Summary
The antioxidant activity of three types of pumpkin seed oil or oil mixtures (cold- -pressed, produced from roasted seed paste and salad) produced in the northern part of Croatia and the kinetics of their behaviour as free radical scavengers were investigated using DPPH˙. In addition, the involvement of oil tocopherol isomers (α-, γ- and δ-) in different steps of DPPH˙ disappearance and their impact on the rate of reaction were analysed. The kinetics of DPPH˙ disappearance is a two-step process. In the first step, rapid disappearance of DPPH˙ occurs during the first 11 min of the reaction, depending on the oil type, followed by a slower decline in the second step. To describe DPPH˙ disappearance kinetics, six mathematical models (mono- and biphasic) were tested. Our findings showed that γ- and δ-tocopherols affected DPPH˙ disappearance during the first step, and α-tocopherol in the second step of the reaction. Moreover, α-tocopherol demonstrated 30 times higher antioxidant activity than γ- and δ-tocopherols. The results indicated the biphasic double-exponential behaviour of DPPH˙ disappearance in oil samples, due to the complexity of reactions that involve different tocopherol isomers and proceed through different chemical pathways.
Key words: DPPH˙ disappearance kinetics, mathematical modelling, pumpkin seed oil, tocopherols
Introduction
The northern part of Croatia is known for a long tradition of pumpkin (Cucurbita pepo L.) cultivation primarily for the production of high quality pumpkin seed oil. Hulless or naked seeds and husk, raw or roasted, can be eaten by themselves or used in the production of oil. There are two main methods of pumpkin seed oil production, resulting in two different products. In the first method maximum temperature during the procedure is 50 °C and the oil is pressed from dry, unroasted seeds, resulting in an olive oil-like greenish colour and a characteristic pumpkin taste (1). This oil, called cold-pressed, is mainly used therapeutically. In the second method, the oil is pressed from the seed paste previously roasted at 100–120 °C. Resulting pumpkin seed oil has a dark green to brown colour and a nutty and roasted flavour (2). In the last few years, this oil has been called virgin pumpkin seed oil and is usually consumed without further treatment mainly as a salad oil (in Austria, Croatia, Slovenia and Hungary) or as a cooking oil (in the Middle East and some African countries) (3). The main components of pumpkin seed oil are triacylglycerols (98% of pumpkin seed oil), while the rest is made up of minor components such as tocopherols, phytosterols, phospholipids, squalene, carotenoids, pigments, phenolic compounds and minor glyceridic compounds (4–8). Containing predominantly linoleic and oleic acids, pumpkin seed oil is considered as highly unsaturated oil. Linolenic and other highly unsaturated fatty acids are present at low levels, providing this oil with high oxidative stability and low free radical production. Among the biologically active compounds in pumpkin seed oil, tocopherols and tocotrienols, collectively known as tocols, have one of the most important roles in human diet and health protection (9). They are lipophylic compounds, having a chromane head with two rings: a phenolic and a heterocyclic, and a phytyl tail saturated in tocopherols and possessing three double bonds in tocotrienols. Isomers also differ in the number and position of methyl groups on the chromane head and are classified as α- (5,7,8-trimethyl), β- (5,8-dimethyl), γ- (7,8-dimethyl) and δ- (8-methyl) tocols. In general, in vegetable oil an abundance of α-, γ- and δ-tocopherols can be found as well as β-isomer and tocotrienols in minor amounts (9). In both types of pumpkin seed oil, cold-pressed and virgin oil, γ-tocopherol was found to be the dominant isomer, while other isomers and γ-tocotrienol occur in varying proportions (7, 10–13). Additionally, in the last few years two new compounds, γ-tocomonoenol and α-tocomonoenol, have also been determined (12) in virgin pumpkin seed oil. Protective effects of tocols achieved through the antioxidant activity are manifested in the suppression of lipid oxidation in foods. Their high antioxidant activity is the result of the ability of chromane head to easily release and donate phenolic hydrogen atoms to the free radicals in lipids. In the literature, a great deal of information about the antioxidant activity of tocopherols in in vivo and in vitro studies can be found. In vivo studies give almost identical results indicating that the antioxidant activity of tocopherols decreases in the following order α>β>γ>δ (14). However, the results obtained by in vitro studies are contradictory. Thus, some scientists found that the α-isomer is more potently antioxidant than β-, γ- and δ-isomers, while others contradict this (15–17). The reason for this different behaviour in in vitro studies is not only the chemical reactivity of tocopherols but also other factors, such as their concentration, temperature, light, type of solvent, and the presence of chemical substances that act antagonistically or synergistically on the system (15, 17).
Although many methods have been developed to study the antioxidant activity of different compounds and foods in vitro, the method using the 2,2-diphenyl-1- -picrylhydrazyl radical (DPPH˙) is widely used today to test the ability of compounds to act as free radical scavengers or hydrogen donors, as well as to evaluate antioxidant activity of either single compounds or complex mixtures that food contains (18, 19). In order to describe and predict the behaviour of radicals in the food as a matrix, mathematical modelling is used to analyse DPPH˙ disappearance kinetics. The monophasic models, such as zero- -order (20), single first-order (20, 21) and pseudo-first order (22, 23) have been dominant for decades due to their simplicity. However, in practice, the disappearance of DPPH˙ takes place in two distinct but concurrent steps: the first is rapid and the second slow. Espin et al. (24) assumed that the reason for this behaviour was related to the presence of two groups of antioxidants, which possess the ability to scavenge radicals at different rates. Therefore, alternative biphasic kinetic models, such as the Weibull model (20, 25), the first-order double exponential model (24) and the Gustafson and Holden model (26), should be used.
