg per g of FM.
Surface colour
The surface colour of the fruit was measured using a colour-measurement device (Minolta CR-400, Minolta, Kyoto, Japan). At the beginning of the study and after 7 days of storage, L*, a* and b* were measured. L* represents the lightness of the colour, a* the position between red (+) and green (–), and b* the scale between blue (–) and yellow (+). These colour measurements were taken at four locations on each of three individual fruit samples (N=12).
Firmness
The force required to penetrate the apple fruit was measured at four locations on each peeled apple, using a 53200SP penetrometer (T.R. Turoni, Forlě, Italy). To determine the firmness of the tomato and bell pepper fruit, a Kramer shear cell (Stable Micro Systems, Godalming, Surrey, UK) was used. The firmness was measured in kg/m2 at the beginning of the study, before storage, and after 7 days of storage.
Statistical analysis
The data were analyzed according to the method of least squares, using a general linear model (GLM) procedure, SAS Software v. 8.01 (35). All of the measurements were performed in triplicate (N=3). Differences at p<0.05 were considered as statistically significant.
Results and Discussion
Recent studies have confirmed the influence of LED light irradiation on plant growth and development. In the present study, we investigated the effects of yellow LED light (590 nm) on several biochemical compounds and quality parameters of apple, tomato and red bell pepper fruit. Our data show differences between the control (stored in the dark) and LED light-irradiated fruit for most of the studied parameters. The mechanisms of the influence of LED light on plant metabolism have been well studied in the plants grown in glasshouses, but they have not been studied in any great extent during fruit storage after harvest.
The data from the analyses of AAC, AOP, TPC and TFC of the control (dark storage) and LED light-irradiated fruit are given in Table 1. The effects of the yellow LED light under the controlled conditions on the compounds analysed in apple peel, tomato and bell pepper fruit varied considerably.
Table 1. Mass fractions of the ascorbic acid (AAC), total phenols (TPC) and flavonoids (TFC), and antioxidant potential (AOP) of the fruit samples before (start) and after 7 days (final) of storage in the dark (control) or under LED light irradiation (590 nm).
| Analysis | Sampling point |
Apple peel | Tomato | Red bell pepper | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control | LED light | Control | LED light | Control | LED light | ||||
| w(AAC)/(mg/100 g FM) | Start | 36.27±0.07 | 20.83±3.94 | 194.36±6.70 | |||||
| Final | 36.97±3.72 | 39.55±1.05 | 11.37±0.80 | 14.08±2.70 | 237.99±14.89 | 261.35±18.15 | |||
| AOP/(µmol TE/g FM) | Start | 5.27±1.07 | 0.80±0.23 | 8.20±0.10 | |||||
| Final | (5.20±1.01)b | (6.95±0.04)a | 0.95±0.05 | 0.99±0.11 | (9.60±0.01)b | (10.23±0.18)a | |||
| w(TPC)/(mg GAE/100 g FM) | Start | 118.4±3.26 | 24.22±0.93 | 133.91±4.03 | |||||
| Final | (178.20±17.95)b | (204.20±3.26)a | (19.75±1.75)b | (23.29±1.90)a | 171.87±0.79 | 176.32±5.35 | |||
| w(TFC)/(mg QE/100 g FM) | Start | 7.15±0.55 | 2.57±0.56 | 16.6±0.33 | |||||
| Final | 14.69±4.25 | 13.30±0.76 | 2.67±0.25 | 2.43±0.50 | 17.74±0.87 | 16.60±0.33 | |||
Data are mean value±standard deviation (N=3). Mean values with different letters within each row are significantly different (p<0.05). LED=light-emitting diode, FM=fresh mass, TE=Trolox equivalents, GAE=gallic acid equivalents, QE=quercetin equivalents
As shown in Table 1, there was higher AAC in the LED light-irradiated apple peel, tomato and red bell pepper fruit compared to the control samples stored in the dark, although these AAC differences did not reach statistical significance. A previous investigation reported higher AAC in tomato fruit when blue light (450 nm) was applied (19). Kim et al. (36) determined higher AAC in strawberry fruit under light at 470 and 525 nm. On the other hand, Samuolienė et al. (13) reported that supplemental LED light colours at 380 and 595 nm had negative effects on AAC in romaine baby leaf lettuce during the growth period. Relatively high standard deviations were observed among the individual fruit samples in the present study, which might have arisen from different factors, including cultivar, genotype, growing season, harvest conditions, storage, and environmental conditions, such as location, growing temperature, soil and light (37–39).
