Summary
The reaction system for octyl-β-glucoside synthesis catalysed by the almond-β-glucosidase has been characterised. The monophasic octanol saturated with different amounts of water served as a reaction medium. Both the glucose and the activated substrate p-nitrophenyl-β-glucoside were examined as glycon donors. The reverse hydrolysis and the transglycosylation were both used as reaction models for this enzymatically catalysed alkyl-β-glucoside synthesis. The rate of synthesis of octyl-β-glucoside (vS), the rate of hydrolysis, i.e. the glucose formation (vH) and the predicted yield (Y) were determined. The effect of water activity on the synthetic and hydrolytic activity of the enzyme was investigated. Both the rate of synthesis and the rate of hydrolysis increased with the increase of the water activity in the reaction system, showing their maximum values at the water activity close to the saturation level. Thus, the maximum ratio of vS/vH=0.165 was achieved at the water activity of 0.94. The predicted yields were 0.5, 0.75 and 14.19% and were lower than the actually achieved yields of 19.45, 38 and 36.40% at the water activities of 0.75, 0.84 and 0.94, respectively. The yield of octyl-β-glucoside in the reverse hydrolysis was only 15.2%, i.e. 3.25 times lower than the yield obtained in the transglycosylation reaction with the water activity of 0.94. The solubility of glucose in pure octanol was only 1.5 mmol/L at the saturation level of 12 mmol/L in the presence of 10 mmol/L of p-nitrophenyl-β-glucoside, and it increased to 15.5 mmol/L in the presence of octyl-β-glucoside.
Key words: enzymatic synthesis, octyl-β-glucoside, biosurfactant, almond-β-glucosidase, monophasic octanol/water mixtures
Introduction
Clean production of chemicals or the so-called green chemistry is an environmentally friendly area of chemistry and it refers to a design of chemical products and processes that reduce or eliminate the use and generation of hazardous substances (1). It is characterised with: minimal waste production, minimal energy consumption, efficient raw material use such as that of renewable resources. Prevention of pollution instead of cleaning the already damaged environment is the paradigm of the green chemistry processes. Enzymatic synthesis of biosurfactants belongs to this kind of processes and biosurfactants are certainly products that are extremely environmentally friendly due to their biocompatibility and biodegradability. Therefore, these specialty biocompounds have a very broad potential application, especially in pharmacy and food industry as well as in medicine (2–4). Alkyl glycosides are environmentally friendly, non-ionic surfactants with favourable properties like biodegradability and chemical stability (5). These compounds are extensively used in personal care products, pharmaceutical preparations and membrane protein research. The chemical route for their synthesis involves extremes of temperature, pressure, use of toxic catalysts, and multiple steps of protection, deprotection and activation. β-Glycosidases provide an alternative method of enzymatic synthesis as these are easily available from microbial systems and exhibit broad substrate specificity and high stereoselectivity. Application of enzymes in unconventional media involves biotechnological processes characterised with very mild reaction conditions, minimal side product generation and production of high quality biocompounds. What is even more interesting, these processes offer a possibility for production of specialty products by using the unique property of the enzymes, their selectivity (6). Glycosidases, such as alkyl glycosidases, are enzymes that are commonly used for biotechnological production of biodegradable and biocompatible surfactants (7–9). Some of these long-chained biosurfactants are very difficult to synthesise via the usual chemical synthesis because of the incompatibility of the two substrates, the hydrophilic glucose and the hydrophobic alcohol molecule (10, 11). The differences in solubility of the two substrates are the reason why for a very long time they were synthesised exclusively in two-phase systems with both free and immobilised β-glycosidases, and via both reverse hydrolysis and transglycosylation reactions. In the water-saturated reaction systems for alkyl-glycoside production, hydrolysis of the already formed alkyl-β-glucoside, the so-called secondary hydrolysis, was responsible for low product yields (12–18). While the reverse hydrolysis, or the so- -called