Pumpkin seed oil production in Croatia has a long tradition and the oil has been well characterized in some studies (2, 13), but the data about its kinetic behaviour as an antioxidant in scavenging radicals are scarce. Therefore, in the present study, in order to ascertain antioxidant behaviour of pumpkin seed oil or mixtures, six mathematical models (three monophasic and three biphasic) were tested. The used oil samples differ in production methods and in geographic origin. The possible involvement of tocopherol isomers (α-, γ- and δ-) and specific fatty acids (oleic and linoleic) in DPPH˙ disappearance as well as their impact on the rate of reaction were evaluated. Concerning the differences in the standard composition parameters of these oil samples, different kinetics of DPPH˙ disappearance was expected and tested.
Materials and Methods
Standards and chemicals
Standards of fatty acid methyl esters and DPPH˙ were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA), while single tocopherol standards (α, γ and δ) were from Supelco Inc. (Bellefonte, PA, USA). All other chemicals and solvents used were of analytical grade and were obtained from Carlo Erba Reagents (Milan, Italy), except acetonitrile, methanol and chloroform (J.T.Baker, Deventer, The Netherlands), which were of chromatographic grade.
Samples
Nine samples of pumpkin seed oil or oil mixtures of three types were used. The first type comprised two samples of cold-pressed pumpkin seed oil (CP1 and CP2) produced from unroasted seeds, the second contained five oil samples made from roasted seed paste (RS1, RS2, RS3, RS4 and RS5) and the third two samples of salad oil (S1 and S2). Salad oil was a mixture of 20% of pumpkin seed oil (produced from roasted seed paste) and 80% of some other (sunflower or soybean) oil. All types of oil were supplied from the local producers from the continental region of Croatia. Oil samples RS1, RS5, CP1 and S1 were from Međimurje, oil RS2 was from Zagorje, RS3 and CP2 from Podravina, and RS4 and S2 from Slavonija. In all of these geographical regions, pumpkin seed oil is produced mainly from the seeds of the plant Cucurbita pepo var. styriaca.
Determination of pumpkin seed oil quality parameters
To evaluate the quality of pumpkin seed oil, free fatty acid (FFA) content and peroxide value (PV) were determined according to the ISO methods 660:2009 and 3960:2007, respectively (27, 28).
Determination of fatty acid composition
Fatty acids in the form of their methyl esters (FAMEs) were determined by gas chromatography according to the ISO method 12966-2:2011 (29). FAMEs were analysed by Auto System XL (Perkin-Elmer, Norwalk, CT, USA), gas chromatograph equipped with a flame-ionization detector, a capillary column model SP-2330 (30 m×0.32 mm×0.2 µm; Supelco, Bellefonte, PA, USA) and a Turbochrom 4 chromatography software (Perkin-Elmer). Injector and detector temperatures were set at 300 and 350 °C, respectively. The analyses were carried out in a programmed temperature mode as follows: 1 min isothermally at 140 °C (initial oven temperature), 5 °C/min to 220 °C and then isothermally for 25 min at 220 °C. Helium (5.0 grade; Messer, Sulzbach, Germany) was used as the carrier gas. The injected sample volume was 1 µL with split injection (100:1). FAMEs were identified by comparison with the commercial standard mixture (Lipid standards: fatty acid methyl ester mixtures C8:0–C24:0, Sigma- -Aldrich). Analysis was done at least in triplicate and the data reported as relative area percentages.
Determination of tocopherols
The tocopherols (α-, γ- and δ-) were determined according to the method proposed by Xu (30) with modifications. Before tocopherol extraction, oil samples were subjected to alkaline hydrolysis with potassium hydroxide. After the incubation in a water bath, samples were extracted with n-hexane and centrifuged (Jouan BR4i multifunction centrifuge, Thermo Electron Corporation, Saint-Herblain, France) to separate hexane layer. Hexane supernatant was first evaporated, then reconstituted with 1 mL of mobile phase (a mixture of acetonitrile and methanol, 75:25, by volume) and filtered through a 0.45-µm polypropylene hydrophilic filter (WhatmanTM PuradiscTM 25 TF, Sigma-Aldrich) prior to analysis. An aliquot (20 µL) was analysed by Spectra System (Thermo Scientific (Thermo Separation Products), Waltham, MA, USA) liquid chromatograph equipped with a P2000 gradient binary pump and a UV2000 UV/Vis detector operating at 295 nm. For tocopherol isomer separation a 150 mm×4.6 mm i.d., particle size 5 µm reversed phase, Hypersil Gold C18 column was used, with a 10 mm×4.6 mm i.d. guard column of the same material (Thermo Electron Corporation, Runcorn, UK). Isocratic elution was carried out at a flow rate of 1.2 mL/min with a mixture of acetonitrile and methanol (75:25, by volume) as mobile phase. Under these conditions, the retention times of α-, γ- and δ-tocopherol were 19.99, 16.99 and 14.16 min, respectively. Calibration curves, used in identification and quantification of tocopherols, were linear from 5 to 400 µg/L, from 6.25 to 50 µg/L and from 0.25 to 5 µg/L for α-, γ- and δ-tocopherol, respectively, with regression coefficients of R2> 0.997 (at least six calibration points, in triplicate).