Similarly, there was higher AOP in all of the LED light-irradiated samples; indeed, the differences in AOP were statistically significant compared to the controls for apple peel and red bell pepper fruit (13). In the previously mentioned study on romaine baby leaf lettuce during the growth period significantly higher AOP was also reported when supplemental green light (530 nm) was used, compared to yellow light in the present study (590 nm). On the other hand, the same authors reported lower AOP when LED light at 455 and 470 nm was applied.
Again, as for AAC and AOP, there was higher TPC in all of the LED light-irradiated samples, with statistically significant differences observed for apple peel and tomato fruit. In comparison with the data of the present study, other studies have shown slightly lower TPC values (but higher TFC; see also below) in the fruit of several pepper cultivars (40). Samuolienė et al. (13) again reported that supplemental orange light (622 nm) enhanced the synthesis of phenolic compounds in romaine baby leaf lettuce during the growth period. They also reported in another study (21) that supplemental LED light at 505 nm promoted significant increases in TPC, but on the other hand, LED light at 535 nm resulted in significantly lower TPC. Also in baby leaf lettuce, Li and Kubota (11) showed an increase in TPC after exposure to LED light from 600 to 700 nm. Using LED light at 470 nm, Kim et al. (36) reported significantly higher TPC in strawberry fruit, and as reported by Zhan et al. (41), storage of broccoli under fluorescent light increased TPC.
Here, lower TFC was observed in LED light-irradiated apple peel, tomato and red bell pepper fruit samples, as compared to the controls, although again, these differences were not statistically significant. In a previous study, use of a wavelength near the λmax of flavonoids (i.e. UV-A light at 380–320 nm) increased TFC in radish sprouts, parsley and Indian spinach (42). Also, Harbaum--Piayda et al. (43) investigated preharvest UV-B irradiation (at 280–380 nm) of pak choi (Brassica campestris L. ssp. chinensis var. communis) and demonstrated a twofold increase in TFC compared to the non-irradiated control.
The pigments and tocopherols in the individual samples here showed different responses to these yellow LED light conditions during storage. We analysed 12 different pigments in these samples, the data for which are shown in Table 2.
Table 2. Mass fractions of compounds (per fresh mass) studied in the fruit samples after 7 days of storage in the dark (control) or under LED light irradiation (590 nm).