condensation reaction (19, 20) is thermodynamically controlled and is a direct reversal of hydrolysis, the glycosyl transfer reaction, called also transglycosylation, is kinetically controlled. Thus, it is the kinetics of the desired reaction and of other possible reactions that determine the yield. In the transglycosylation reaction the properties of the enzyme are of prime importance (21, 22). Other parameters that affect the glycosyl transfer reactions are: reaction temperature, reaction pH, nature and concentration of the acceptor and the donor, solvent involved, water activity and whether the enzyme is free or immobilized (23). The Amberlite® IRC-50, XAD-4, XAD- -16 and other hydrophobic crosslinked polystyrene copolymer resins are some of the most frequently used matrices for immobilization of glycosidases in synthetic reactions in unconventional media (24, 25). Especially the application of the inorganic Celite 545 as an immobilization matrix of different glycosidases with a simple adsorption method resulted in enzyme preparations with very good performances valuable for their use in synthetic reactions. It has been shown that even the additives used in the commercial enzyme preparations as stabilizers have a positive effect on the enzyme dispersibility in hydrophobic media (26). Simple and mixed AOT/PEG reverse micelles are also proved as efficient catalysts in transglycosylation reaction with β-galactosidases as catalysts (27). There is no doubt that the choice of the immobilization matrix and technique influences both the enzyme activity and stability when the enzyme is to be applied in unconventional media. The number of reports about biosurfactant synthesis in one-phase systems has increased lately and they refer mostly to the monophase hexanol and few to heptanol reaction systems (28–30). However, due to the already mentioned reasons of low solubility of sugar substrates in very hydrophobic alcohols, there are not many reports about the alkyl-β-glycoside synthesis in pure monophasic octanol reaction system and those few refer to the reaction of reverse hydrolysis and not to the transglycosylation reaction (14, 24). In the present work, the monophasic octanol system was used as a reaction medium for octyl-β-glucoside synthesis by the almond β-glucosidase via two reactions: the reverse hydrolysis (direct condensation) and the transglycosylation reaction. The total characterisation of the reaction system was performed and the rates of p-nitrophenyl-β-glucoside depletion, glucose formation and octylglucoside synthesis were all determined. The effect of water activity on both the rate of synthesis (the octyl-β-glucoside formation) and the rate of hydrolysis (the glucose formation) as well as on the total reaction (synthesis and hydrolysis) rate was investigated and double checked by using two analytical techniques: the HPLC and the spectrophotometric analysis. The predicted yield of the product was calculated and compared to the experimentally obtained yield. The transglycosylation reaction system was compared to the reaction system where the reverse hydrolysis was used as a model reaction and glucose was used as a sugar substrate instead of the p-nitrophenyl-β-glucoside. The glucose solubility and the performances of the two reaction systems were investigated and compared in detail.
Materials and Methods
Materials
The β-glucosidase used in this work was of plant origin, the almond β-glycosidase, and it was used in its lyophilised form obtained from Sigma-Aldrich (St. Louis, MO, USA). The solvent acetonitrile used in the HPLC analysis was obtained from Merck (Darmstadt, Germany). The sugar substrates p-nitrophenyl-β-d-glucoside and d-glucose, as well as the standard of octyl-β-d-glucoside and the molecular sieves (UOP type 3 Ĺ) for drying solvents (diameter 0.3 nm) were also purchased from Sigma-Aldrich (St. Louis, USA).
Enzymatic reaction
The substrates d-glucose and p-nitrophenyl-β-d-glucopyranoside were dissolved in octanol (dried with 3 Ĺ molecule sieves) to a concentration of 10 mmol/L. Aqueous buffer of 40–80 µL that corresponded to the selected value of water activity (50 mmol/L of citrate buffer, pH= 5.0) contained 4.4 mg of enzyme. The final reaction volume was 2 mL. The reactions were carried out in closed glass vials, in an oven at 50 °C, with vigorous shaking at 800 rpm. At different reaction times, samples were withdrawn from the reaction mixture, put in the freezer for 30 min, diluted with mobile phase, and analyzed by HPLC and spectrophotometer.