Determination of DPPH˙ radical scavenging activity and kinetic analysis
The radical scavenging activity (RSA) of the oil was determined by measuring the DPPH˙ disappearance due to the effect of radical scavengers present in oil as described by Kalantzakis et al. (31). Oil samples were added to the freshly prepared solution of 0.1 mmol/L of DPPH˙ in ethyl acetate in order to obtain a 4% (by mass per volume) oil solution. After 10 s of vigorous mixing on a vortex agitator, the absorbance of the mixture was measured at 517 nm, using Cary 100 Bio WinUV (Agilent Technologies, Santa Clara, CA, USA) spectrophotometer. The absorbance of the mixture was measured at 0, 0.5 and every 1 min for 20 min when the reaction reached a steady state. The total RSA of the oil was calculated as the percentage of DPPH˙ inhibition after 20 min according to the following equation:
where A0 and Asample are the absorbance of the control solution and of sample at time t, respectively. A0 was calculated as the sum of the absorbance of freshly prepared DPPH˙ solution multiplied by 0.8 and absorbance of 4% oil solution in ethyl acetate. The correction factor 0.8 was determined experimentally.
In order to find the mathematical model that best describes DPPH˙ disappearance kinetics in pumpkin seed oil samples, six mono- (Eqs. 2–4) and biphasic (Eqs. 5–7) mathematical models were tested:
where DPPH˙0, DPPH˙t, DPPH˙1, DPPH˙2 and DPPH˙r are the ratios of absorbance at the beginning of the experiment, at time t, in steps 1 and 2 at t=0, and remaining value, respectively, k0, k1 and k2 are kinetic constants, kα and β are shape and location parameter, respectively, and t is the reaction time. Curves obtained with predicting models were plotted with the non-linear regression procedures, available with Wolfram Research Mathematica® (32).
Statistical analysis
All results were expressed as mean value±standard deviation (N=3). All statistical analyses were performed using the STATISTICA® v. 8 (33). The effects of tocopherol isomers on DPPH˙ disappearance kinetics in pumpkin seed oil samples were tested by a nonparametric correlation test (Kendall-Tau) and by multiple linear regression analysis. Furthermore, differences between types of pumpkin seed oil samples in the ability to scavenge DPPH˙ were tested by one-way ANOVA test with post hoc comparison (Tukey’s HSD test). The results were considered statistically significant at p<0.05. The goodness of fit of the tested mathematical models to the experimental data was evaluated by the coefficient of determination (R2), the scaled root mean square error (SRMSE; Eq. 8), and error of chi-square test (χ2; Eq. 9):
where Mexp and Mpred are experimental and predicted data, Mexp,i is the mean of all experimental data, and N number of measurements. The similarities between mathematical models were determined with hierarchical agglomerative cluster analysis.
Results and Discussion
Standard quality and composition of pumpkin seed oil
The cold-pressed pumpkin seed oil samples were light green in colour with characteristic pumpkin odour and taste, while the samples of oil produced from roasted seed paste had a deep greenish brown colour, with a specific roasted and nutty aroma. Salad oil had less intense greenish brown colour and aroma. The standard quality parameters showed that all oil samples had FFA content <1 g per 100 g of oil, and PV<7.5 mmol of O2 per kg of oil (Table 1). Similar values of quality parameters of cold- -pressed oil and oil produced from roasted seed paste were found in the literature (3, 7, 13).
Table 1. Standard quality and composition parameters (FFA and PV), fatty acid and tocopherol contents in pumpkin seed oil samples.
| Oil sample | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CP1 | CP2 | RS1 | RS2 | RS3 | RS4 | RS5 | S1 | S2 | |
| FFA/(mg KOH/g oil) | 0.90±0.04 | 0.6±0.1 | 0.9±0.1 | 0.72±0.02 | 0.70±0.05 | 0.67±0.04 | 0.65±0.02 | 0.59±0.02 | 0.6±0.1 |
| PV/(mg O2/kg oil) | 3.0±0.1 | 2.5±0.1 | 5.6±0.1 | 5.4±0.1 | 2.5±0.4 | 4.7±0.5 | 5.0±0.2 | 4.9±0.5 | 3.3±0.1 |
| w(fatty acid)/% | |||||||||