| Compound | w/(µg/g) | |||||||
|---|---|---|---|---|---|---|---|---|
| Apple peel | Tomato | Red bell pepper | ||||||
| Control | LED light | Control | LED light | Control | LED light | |||
| Lycopene | <LOD | <LOD | 475.60±14.57 | 461.29±2.93 | <LOD | <LOD | ||
| α-Carotene | <LOD | <LOD | <LOD | <LOD | 2.49±0.09 | 2.78±0.21 | ||
| β-Carotene | (8.18±0.08)b | (10.73±0.13)a | (75.64±0.46)a | (69.81±0.58)b | (115.98±3.15)b | (152.17±0.79)a | ||
| δ-Tocopherol | 14.28±2.06 | 13.82±0.48 | <LOD | <LOD | 1.51±0.40 | <LOD | ||
| γ-Tocopherol | 4.37±0.02 | 4.26±0.20 | (6.32±0.08)a | (4.63±0.07)b | (5.29±0.09)b | (8.61±0.01)a | ||
| α-Tocopherol | 89.25±0.88 | 77.93±4.85 | 116.45±0.00 | 112.21±3.79 | (135.71±1.06)b | (152.59±1.80)a | ||
| Neoxanthin | 1.42±0.15 | 1.47±0.22 | 2.49±0.36 | 3.36±0.19 | <LOD | <LOD | ||
| Violaxanthin | 4.87±0.61 | 5.70±0.69 | 4.35±0.76 | 4.17±0.06 | 20.04±1.31 | 22.61±1.62 | ||
| Antheraxanthin | <LOD | <LOD | <LOD | <LOD | 6.14±0.55 | 7.29±0.21 | ||
| Lutein | (8.59±0.08)b | (9.86±0.09)a | 9.47±0.02 | 9.24±0.08 | (2.46±0.04)b | (3.60±0.18)a | ||
| Chlorophyll b | (18.57±0.63)b | (21.91±0.14)a | (3.58±0.09)a | (1.23±0.74)b | (8.87±0.69)a | (5.96±1.10)b | ||
| Chlorophyll a | (59.54±0.04)b | (72.38±0.78)a | 25.39±0.59 | 21.64±2.00 | (67.53±0.19)b | (78.66±1.89)a | ||
Data are mean value±standard deviation (N=3). Mean values with different letters within each row are significantly different (p<0.05). LED=light-emitting diode, LOD=limit of detection
In apple peel, nine different compounds were detected and quantitatively evaluated, which showed decreasing order of abundance in the control: α-tocopherol>chlorophyll a>chlorophyll b>δ-tocopherol>lutein>β-carotene>violaxanthin>γ-tocopherol>neoxanthin. The content of α-tocopherol and chlorophyll was 89.25 and 59.54 µg/g of FM, respectively, while the content of violaxanthin, γ-tocopherol and neoxanthin was <5 µg per g of FM.
Significant increases were detected of lutein (14.8%), chlorophyll b (17.9%), chlorophyll a (21.6%) and β-carotene (31.2%) mass fractions between the control and the LED light-irradiated samples of apple peel. Although violaxanthin and neoxanthin contents were higher in the LED light-irradiated apple peel, these differences were not significant. While this light exposure induced synthesis or slowed down the degradation of the above-mentioned pigments, a decrease in the tocopherol level was observed, although it did not reach statistical significance. Wu et al. (18) irradiated pea seedlings with blue LED light (465–470 nm) and reported an increase in total chlorophyll content. Furthermore, they also irradiated pea seedlings with LED light at 625 to 630 nm, and saw an increase in β-carotene content in the stems and leaves.
Nine pigments were detected in the tomato fruit, and their decreasing order of abundance in the control was: lycopene>α-tocopherol>β-carotene>chlorophyll a>lutein> γ-tocopherol>violaxanthin>chlorophyll b>neoxanthin. The most abundant pigments were (in µg per g of FM): lycopene 475.60, followed by α-tocopherol 116.45 and β-carotene 75.64. The least represented tomato pigments were violaxanthin, chlorophyll b and neoxanthin, with mass fractions <5 µg per g of FM.
LED light irradiation resulted in an increase of only the neoxanthin content in the tomato fruit (34.9%), although this was not significant. However, significant decreases in the LED light-irradiated tomato samples were observed in β-carotene (7.7%), γ-tocopherol (26.7%) and chlorophyll b (65.6%). The lycopene, α-tocopherol, lutein and violaxanthin mass fractions also decreased, but these differences were not significant. Using different LED light wavelengths (i.e. 650–660 nm), Dhakal and Baek (20) reported an increase in lycopene in mature green tomato fruit. Li and Kubota (11) also showed an increase in xanthophyll and β-carotene in baby leaf lettuce as a result of LED light irradiation at 476 nm, along with a decrease in xanthophyll, β-carotene and chlorophyll content after LED light irradiation at 734 nm.