HPLC analysis
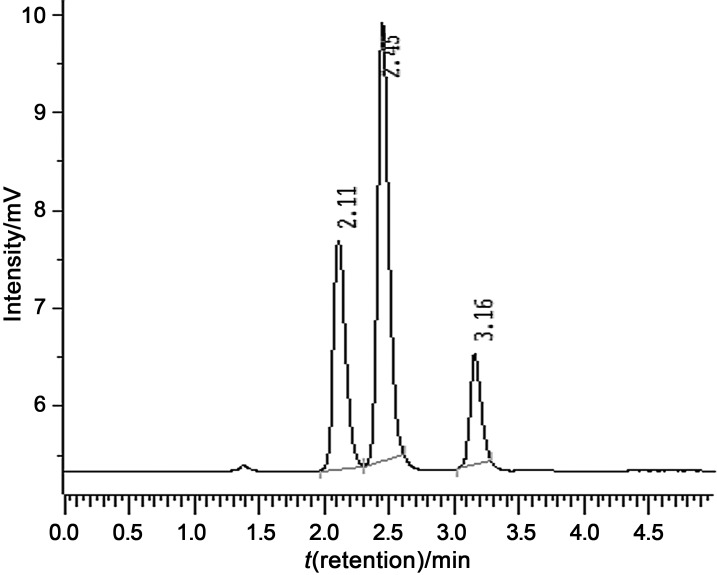
The HPLC system used was a LaChrom system with an LC-7100 pump, an L-7250 autosampler, and a D-7000 Interface system (all from Merck Hitachi, Darmstadt, Germany). The detector was a 500-ELSD evaporative light scattering detector (Alltech Associates, Inc., Deerfield, MI, USA) under the following operating conditions: nebulizer gas flow 3.1 L/min and drift tube temperature 100 °C. The other analytical conditions were: LiChrospher® 100 RP-18 column (4 mm×250 mm, 5 mm; Merck, Solna, Sweden) at room temperature, and flow rate of 1 mL/min. The composition of the mobile phase was acetonitrile/water in a ratio of 60:40. The retention times were: glucose 2.11 min, p-nitrophenyl-β-d-glucoside 2.45 min and octyl-β-d-glucoside 3.16 min (Fig. 1).
Fig. 1.
The chromatographic resolution of the reaction mixture: glucose (2.11 min), p-nitrophenyl-β-d-glucoside (2.45 min) and octyl-β-d-glucoside (3.16 min)
Spectrophotometric analysis
A spectrophotometric method for measuring the total enzyme activity (hydrolytic and transglycosylation) was developed. The concentration of depleted p-nitrophenyl- -β-d-galactoside was calculated from the concentration of liberated p-nitrophenol. The reaction was followed by measuring the increase of the absorbance at 405 nm, using Varian Cary® 50 UV-Vis spectrophotometer (Agilent Technologies Santa Clara, CA, USA). The molar absorption coefficient of p-nitrophenol at pH=5 and 50 °C was ε405 nm= 0.204 L/(mmol·cm).
Calculations of water and octanol activities
The water and octanol activity coefficients in the reaction mixtures were calculated using the UNIFAC Activity Coefficient Calculator v. 3.0 (29).
Calculation of the predicted octyl-β-glucoside yield
The experimentally obtained maximal yield was compared with the predicted yield (Y) calculated from the initial reaction rates by the following equation:
where vS is the rate of synthesis of octyl-β-glucoside and vH is the rate of hydrolysis and it refers to the rate of glucose formation.
Results and Discussion
Transglycosylation reaction
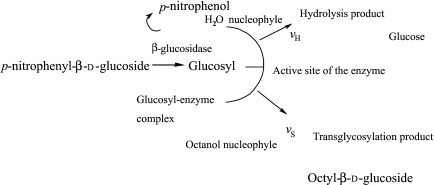
The almond β-glucosidase was used as a catalyst for octyl-β-glucoside synthesis via a transglycosylation reaction. The enzyme belongs to the glycosyl hydrolase family 1 and has a retaining reaction mechanism. The transglycosylation reaction is believed to proceed via a formation of glycosyl enzyme intermediate (Fig. 2) that might react either with the water nucleophile and liberate a glucose molecule (hydrolysis product) or with the alcohol nucleophile and form an octyl-β-d-glucoside molecule (transglycosylation product).
Fig. 2.
Schematic presentation of the conversion of p-nitrophenyl--β-d-glucoside to octanol by almond β-glucosidase. The hydrolysis (formation of glucose) and the secondary hydrolysis (the hydrolysis of the already formed octyl-β-d-glucoside) were side reactions
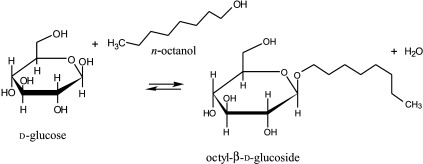
The ratio of vS/vH is actually a measure of the enzyme selectivity towards a certain nucleophile donor. In the transglycosylation reactions the reaction conditions and the properties of the enzyme are the crucial parameters that influence the product yield. In these kinetically controlled reactions the initial reaction rate is fast, thus the yield is higher than the thermodynamically predicted one. The product concentration reaches maximum when the rate of synthesis vS and the rate of hydrolysis vH become equal. This is the optimal reaction time for achieving maximum product yield. Unlike the kinetically controlled reactions (Fig. 2), in the thermodynamically controlled reactions (Fig. 3) the rates are slower, the yields are strongly dependent on the water activity in the system and the properties of the enzyme do not influence the yield, only the reaction time needed for reaching the equilibrium. The yield is determined by the equilibrium constant for the reaction and by the concentrations of the reactants.