| C16:0 | 11.1±0.2 | 12.3±0.1 | 12.0±0.3 | 9.0±0.1 | 10.0±0.3 | 10.11±0.01 | 11.3±0.2 | 7.4±0.2 | 7.84±0.04 |
| C18:0 | 3.8±0.1 | 4.6±0.1 | 3.5±0.2 | 3.0±0.1 | 2.9±0.1 | 4.02±0.04 | 4.1±0.4 | 2.2±0.1 | 3.4±0.1 |
| C18:1 | 35.7±0.4 | 36.70±0.04 | 33.6±0.2 | 32.52±0.01 | 28.79±0.04 | 39.1±0.0 | 33.3±0.7 | 31.7±0.2 | 31.0±0.2 |
| C18:2 | 48.0±0.2 | 45.6±0.1 | 49.6±0.1 | 54.40±0.04 | 56.2±0.2 | 45.87±0.03 | 50.9±1.4 | 54.49±0.07 | 57.4±0.6 |
| C18:3 | 0.8±0.5 | 0.1±0.1 | 0.73±0.07 | 0.4±0.2 | 1.5±0.1 | 0.4±0.0 | 0.2±0.1 | 3.23±0.02 | 0.28±0.04 |
| C20:0 | 0.44±0.07 | 0.6±0.3 | 0.4±0.0 | 0.35±0.01 | 0.32±0.02 | 0.4±0.0 | 0.10±0.04 | 0.54±0.01 | 0.07±0.04 |
| C22:0 | 0.08±0.02 | 0.04±0.01 | 0.2±0.1 | 0.36±0.02 | 0.37±0.01 | 0.1±0.0 | 0.02±0.02 | 0.44±0.02 | 0.06±0.02 |
| Σ(unsaturated fatty acids) | 84.5±0.3 | 82.46±0.09 | 84.0±0.1 | 87.33±0.07 | 86.4±0.1 | 85.29±0.01 | 84.4±0.8 | 89.4±0.2 | 88.7±0.4 |
| w(tocopherol)/(mg/kg) | |||||||||
| α | 10.1±0.9 | 11.5±3.1 | 32.4±5.9 | 85.1±2.1 | 160.2±5.7 | 89.3±5.3 | 95.0±5.8 | 198.8±4.3 | 237.2±0.1 |
| γ | 251.0±1.1 | 332.9±5.3 | 401.7±9.5 | 174.7±5.6 | 275.4±7.7 | 315.0±6.8 | 242.8±1.7 | 248.5±3.4 | 83.5±2.4 |
| δ | 2.3±0.2 | 2.2±0.2 | 30.8±1.3 | 7.1±0.4 | 18.5±0.8 | 15.7±0.1 | 3.70±0.04 | 10.1±0.4 | 3.0±0.3 |
| Σ(tocopherols) | 263.4±3.1 | 346.6±1.8 | 464.9±10.6 | 266.9±3.42 | 454.1±10.0 | 420.0±7.1 | 374.8±3.9 | 457.4±3.4 | 323.7±1.8 |
All parameters are expressed as mean value±standard deviation (N=3). Pumpkin seed oil samples: CP1 and CP2=cold-pressed, produced from unroasted seeds, RS1–RS5=oil mixtures produced from roasted seed paste, and S1 and S2=salad oil mixtures. FFA=free fatty acids, PV=peroxide value
The fatty acid composition of the tested oil samples is shown in Table 1. All oil samples had high content of unsaturated fatty acids (comprising more than 82% of total fatty acids), with predominant linoleic acid (C18:2) and high content of oleic acid (C18:1). Saturated fatty acids were within a range of 10.6 in salad oil mixture to 17.5% of total fatty acids in cold-pressed oil, with the palmitic acid (C16:0) as the main type. These results are consistent with the results found in the literature (2, 8, 13). The highest content of linoleic acid (C18:2) and the lowest of palmitic acid (C16:0) in the S1 oil was a consequence of the addition of sunflower oil that contains more than 65% of linoleic acid and nearly 6% of palmitic acid (data not shown). The content of these two fatty acids can be directly related to the content of the sunflower oil added during the production. The S2 oil is actually a soybean oil mixture; the presence of soybean oil is revealed in the high content of linolenic (C18:3) acid. It is known that during mechanical extraction in the pumpkin seed oil production, sunflower, soybean or any other oil can be added, depending on the producer. The addition of another oil, because of the characteristic fatty acid profile, is reflected in the fatty acid profile of the produced pumpkin seed oil and can be a measure of oil adulteration (34).
In all analysed oil samples or mixtures, α-, γ- and δ-tocopherols, known as important biological antioxidants, were identified. Although in very low amounts, in some samples β-tocopherols (in sunflower mixture S1) were also identified. The mass fraction of quantified tocopherols (α-, γ- and δ-isomers) in the analysed samples ranged between 263 and 465 mg/kg, as shown in Table 1, which is consistent with the literature (2, 10, 12). The γ-tocopherol prevailed in cold-pressed oil, making more than 95% of total tocopherol content, and in the oil produced from roasted seed paste in which it accounted for 60.6% (275 mg/kg) in RS3 to 86.4% (243 mg/kg) in RS5 of total tocopherol content. In salad oil mixtures, the mass fraction of α-tocopherol was higher compared to other oil types, due to the addition of other types of oil: sunflower (in oil sample S2) and soybean (in sample S1). The adulteration or admixture of soybean oil was visible in the higher mass fraction of δ-tocopherol isomer in samples S1 and RS3.