In red bell pepper fruit, ten pigments were detected, in decreasing order of abundance in the control: α-tocopherol>β-carotene>chlorophyll a>violaxanthin>chlorophyll b>antheraxanthin>γ-tocopherol>α-carotene>lutein>δ-tocopherol. Thus α-tocopherol was present at mass fraction (in µg per g of FM) 135.71, followed by β-carotene at 115.98 and chlorophyll at 67.53. Mass fractions of lutein, α-carotene and δ-tocopherol were low (all <3 µg per g of FM); indeed, δ-tocopherol was below the limit of detection.
Significant increases of α-tocopherol (12.4%), chlorophyll a (16.5%), β-carotene (31.2%), lutein (46.3%) and γ-tocopherol (62.8%) mass fractions were observed in LED light-irradiated red bell pepper fruit. The only pigment that was present at lower mass fraction after the LED light irradiation was chlorophyll b, with a significant 32.8% decrease, compared to the control. Gangadhar et al. (44) reported that blue LED light enhanced the synthesis of chlorophyll a and chlorophyll b in chilli pepper fruit, while the combination of red and blue LED light resulted in the highest carotenoid levels. Samuolienė et al. (13) also reported a decrease in tocopherol content in the romaine baby leaf lettuce under LED light conditions.
The colour readings for the apple, tomato and red bell pepper fruit samples are summarised in Table 3. Here, compared to the controls, LED light irradiation had no significant effects on any of the colour parameters determined for the fruit (L*, a* and b*), although there was a tendency to accelerate yellow colour development of apple fruit, and red colour development of red bell pepper fruit. Dhakal and Baek (20) reported that red light (at 650–660 nm) enhanced red colour development of tomato fruit.
Table 3. Colour readings of the fruit samples before (start) and after 7 days (final) of storage in the dark (control) or under LED light irradiation (590 nm).
| Colour parameter |
Sampling point | Apple | Tomato | Red bell pepper | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control | LED light | Control | LED light | Control | LED light | ||||
| L* | Start | 60.06±1.64 | 59.61±1.71 | 45.37±0.99 | 45.30±0.78 | 33.53±1.23 | 33.53±1.23 | ||
| Final | 60.16±1.72 | 59.95±1.98 | 44.15±1.13 | 44.45±0.98 | 32.52±1.31 | 33.41±1.11 | |||
| a* | Start | –17.68±2.42 | –19.05±0.36 | 16.14±1.08 | 15.17±1.60 | 23.83±1.20 | 23.83±1.20 | ||
| Final | –17.49±2.42 | –18.67±0.65 | 17.86±0.62 | 16.93±1.36 | 24.37±1.59 | 25.66±1.42 | |||
| b* | Start | 41.08±2.03 | 41.63±1.38 | 28.03±0.83 | 28.76±1.76 | 13.13±1.03 | 13.13±1.03 | ||
| Final | 41.51±1.75 | 43.06±1.89 | 28.07±0.85 | 28.74±1.54 | 13.86±1.46 | 14.66±0.79 | |||
Data are mean value±standard deviation (N=12). LED=light-emitting diode
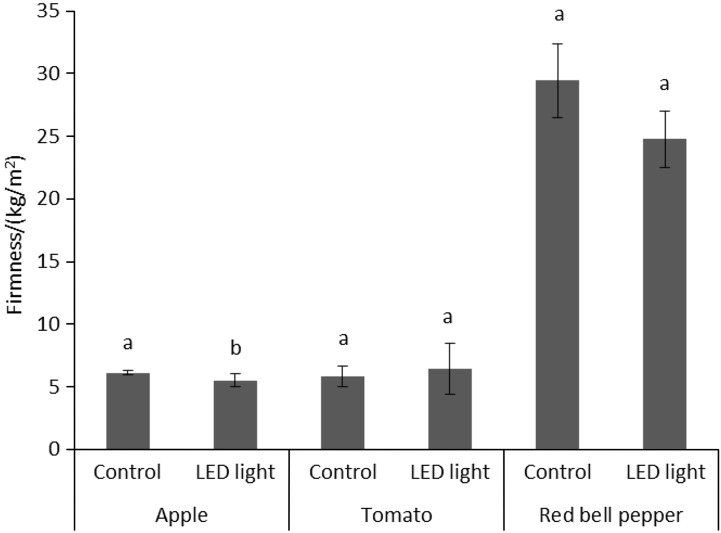
Firmness is an important factor for postharvest storage, and it is also important from a nutritional point of view (45). Fig. 1 shows the firmness of apple, tomato and red bell pepper fruit. Here, there was a significant difference between the apple fruit exposed to LED light, with a 10% lower firmness, and the control. Although lower firmness was also seen of red bell pepper fruit and a slightly increased firmness of tomato fruit, this was significant (Fig. 1). Dhakal and Baek (20) reported that tomato fruit irradiated with blue light (at 440–450 nm) had higher levels of firmness than those irradiated with red light (at 650–660 nm) or kept in darkness.