Fig. 3.
Schematic presentation of the conversion of β-d-glucoside to octanol by almond β-glucosidase in a simple reverse hydrolysis reaction
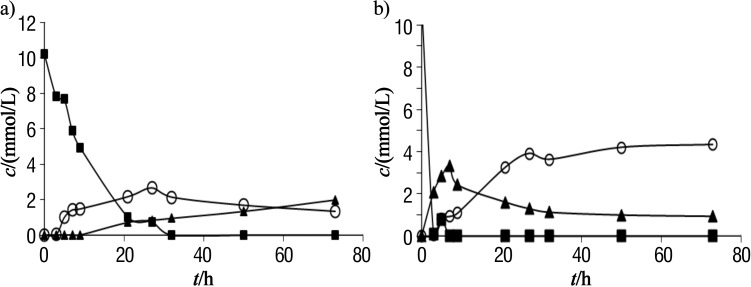
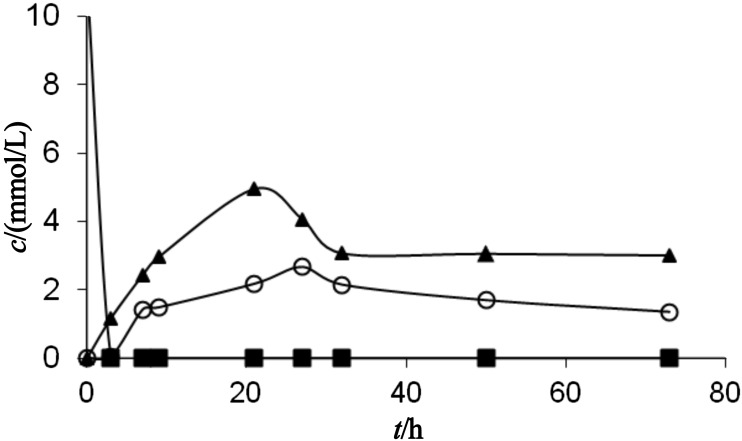
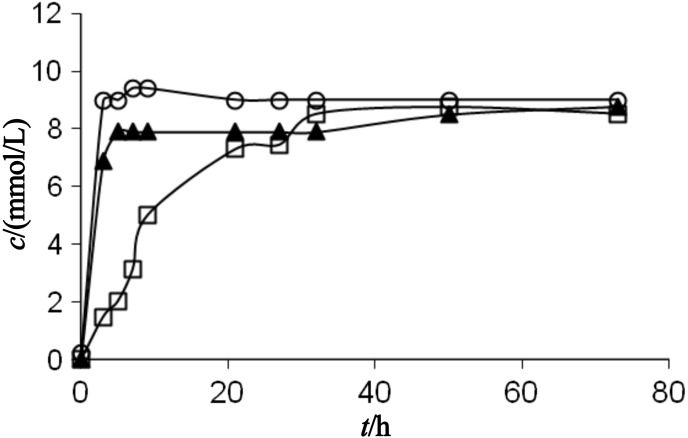
In the present work the influence of water activity on the octyl-β-glucoside synthesis via the transglycosylation reaction was investigated. It was interesting to notice that the enzyme was not significantly active at the water activities below 0.7. The time course of the transglycosylation reaction at the water activities of 0.75, 0.84 and 0.94 is shown in Figs. 4 and 5. Glucose is present in the reaction system in two forms: dissolved and undissolved. The concentration of undissolved glucose is calculated as a difference between the concentration of p-nitrophenyl-β- -d-glucoside (substrate) and the sum of concentrations of octyl-β-d-glucoside and the dissolved glucose (products).
Fig. 4.
Time course of the octyl-β-d-glucoside synthesis via a transglycosylation reaction catalysed by almond β-glucosidase at the water activity of: a) 0.75 and b) 0.84. The substrate p-nitrophenyl-β-d-glucoside (square) was converted to octyl-β-glucoside (triangle) and dissolved glucose (circle)
Fig. 5.