Radical scavenging activity and analysis of DPPH˙ disappearance kinetics
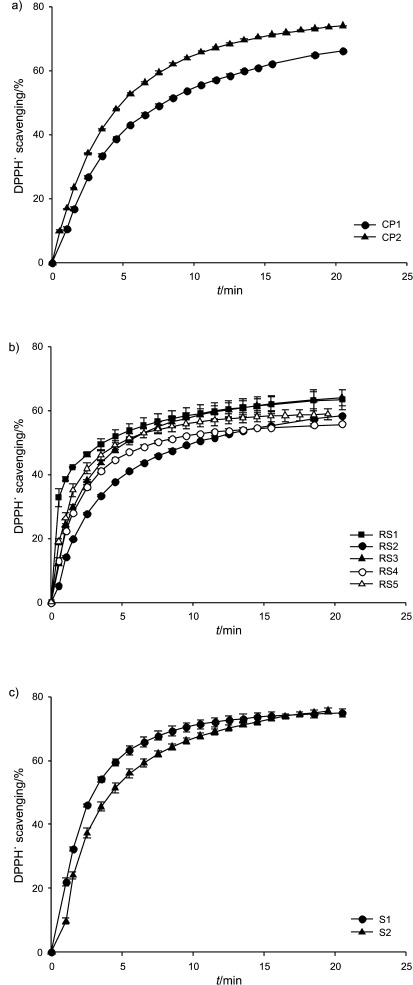
The effect of antioxidants from pumpkin seed oil on the kinetics of DPPH˙ disappearance is shown and compared in Fig. 1. As can be seen in Fig. 1 the kinetics of DPPH˙ disappearance was a two-step process. In the first step, fast disappearance of DPPH˙ occurs during the first 11 min of the reaction, depending on the oil sample, followed by a slower decline in the second step. The time of the rapid step was determined graphically according to the slope of the DPPH˙ disappearance curve (and also confirmed by software).
Fig. 1.
Radical scavenging activity of pumpkin seed oil: a) cold--pressed oil (CP1 and CP2), b) oil produced from roasted seed paste (RS1–RS5), and c) salad oil (S1 and S2). Values are expressed as mean±standard deviation (N=3)
Thus, the longer time of the first step when using cold-pressed and salad oil mixture has been confirmed (8.5–10.5 min, Table 2) compared to the oil obtained from roasted seed paste (4.5–6.5 min, Table 2). Although the time of the first step of DPPH˙ disappearance in the cold- -pressed and salad oil mixture was identical, considerable differences in their antioxidant activity at the end of this step can be found. Higher antioxidant activity was found in salad oil mixture (average of 68.5%) than in the cold- -pressed oil (average of 58.7%). At the end of the reaction the overall depleted DPPH˙ amount in the oil from the roasted seeds was considerably lower (59.7%, Table 2) than in cold-pressed and salad oil (70.3 and 75.1%, Table 2). The results of one-way ANOVA showed that a highly significant difference in RSA between the cold-pressed and oil from roasted seeds (p=0.0017) and between the oil from roasted seeds and salad oil (p=0.0002) existed, but no such difference was observed between cold-pressed and salad oil (p=0.1379) (data not shown).
Table 2. Radical scavenging activity (RSA) of pumpkin seed oil in the first (rapid) step of the reaction and at the end.
| Oil sample | t1/min | RSAa/% | RSAb/% |
|---|---|---|---|
| CP1 | 8.5±0.4 | 51.8±2.6 | 66.4±2.1 |
| CP2 | 10.5±0.1 | 65.7±0.6 | 74.10±0.01 |
| RS1 | 4.5±0.1 | 51.9±1.2 | 63.4±2.1 |
| RS2 | 6.50±0.06 | 43.6±0.6 | 58.3±0.6 |
| RS3 | 6.5±0.2 | 53.0±1.5 | 64.0±2.4 |
| RS4 | 5.5±0.2 | 46.9±1.8 | 53.8±2.5 |
| RS5 | 5.50±0.04 | 51.3±0.5 | 58.8±0.4 |
| S1 | 8.5±0.1 | 69.3±1.4 | 74.9±1.1 |
| S2 | 10.50±0.07 | 67.7±0.2 | 75.3±0.6 |
Values are expressed as mean value±standard deviation (N=3). t1=time of the first step of DPPH˙ disappearance; a=percentage of DPPH˙ depleted in the first step of the reaction; b=percentage of DPPH˙ depleted at the end of the reaction. For oil sample abbreviations see Table 1
In order to explain the mechanisms controlling the antioxidant activity changes of pumpkin seed oil, the experimental data were fitted to six mathematical models, three monophasic and three biphasic, as explained in the Materials and Methods section. The data obtained by models were compared with experimental data and evaluated by statistical indices (R2, SRMSE and χ2 error). As previously noted, in all oil samples the DPPH˙ disappearance took place in two steps. It can be suggested that the biphasic kinetic models (Weibull, first-order double-exponential and Gustafson and Holden model) are more applicable in the case of the biphasic pattern of DPPH˙ disappearance than the monophasic models (zero-order, single-first order and logarithmic model). The values of the parameters obtained by regression analysis and statistical indices are presented in Tables 3 (for monophasic) and 4 (for biphasic models). The better applicability of biphasic models in the description of DPPH˙ disappearance is confirmed by the high coefficients of determination and low error values (R2>0.9947, SRMSE=0.001–0.077; χ2 error=0.03–6.37, Table 4). The biphasic first-order double- -exponential model had the smallest χ2 error values (Table 4) in almost all tested samples, except for samples RS1 and S2, for which the Weibull distribution model described the DPPH˙ disappearance most satisfactorily. Contrastingly, monophasic models provide a relatively poor data fit (SRMSE=0.094–0.311; χ2 error=7.79–25.79, Table 3) except for the logarithmic model in which low errors were obtained (SRMSE=0.019–0.084; χ2 error=1.60–7.17). All the above statistical indices evince that there were no large differences in the applicability among biphasic mathematical models (Weibull, first-order double-exponential model and Gustafson and Holden model) and that they may be successfully used in the description of DPPH˙ disappearance in the tested oil samples.