Fig 1.
Firmness measurements of the fruit samples after 7 days of storage in the dark (control) or under LED light irradiation (590 nm). Data are mean value±standard deviation (N=3). Mean values with different letters are significantly different (p<0.05)
Further studies are now needed to take into account, in particular, the wavelength and power output of the LED light irradiation.
Conclusions
In the present study, the focus was on some bioactive compounds and their change after the exposure to yellow LED light irradiation. The statistically significant effects of the yellow LED light on the irradiated apple peel and red bell pepper fruit during storage were found for antioxidant potential compared to the control samples stored in the dark. Also, there were significant differences obtained in total phenolic content in LED light-irradiated apple peel and tomato fruits. The analysis of pigments and tocopherols showed significantly higher mass fractions of lutein, chlorophyll a, chlorophyll b and β-carotene in LED light-irradiated samples of apple peel, and of β-carotene, α-tocopherol, γ-tocopherol, chlorophyll a and lutein in LED light-irradiated red bell pepper fruits. The firmness of LED light-irradiated apple fruit was also significantly lower. The effects of yellow LED light on postharvest processes are not well elaborated in literature. We have shown that yellow LED light irradiation affects mass fractions of ascorbic acid, total phenolics, total flavonoids, pigments, tocopherols and antioxidant potential, thus increasing the nutritional value of food.
Acknowledgement
This work was financed by Slovenian research agency in the frame of the programme Integrated food technology and nutrition. Authors greatly acknowledge the skilful technical assistance from Gorenje, d.o.o., Velenje, Slovenia.
References
- 1.Moo-Huchin VM, Moo-Huchin MI, Estrada-León RJ, Cuevas-Glory L, Estrada-Mota IA, Ortiz-Vázquez E, et al. Antioxidant compounds, antioxidant activity and phenolic content in peel from three tropical fruits from Yucatan, Mexico. Food Chem. 2015;166:17–22. 10.1016/j.foodchem.2014.05.127 [DOI] [PubMed] [Google Scholar]
- 2.Atmani D, Chaher N, Berboucha M, Ayouni K, Lounis H, Boudaoud H, et al. Antioxidant capacity and phenol content of selected Algerian medicinal plants. Food Chem. 2009;112:303–9. 10.1016/j.foodchem.2008.05.077 [DOI] [Google Scholar]
- 3.Sinkovič L, Demšar L, Žnidarčič D, Vidrih R, Hribar J, Treutter D. Phenolic profiles in leaves of chicory cultivars (Cichorium intybus L.) as influenced by organic and mineral fertilizers. Food Chem. 2015;166:507–13. 10.1016/j.foodchem.2014.06.024 [DOI] [PubMed] [Google Scholar]
- 4.Hollman PCH, Katan MB. Dietary flavonoids: intake, health effects and bioavailability. Food Chem Toxicol. 1999;37:937–42. 10.1016/S0278-6915(99)00079-4 [DOI] [PubMed] [Google Scholar]
- 5.Cieślik E, Gręda A, Adamus W. Contents of polyphenols in fruit and vegetables. Food Chem. 2006;94:135–42. 10.1016/j.foodchem.2004.11.015 [DOI] [Google Scholar]