Time course of the octyl-β-d-glucoside synthesis via a transglycosylation reaction catalysed by almond β-glucosidase at the water activity of 0.94. The substrate p-nitrophenyl-β-d- -glucoside (square) was converted to octyl-β-d-glucoside (triangle) and dissolved glucose (circle)
It can be noticed from the presented results that the hydrolytic and synthetic activities, as well as the product yield, increased when the water activity increased. The initial rate of octyl-β-d-glucoside synthesis (vS) in the system with water activity of 0.75 was very low and its value was only 0.5 µmol/min. The rate of hydrolysis (vH) at 9.5 µmol/min, on the other hand, was 19 times higher than the rate of synthesis. This means that at relatively low water activities, water is a much better nucleophile, since the water molecule is smaller than the octanol molecule. The selectivity of the enzyme towards the smaller nucleophile is pronounced at low water activity, when the enzyme is in its very rigid conformation surrounded with the almost non aqueous octanol environment. The ratio of vS/vH also increased when the water activity increased. Thus, at the water activity of 0.84, this ratio was 0.076, while at the water activity of 0.94 it was 0.165. However, the ratio in all examined cases was below 1, which depends on the enzyme selectivity towards the certain nucleophile donor.
The liberation of p-nitrophenol at different water activities in octanol is shown in Fig. 5. The concentration of p-nitrophenol was determined spectrophotometrically.
The total enzyme activity is a sum of the synthetic and hydrolytic activities of the enzyme. Since the rate of liberation of p-nitrophenol in octanol is a measure of the total enzyme activity, it is obvious that the total enzyme activity was the highest at the highest water activity examined (aw=0.94). This is another confirmation of the assumption that the total enzyme activity increased when the water activity increased, as has been already shown for the rate of synthesis and hydrolysis, separately (Figs. 4 and 5).
That the transglycosylation activity of glycosidases increases with the water activity increase has already been reported (29, 30). Hansson et al. (29), while investigating several glycosidases from wild and genetically modified microorganisms in hexyl glycoside synthesis, found that all examined enzymes showed increased activity at very high water activity, having their maximum at the water saturation level. Mladenoska et al. (30), while investigating several microbial galactosidases in transgalactosylation synthesis of hexyl-β-galactoside, noticed the same pattern of increased enzyme activity at higher water activities. These researchers also noticed the increased values of the vS/vH ratio at increased water activities. Most of the researchers explain this phenomenon with the increased enzyme flexibility at higher water activities (24, 29, 30). Thus, it seems that the more flexible enzyme at higher water activities readily accepts both the water and the alcohol nucleophile.
The values for the predicted yield, calculated according to Eq. 1, and the experimentally obtained yield are shown in Table 1.
Table 1. Experimental yield of octyl-β-d-glucoside compared to the predicted yield calculated according to Eq. 1 at different water activities. The conversion of p-nitrophenyl-β-d-glucoside in a transglycosylation reaction was catalysed by the almond β-glucosidase.
| aw | Y(octyl-β-d-glucoside)/% | |
|---|---|---|
| predicted | experimental | |
| 0.75 | 0.50 | 19.45 |
| 0.84 | 0.75 | 38.00 |
| 0.94 | 14.19 | 36.39 |
It can be noticed that in all examined cases the experimentally obtained yields are higher than the predicted. It can be assumed that this is due to higher glucose solubility in the presence of the product octyl-β-d-glucoside, which is why the yields higher than equilibrium can be achieved in this type of reactions catalysed by transferase. Thus, it seems that the glucose liberated in the hydrolytic reaction condenses with the octanol substrate and creates the product octylglucoside via a second reverse hydrolysis, which has already been reported (29). When Hansson et al. (29) examined the ability of various glycosidases to synthesise hexyl-β-glucoside, they found that the experimental yields of the obtained product were in several cases higher than predicted. They explained this phenomenon with the fact that the concentration of galactose in hexanol became higher than its equilibrium concentration and was further condensed with the hexanol forming hexyl-β-d-galactoside.
Comparison of transglycosylation and reverse hydrolysis
In order to compare transglycosylation reaction system (Fig. 6) with the one of reverse hydrolysis, instead of the activated p-nitrophenyl-β-d-glucoside, glucose was used as a substrate for octyl-β-d-glucoside synthesis by almond β-glucosidase.
Fig. 6.