Table 3. Monophasic models, kinetic parameters and statistical indices in the prediction of radical scavenging activity of pumpkin seed oil.
| Model/parameter | Oil sample | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CP1 | CP2 | RS1 | RS2 | RS3 | RS4 | RS5 | S1 | S2 | |
| Zero-order model | |||||||||
| k0 | –0.029 | –0.030 | –0.018 | –0.025 | –0.025 | –0.020 | –0.019 | –0.027 | –0.031 |
| R2 | 0.9792 | 0.9563 | 0.9593 | 0.9816 | 0.9642 | 0.9723 | 0.9654 | 0.9138 | 0.9443 |
| SRMSE | 0.144 | 0.221 | 0.200 | 0.134 | 0.190 | 0.164 | 0.185 | 0.311 | 0.251 |
| χ2 error | 11.96 | 18.57 | 16.62 | 11.15 | 15.78 | 13.62 | 15.47 | 25.79 | 20.94 |
| First-order model | |||||||||
| k1 | 0.062 | 0.081 | 0.044 | 0.050 | 0.058 | 0.043 | 0.043 | 0.097 | 0.090 |
| R2 | 0.9912 | 0.9832 | 0.9663 | 0.9892 | 0.9773 | 0.9792 | 0.9724 | 0.9564 | 0.9764 |
| SRMSE | 0.094 | 0.137 | 0.182 | 0.103 | 0.151 | 0.142 | 0.165 | 0.221 | 0.163 |
| χ2 error | 7.79 | 11.51 | 15.13 | 8.54 | 12.57 | 11.81 | 13.81 | 18.34 | 13.62 |
| Logarithmic model | |||||||||
| k1 | 0.188 | 0.224 | 0.833 | 0.236 | 0.344 | 0.412 | 0.504 | 0.369 | 0.255 |
| R2 | 0.9996 | 0.9997 | 0.9923 | 0.9995 | 0.9986 | 0.9994 | 0.9986 | 0.9996 | 0.9983 |
| SRMSE | 0.019 | 0.019 | 0.084 | 0.022 | 0.036 | 0.024 | 0.036 | 0.021 | 0.043 |
| χ2 error | 1.60 | 1.65 | 7.17 | 1.90 | 3.09 | 2.04 | 3.07 | 1.79 | 3.66 |
k0 and k1=rate constants, R2=determination coefficient, SRMSE=scaled root mean square error, χ2 error=error of chi-square test. For oil sample abbreviations see Table 1
Table 4. Biphasic models, kinetic parameters and statistical indices in the prediction of radical scavenging activity of pumpkin seed oil.
| Model/parameter | Oil sample | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CP1 | CP2 | RS1 | RS2 | RS3 | RS4 | RS5 | S1 | S2 | |
| Weibull distribution model | |||||||||
| kα | 4.054 | 2.629 | 0.813 | 4.479 | 2.370 | 2.907 | 1.866 | 1.863 | 2.668 |
| β | –0.550 | –0.606 | –0.249 | –0.436 | –0.413 | –0.333 | –0.311 | –0.627 | –0.685 |
| R2 | 0.9999 | 0.9995 | 0.9999 | 0.9998 | 0.9994 | 0.9986 | 0.9984 | 0.9947 | 0.9983 |
| SRMSE | 0.012 | 0.023 | 0.006 | 0.014 | 0.025 | 0.036 | 0.039 | 0.077 | 0.001 |
| χ2 error | 0.96 | 1.93 | 0.47 | 1.17 | 2.08 | 2.97 | 3.28 | 6.37 | 0.03 |
| First-order double-exponential model | |||||||||
| k1 | 0.067 | 0.417 | 3.671 | 0.484 | 0.866 | 0.953 | 0.239 | 0.431 | 0.356 |
| k2 | 0.307 | 0.131 | 0.186 | 0.094 | 0.160 | 0.217 | 1.302 | 0.072 | 0.069 |
| R2 | 0.9999 | 0.9999 | 0.9999 | 0.9995 | 0.9997 | 0.9999 | 0.9999 | 0.9999 | 0.9988 |
| SRMSE | 0.008 | 0.003 | 0.008 | 0.022 | 0.018 | 0.003 | 0.009 | 0.011 | 0.037 |
| χ2 error | 0.73 | 0.27 | 0.73 | 1.96 | 1.62 | 0.28 | 0.82 | 0.97 | 3.34 |
| Gustafson and Holden model | |||||||||