- 6.Lister CE, Lancaster JE, Sutton KH, Walker JRL. Developmental changes in the concentration and composition of flavonoids in skin of a red and a green apple cultivar. J Sci Food Agric. 1994;64:155–61. 10.1002/jsfa.2740640204 [DOI] [Google Scholar]
- 7.Riahi A, Hdider C. Bioactive compounds and antioxidant activity of organically grown tomato (Solanum lycopersicum L.) cultivars as affected by fertilization. Sci Hortic (Amsterdam). 2013;151:90–6. 10.1016/j.scienta.2012.12.009 [DOI] [Google Scholar]
- 8.George B, Kaur C, Khurdiya DS, Kapoor HC. Antioxidants in tomato (Lycopersium esculentum) as a function of genotype. Food Chem. 2004;84:45–51. 10.1016/S0308-8146(03)00165-1 [DOI] [Google Scholar]
- 9.Lin JY, Tang CY. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007;101:140–7. 10.1016/j.foodchem.2006.01.014 [DOI] [Google Scholar]
- 10.Sun T, Xu Z, Wu CT, Janes M, Prinyawiwatkul W, No HK. Antioxidant activities of different colored sweet bell peppers (Capsicum annuum L.). J Food Sci. 2007;72:S98–102. 10.1111/j.1750-3841.2006.00245.x [DOI] [PubMed] [Google Scholar]
- 11.Li Q, Kubota C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ Exp Bot. 2009;67:59–64. 10.1016/j.envexpbot.2009.06.011 [DOI] [Google Scholar]
- 12.Fan XX, Xu ZG, Liu XY, Tang CM, Wang LW, Han XL. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci Hortic (Amsterdam). 2013;153:50–5. 10.1016/j.scienta.2013.01.017 [DOI] [Google Scholar]
- 13.Samuolienė G, Brazaitytė A, Sirtautas R, Viršilė A, Sakalauskaitė J, Sakalauskienė S, et al. LED illumination affects bioactive compounds in romaine baby leaf lettuce. J Sci Food Agric. 2013;93:3286–91. 10.1002/jsfa.6173 [DOI] [PubMed] [Google Scholar]
- 14.Moretti CL, Mattos LM, Calbo AG, Sargent SA. Climate changes and potential impacts on postharvest quality of fruit and vegetable crops: a review. Food Res Int. 2010;43:1824–32. 10.1016/j.foodres.2009.10.013 [DOI] [Google Scholar]
- 15.Hribar J, Vidrih R. Impacts of climate change on fruit physiology and quality. In: Proceedings of the 50th Croatian and 10th International Symposium on Agriculture; 2015 February 16–20; Opatija, Croatia. Zagreb, Croatia: Faculty of Agriculture; 2015. pp. 42–5. [Google Scholar]
- 16.Thewes FR, Both V, Brackmann A, Weber A, de Oliveira Anese R. Dynamic controlled atmosphere and ultralow oxygen storage on ‘Gala’ mutants quality maintenance. Food Chem. 2015;188:62–70. 10.1016/j.foodchem.2015.04.128 [DOI] [PubMed] [Google Scholar]
- 17.Lin KH, Huang MY, Huang WD, Hsu MH, Yang ZW, Yang CM. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci Hortic (Amsterdam). 2013;150:86–91. 10.1016/j.scienta.2012.10.002 [DOI] [Google Scholar]
- 18.Wu MC, Hou CY, Jiang CM, Wang YT, Wang CY, Chen HH, et al. A novel approach of LED light radiation improves the antioxidant activity of pea seedlings. Food Chem. 2007;101:1753–8. 10.1016/j.foodchem.2006.02.010 [DOI] [Google Scholar]
- 19.Xu Hl, Xu Q, Li F, Feng Y, Qin F, Fang W. Applications of xerophytophysiology in plant production—LED blue light as a stimulus improved the tomato crop. Sci Hortic (Amsterdam). 2012;148:190–6. 10.1016/j.scienta.2012.06.044 [DOI] [Google Scholar]