Time course of the release of p-nitrophenol from p-nitrophenyl-β-d-glucoside in octanol as medium at the water activity of: (square) 0.75, (triangle) 0.84 and (circle) 0.94
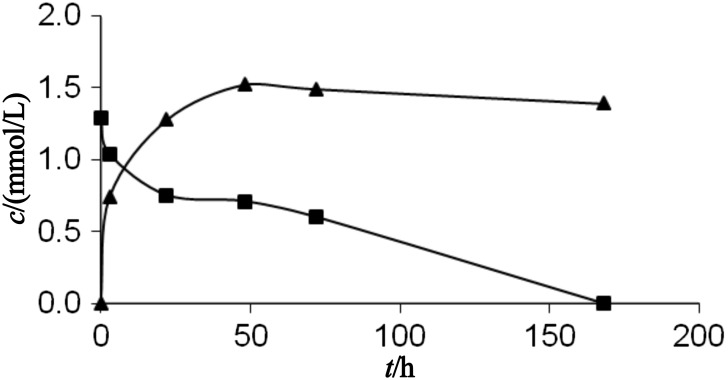
The time course of reverse hydrolysis at water activity of 0.94 is presented in Fig. 7.
Fig. 7.
Time course of octyl-β-d-glucoside synthesis (triangle) via reverse hydrolysis catalysed by almond β-glucosidase in monophasic octanol system. Glucose (square) was used as a sugar substrate
The yield of the desired product was only 15.2%, which is 3.25 times lower than the yield obtained in the transglycosylation reaction at the water activity of 0.94. It can be suggested that the lower product yield (only 1.28 mmol/L) is the result of lower glucose solubility in octanol, which is obvious from Fig. 5. It is clear that the glucose solubility influenced strongly the product yield and was one of the limiting factors in the enzymatic synthesis of octyl-β-d-glucoside.
In order to study in detail the glucose solubility in the reaction system and the effect of the components of the reaction mixture on it, model reaction systems were created that contained the substrate and the product of the reaction, namely the reaction system of: (i) octanol with only glucose, (ii) octanol with glucose and p-nitrophenyl- -β-d-glucoside, and (iii) octanol with glucose and octyl-β--d-glucoside. It was found that while the solubility of glucose in pure octanol was only 1.5 mmol/L at the level of saturation, it increased to 12 mmol/L in the presence of 10 mmol/L of p-nitrophenyl-β-d-glucoside and up to 15.5 mmol/L in the presence of octyl-β-d-glucoside. Thus, it seems that both the substrate p-nitrophenyl-β-d-glucoside and the product octyl-β-d-glucoside increased the solubility of hydrophilic glucose in the hydrophobic octanol because they acted as strong surface-active agents. This is probably the reason why the solubility of glucose in the transglycosylation reaction system was higher than the equilibrium and why the yields higher than predicted can be obtained only in the transglycosylation reaction system.
Rate limitation due to low glucose solubility is one of the most reported limitation factors of the alkyl glycoside synthesis, together with product hydrolysis, denaturation of enzymes with the solvent and the mass transfer limitations (16–18).
Conclusion
A successful method for enzymatic synthesis of octyl--β-d-glucoside, a very powerful biocompatible and biodegradable surfactant, was developed using a monophasic octanol reaction system and transglycosylation reaction. Yields higher than predicted were achieved by using this reaction system. The transglycosylation reaction was proved to be much better than the reverse hydrolysis. In the initial reaction phase, yields achieved by transglycosylation were much higher than those achieved by reverse hydrolysis. In the reverse hydrolysis glucose solubility was the main rate-limiting factor. The transglycosylation reaction in monophasic octanol system at high water activities proved to be a suitable system for octyl-β-d-glucoside synthesis by almond β-glucosidase. The monophasic reaction system was proved as a successful one for the synthesis of long-chained alkyl-β-glucoside, because the hydrolysis of the already formed product has very low rates due to the lack of a water phase. The immobilization of the enzyme might be considered for a future research, since the immobilized enzyme preparation would be more easily recoverable and reusable.
Acknowledgements
The author would like to thank Professor Patrick Adlercreutz from the Department of Biotechnology, Centre for Chemistry and Chemical Engineering, Lund University, Lund, Sweden, for all the advice and support given during the research presented in this work.