| kα | 0.564 | 0.651 | 0.175 | 0.336 | 0.315 | 0.226 | 0.216 | 0.536 | 0.705 |
| β | 3.207 | 2.580 | 0.065 | 1.423 | 0.675 | 0.399 | 0.238 | 1.146 | 2.554 |
| R2 | 0.9998 | 0.9996 | 0.9999 | 0.9998 | 0.9954 | 0.9992 | 0.9990 | 0.9966 | 0.9973 |
| SRMSE | 0.015 | 0.012 | 0.010 | 0.070 | 0.021 | 0.028 | 0.030 | 0.060 | 0.054 |
| χ2 error | 1.30 | 1.70 | 0.84 | 5.94 | 1.77 | 2.34 | 2.56 | 5.08 | 4.59 |
k1 and k2=rate constants, kα and β=shape and location parameters, R2=determination coefficient, SRMSE=scaled root mean square error, χ2 error=error of chi-square test. For oil sample abbreviations see Table 1
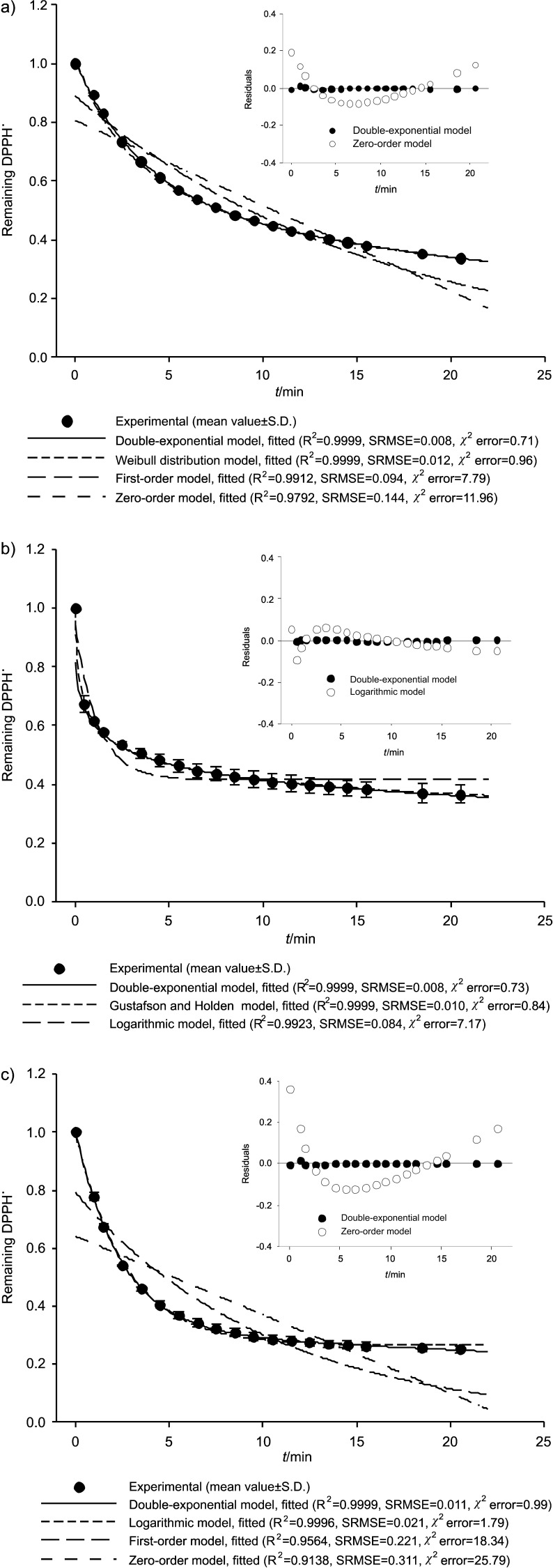
A more detailed study of biphasic DPPH˙ disappearance kinetics was carried out in CP1, RS1 and S1 oil samples, which are selected as representative samples of each type of oil. The kinetics of DPPH˙ disappearance in these samples is shown in Fig. 2, where experimental data for depleted DPPH˙ are shown together with the curves simulated by the best and worst fit (approved also by hierarchical agglomerative cluster analysis, Fig. 3).
Fig. 2.
Experimental data and theoretical curves from kinetic models of DPPH˙ disappearance in pumpkin seed oil: a) cold- -pressed oil (CP1), b) oil produced from roasted seed paste (RS1) and c) salad oil (S1). Values are expressed as mean±standard deviation (N=3). The inserted figures represent residual plot for experimental data against values predicted by the model for the best and worst fit. S.D.=standard deviation
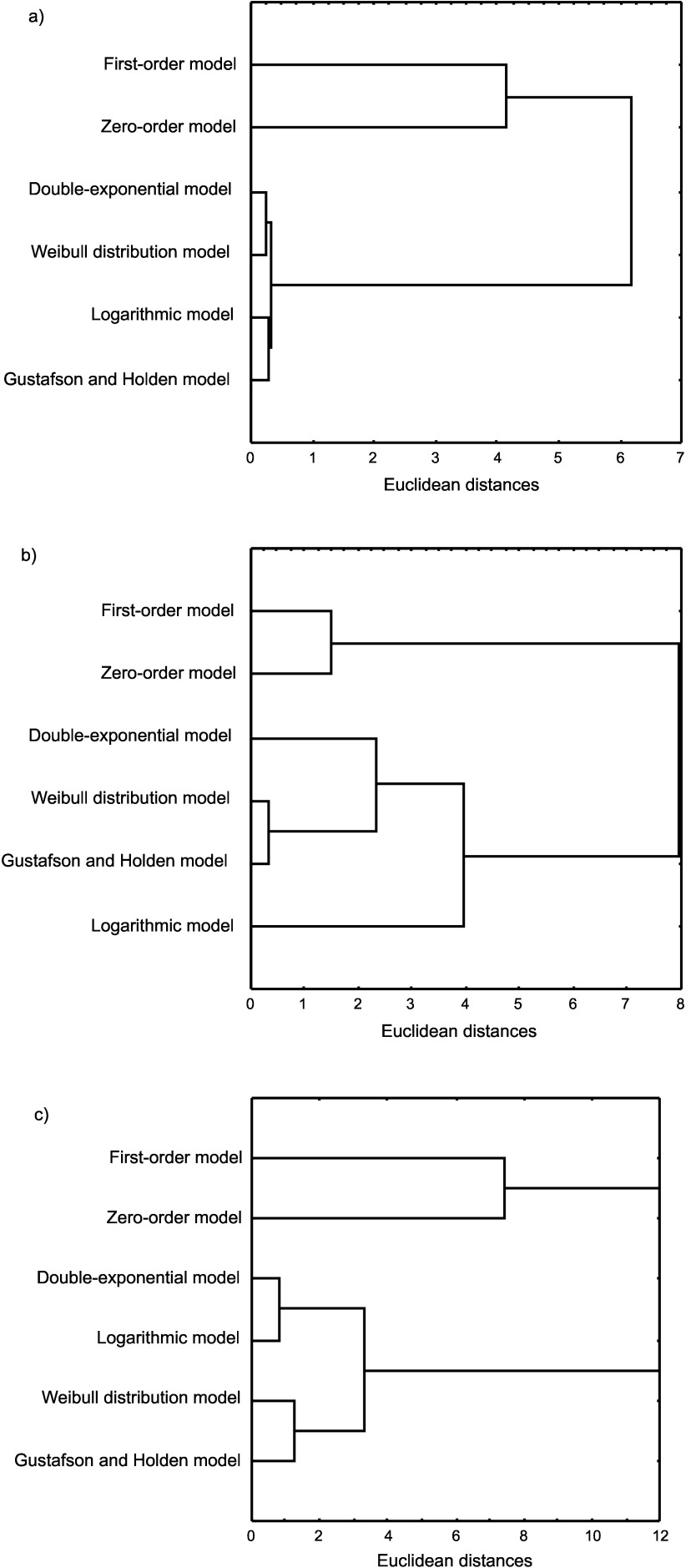
Fig. 3.
Hierarchical agglomerative cluster analysis of mathematical models used in the prediction of radical scavenging activity of pumpkin seed oil: a) cold-pressed oil, b) oil produced from roasted seed paste, and c) salad oil. Proximity measure: squared Euclidean distance. Clustering method: similarities between models
The model that best explains the kinetic behaviour of DPPH˙ in representative samples was ascertained by cluster analysis showing the relationships between the models used on the basis of statistical indices (R2, SRMSE and χ2 error). For predicting DPPH˙ disappearance in sample CP1, the lowest errors (SRMSE=0.008 and χ2 error=0.73) and the highest R2 values (R2=0.9999) were obtained by the first-order double-exponential model, followed by the Weibull distribution model and the Gustafson and Holden model (Table 4). This is also obvious from Fig. 2a, where the residual plots (inserts in Fig. 2) for the biphasic kinetic models (Weibull distribution and first-order double-exponential models) are closer to zero than the monophasic models (zero-order and first-order models).