- 20.Dhakal R, Baek KH. Short period irradiation of single blue wavelength light extends the storage period of mature green tomatoes. Postharvest Biol Technol. 2014;90:73–7. 10.1016/j.postharvbio.2013.12.007 [DOI] [Google Scholar]
- 21.Samuolienė G, Sirtautas R, Brazaitytė A, Duchovskis P. LED lighting and seasonality effects antioxidant properties of baby leaf lettuce. Food Chem. 2012;134:1494–9. 10.1016/j.foodchem.2012.03.061 [DOI] [PubMed] [Google Scholar]
- 22.Lu N, Maruo T, Johkan M, Hohjo M, Tsukagoshi S, Ito Y, et al. Effects of supplemental lighting with light-emitting diodes (LEDs) on tomato yield and quality of single-truss tomato plants grown at high planting density. Envrion Control Biol. 2012;50:63–74. 10.2525/ecb.50.63 [DOI] [Google Scholar]
- 23.Samuolienė G, Brazaitytė A, Duchovskis P, Viršilė A, Jankauskienė J, Sirtautas R, et al. Cultivation of vegetable transplants using solid-state lamps for the short-wavelength supplementary lighting in greenhouses. Acta Hortic. 2012; (952):885–92. 10.17660/ActaHortic.2012.952.112 [DOI] [Google Scholar]
- 24.Ménard C, Dorais M, Hovi T, Gosselin A. Developmental and physiological responses of tomato and cucumber to additional blue light. Acta Hortic. 2005; (711):291–6. 10.17660/ActaHortic.2006.711.39 [DOI] [Google Scholar]
- 25.Brown CS, Schuerger AC, Sager JC. Growth and photomorphogenesis of pepper plants under red light-emitting diodes with supplemental blue or far-red lighting. J Am Soc Hortic Sci. 1995;120:808–13. [PubMed] [Google Scholar]
- 26.Ahn SY, Kim SA, Choi SJ, Yun HK. Comparison of accumulation of stilbene compounds and stilbene related gene expression in two grape berries irradiated with different light sources. Hortic Environ Biotechnol. 2015;56:36–43. 10.1007/s13580-015-0045-x [DOI] [Google Scholar]
- 27.Park S, Chang M, Choi J, Kim B. Effect of a Refrigerator with LED on functional composition changes and freshness prolongation of cabbage. Korean J Food Preserv. 2007;14:113–8. [Google Scholar]
- 28.Lee YJ, Ha JY, Oh JE, Cho MS. The effect of LED irradiation on the quality of cabbage stored at a low temperature. Food Sci Biotechnol. 2014;23:1087–93. 10.1007/s10068-014-0149-6 [DOI] [Google Scholar]
- 29.Kanazawa K, Hashimoto T, Yoshida S, Sungwon P, Fukuda S. Short photoirradiation induces flavonoid synthesis and increases its production in postharvest vegetables. J Agric Food Chem. 2012;60:4359–68. 10.1021/jf300107s [DOI] [PubMed] [Google Scholar]
- 30.Vänninen I, Pinto DM, Nissinen AI, Johansen NS, Shipp L. In the light of new greenhouse technologies: 1. Plant-mediated effects of artificial lighting on arthropods and tritrophic interactions. Ann Appl Biol. 2010;157:393–414. 10.1111/j.1744-7348.2010.00438.x [DOI] [Google Scholar]
- 31.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT –. Food Sci Technol (Campinas). 1995;28:25–30. 10.1016/S0023-6438(95)80008-5 [DOI] [Google Scholar]
- 32.Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–58. [Google Scholar]