References
- 1.Hatti-Kaul R, Törnvall U, Gustafsson L, Börjesson P. Industrial biotechnology for the production of bio-based chemicals – a cradle-to-grave perspective. Trends Biotechnol. 2007;25:119–24. 10.1016/j.tibtech.2007.01.001 [DOI] [PubMed] [Google Scholar]
- 2.de Roode BM, Franssen MCR, van der Padt A, Boom RM. Perspectives for the industrial enzymatic production of glycosides. Biotechnol Prog. 2003;19:1391–402. 10.1021/bp030038q [DOI] [PubMed] [Google Scholar]
- 3.Rather MY, Mishra S, Chand S. β-Glucosidase catalyzed synthesis of octyl-β-d-glucopyranoside using whole cells of Pichia etchellsii in micro aqueous media. J Biotechnol. 2010;150:490–6. 10.1016/j.jbiotec.2010.09.933 [DOI] [PubMed] [Google Scholar]
- 4.Rather MY, Mishra S. β-Glycosidases: an alternative enzyme based method for synthesis of alkyl-glycosides. Sustain Chem Process. 2013;1:7 10.1186/2043-7129-1-7 [DOI] [Google Scholar]
- 5.Hansson T, Adlercreutz P. Enhanced transglycosylation/hydrolysis ratio of mutants of Pyrococcus furiosus β-glucosidase: effects of donor concentration, water content, and temperature on activity and selectivity in hexanol. Biotechnol Bioeng. 2001;75:656–65. 10.1002/bit.10043 [DOI] [PubMed] [Google Scholar]
- 6.Adlercreutz P. Biocatalysts in non-conventional media. In: Straathof AJJ, Adlercreutz P, editors. Applied Biocatalysis. Boca Raton, FL, USA: CRC Press; 2000. pp. 295–315. [Google Scholar]
- 7.van Rantwijk F, Woudenberg-van Oosterom M, Sheldon RA. Glycosidase-catalysed synthesis of alkyl glycosides. J Mol Catal, B Enzym. 1999;6:511–32. 10.1016/S1381-1177(99)00042-9 [DOI] [Google Scholar]
- 8.Hansson T. β-Glycosidases from hyperthermophiles as biocatalysts. [PhD Thesis]. Lund, Sweden: Lund University; 2002. [Google Scholar]
- 9.Mladenoska I, Elovson Grey C, Winkelhausen E, Kuzmanova S, Adlercreutz P. Competition between transglycosylation and hydrolysis in almond β-glucosidase-catalyzed conversion of p-nitrophenyl-β-d-glucoside in monophasic water/alcohol mixtures. Biocatalysis Biotransform. 2007;25:382–5. 10.1080/10242420701510494 [DOI] [Google Scholar]
- 10.Garcia-Garibay M, Lopez-Munguia A, Barzana E. Alcoholysis and reverse hydrolysis reactions in organic one-phase system with hyperthermophilic β-glycosidase. Biotechnol Bioeng. 2000;69:627–32. [DOI] [PubMed] [Google Scholar]
- 11.Svensson D, Ulvenlund S, Adlercreutz P. Efficient synthesis of a long carbohydrate chain alkyl glycoside catalysed by cyclodextrin glycosyltransferase (CGTase). Biotechnol Bioeng. 2009;104:854–61. 10.1002/bit.22472 [DOI] [PubMed] [Google Scholar]
- 12.Kosáry J, Stefanovits-Banay E, Boross L. Reverse hydrolytic process for O-alkylation of glucose catalysed by immobilized α- and β- glucosidases. J Biotechnol. 1998;66:83–6. 10.1016/S0168-1656(98)00160-6 [DOI] [Google Scholar]
- 13.Woudenberg-van Oosterum M, van Belle HJA, van Rantwijk F, Sheldon RA. Immobilised β-galactosidases and their use in galactoside synthesis. J Mol Cat. Chem. 1998;134:267–74. 10.1016/S1381-1169(98)00045-4 [DOI] [Google Scholar]
- 14.Ducret A, Trani M, Lortie R. Screening of various glycosidases for the synthesis of octyl glucoside. Biotechnol Bioeng. 2002;77:752–7. 10.1002/bit.10156 [DOI] [PubMed] [Google Scholar]
- 15.Svasti J, Phongsak T, Sarnthima R. Transglucosylation of tertiary alcohols using cassava β-glucosidase. Biochem Biophys Res Commun. 2003;305:470–5. 10.1016/S0006-291X(03)00793-9 [DOI] [PubMed] [Google Scholar]
- 16.Panintrarux C, Adachi S, Araki Y, Kimura Y, Matsuno R. Equilibrium yield of n-alkyl-β-d-glucoside through condensation of glucose and n-alcohol by β-glucosidase in a biphasic system. Enzyme Microb Technol. 1995;17:32–40. 10.1016/0141-0229(94)00082-3 [DOI] [Google Scholar]
- 17.Ismail A, Linder M, Ghoul M. Optimization of butylgalactoside synthesis by β-galactosidase from Aspergillus oryzae. Enzyme Microb Technol. 1999;25:208–13. 10.1016/S0141-0229(99)00028-9 [DOI] [Google Scholar]
- 18.Papanikolaou S. Enzyme-catalyzed synthesis of alkyl-β- -glucosides in a water-alcohol two-phase system. Bioresour Technol. 2001;77:157–61. 10.1016/S0960-8524(00)00153-X [DOI] [PubMed] [Google Scholar]
- 19.Hansson T, Adlercreutz P. Optimization of galactooligosaccharide production from lactose using β-glycosidases from hyperthermophiles. Food Biotechnol. 2001;15:79–97. 10.1081/FBT-100106830 [DOI] [Google Scholar]
- 20.Kashe V. Mechanism and yields in enzyme catalysed equilibrium and kinetically controlled synthesis of β-lactam antibiotics, peptides and other condensation products. Enzyme Microb Technol. 1986;8:4–16. 10.1016/0141-0229(86)90003-7 [DOI] [Google Scholar]
- 21.Turner P, Svensson D, Adlercreutz P, Nordberg-Karlsson E. A novel variant of Thermotoga neapolitana β-glucosidase B is an efficient catalyst for the synthesis of alkyl glucosides by transglycosylation. J Biotechnol. 2007;130:67–74. 10.1016/j.jbiotec.2007.02.016 [DOI] [PubMed] [Google Scholar]
- 22.Hansson T. Kaper, van der Oost J, de Vos, WM, Adlercreutz P. Improved oligosaccharide synthesis by protein engineering of β-glucosidase CelB from hyperthermophilic Pyrococcus furiosus. Biotechnol Bioeng. 2001;73:203–10. 10.1002/bit.1052 [DOI] [PubMed] [Google Scholar]
- 23.Nikolovska-Nedelkoska D, Mladenoska I, Poposka F, Winkelhausen E, Kuzmanova S. Modification of β-galactosidase for use in organic mono-phase hexanol system. Maced J Chem Chem Eng. 2009;28:91–7. [Google Scholar]
- 24.Ljunger G, Adlercreutz P, Mattiasson B. Enzymic synthesis of octyl-β-glucoside in octanol at controlled water activity. Enzyme Microb Technol. 1994;16:751–5. 10.1016/0141-0229(94)90031-0 [DOI] [Google Scholar]
- 25.Hansson T, Adlercreutz P. Enzymatic synthesis of hexyl glycosides from lactose at low water activity and high temperature using hyperthermostabile β-glycosidases. Biocatal Biotrans. 2002;20:167–78. 10.1080/10242420290020697 [DOI] [Google Scholar]
- 26.Persson M, Mladenoska I, Wehtje E, Adlercreutz P. Preparations of lipases for use in organic solvents. Enzyme Microb Technol. 2002;31:833–41. 10.1016/S0141-0229(02)00184-9 [DOI] [Google Scholar]
- 27.Mladenoska I. Simple and mixed reverse micelles as potential bioreactors for enzymatic synthesis of alkyl glycosides – environmentally friendly surfactants. Food Technol Biotechnol. 2012;50:420–6. [Google Scholar]
- 28.Mladenoska I, Nikolovska-Nedelkoska D, Winkelhausen E, Kuzmanova S. Aspergillus oryzae-β-galactosidase – an efficient catalyst for alkyl-β-galactoside synthesis in organic mono-phase system. Maced J Chem Chem Eng. 2007;26:17–24. [Google Scholar]
- 29.Hansson T, Andersson M, Wehtje E, Adlercreutz P. Influence of water activity on the competition between β-glycosidase-catalysed transglycosylation and hydrolysis in aqueous hexanol. Enzyme Microb Technol. 2001;29:527–34. 10.1016/S0141-0229(01)00421-5 [DOI] [Google Scholar]
- 30.Mladenoska I, Winkelhausen E, Kuzmanova S. Transgalactosylation/hydrolysis ratios of various β-galactosidases catalyzing alkyl-β-galactoside synthesis in single-phased alcohol media. Food Technol Biotechnol. 2008;46:311–6. [Google Scholar]