- 33.Tausz M, Wonisch A, Grill D, Morales D, Jiménez MS. Measuring antioxidants in tree species in the natural environment: from sampling to data evaluation. J Exp Bot. 2003;54:1505–10. 10.1093/jxb/erg175 [DOI] [PubMed] [Google Scholar]
- 34.Wildi B, Lütz C. Antioxidant composition of selected high alpine plant species from different altitudes. Plant Cell Environ. 1996;19:138–46. 10.1111/j.1365-3040.1996.tb00235.x [DOI] [Google Scholar]
- 35.Software SAS. v. 8.01, SAS Institute Inc., Cary, NC, USA; 1999. [Google Scholar]
- 36.Kim BS, Lee HO, Kim JY, Kwon KH, Cha HS, Kim JH. An effect of light emitting diode (LED) irradiation treatment on the amplification of functional components of immature strawberry. Hortic Environ Biotechnol. 2011;52:35–9. 10.1007/s13580-011-0189-2 [DOI] [Google Scholar]
- 37.Jeffery EH, Brown AF, Kurilich AC, Keck AS, Matusheski N, Klein BP, et al. Variation in content of bioactive components in broccoli. J Food Compos Anal. 2003;16:323–30. 10.1016/S0889-1575(03)00045-0 [DOI] [Google Scholar]
- 38.Anttonen MJ, Karjalainen RO. Environmental and genetic variation of phenolic compounds in red raspberry. J Food Compos Anal. 2005;18:759–69. 10.1016/j.jfca.2004.11.003 [DOI] [Google Scholar]
- 39.Howard LR, Talcott ST, Brenes CH, Villalon B. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. J Agric Food Chem. 2000;48:1713–20. 10.1021/jf990916t [DOI] [PubMed] [Google Scholar]
- 40.Medina-Juárez LA, Molina-Quijada DMA, Del Toro-Sánchez CL, González-Aguilar GA, Gámez-Meza N. Antioxidant activity of peppers (Capsicum annuum L.) extracts and characterization of their phenolic constituents. Interciencia. 2012;37:588–93. [Google Scholar]
- 41.Zhan L, Hu J, Li Y, Pang L. Combination of light exposure and low temperature in preserving quality and extending shelf-life of fresh-cut broccoli (Brassica oleracea L.). Postharvest Biol Technol. 2012;72:76–81. 10.1016/j.postharvbio.2012.05.001 [DOI] [Google Scholar]
- 42.Kanazawa K, Hashimoto T, Yoshida S, Sungwon P, Fukuda S. Short photoirradiation induces flavonoid synthesis and increases its production in postharvest vegetables. J Agric Food Chem. 2012;60:4359–68. 10.1021/jf300107s [DOI] [PubMed] [Google Scholar]
- 43.Harbaum-Piayda B, Walter B, Bengtsson GB, Hubbermann EM, Bilger W, Schwarz K. Influence of pre-harvest UV-B irradiation and normal or controlled atmosphere storage on flavonoid and hydroxycinnamic acid contents of pak choi (Brassica campestris L. ssp. chinensis var. communis). Postharvest Biol Technol. 2010;56:202–8. 10.1016/j.postharvbio.2010.01.003 [DOI] [Google Scholar]
- 44.Gangadhar BH, Mishra RK, Pandian G, Park SW. Comparative study of color, pungency, and biochemical composition in chili pepper (Capsicum annuum) under different light-emitting diode treatments. HortSci. 2012;47:1729–35. [Google Scholar]
- 45.Fan G, Zha J, Du R, Gao L. Determination of soluble solids and firmness of apples by Vis/NIR transmittance. J Food Eng. 2009;93:416–20. 10.1016/j.jfoodeng.2009.02.006 [DOI] [Google Scholar]