In addition, following cluster analysis, the double- -exponential and Weibull distribution models were divided into the same cluster (Fig. 3a), leading to the conclusion that these models should be used for description of DPPH˙ loss in sample CP1, rather than other used models. For sample RS1, an excellent fit of the experimental data for the Weibull distribution model and the Gustafson-Holden model was indicated by low errors and high R2 values (SRMSE=0.006–0.010, χ2 error=0.47–0.84, R2=0.9999; Table 4, Fig. 2b). At the same time, these models are classified by cluster analysis in the same group (Fig. 3b). Therefore, these biphasic models can be recommended for the description of DPPH˙ disappearance in sample RS1. A somewhat different situation was found in sample S1, where DPPH˙ disappearance was described equally well by the monophasic logarithmic model and the biphasic first-order double-exponential model, because cluster analysis classifies these models in the same group (Fig. 3c). This is supported by the obtained statistical indices, where fitting of experimental data to the logarithmic model and the first-order double-exponential model (Tables 3 and 4) generated almost identical R2, errors and residual values. According to all these results, it may be concluded that the use of the first-order double- -exponential model and the logarithmic model was preferable for describing the DPPH˙ disappearance in sample S1.
Effect of oil composition on the DPPH˙ disappearance
All the previously mentioned facts about biphasic DPPH˙ disappearance indicate that the scavenging process is governed by two different reaction mechanisms. Since the biphasic first-order double-exponential model gave the best description of DPPH˙ disappearance in the experimental oil samples, it was selected to give a more detailed explanation of the reaction mechanism. Generally, the scavenging reaction between DPPH˙ radical and antioxidants present in the oil starts with the transfer of the most labile H atom from the scavenger molecule to the free radical. The newly formed less reactive radical (A˙) can react with another DPPH˙ or with A˙ via radical disproportionation reaction to form more stable products. Our findings relating to biphasic DPPH˙ disappearance are consistent with several prior studies (24). Espin et al. (24) assumed that the reason for biphasic behaviour can be attributed to the complexity of the reactions, since they involve two different groups of antioxidants and proceed through different chemical pathways. This means that antioxidants present in the oil possess different radical- -suppressing abilities with which they react at different rates and thereby cause two steps of reaction. Each of the two antioxidants (AH1 and AH2) reacts with DPPH˙ according to the following reactions:
In these reactions, the overall reaction rate is characterized by the two rate constants, k1 and k2, which represent the rate of DPPH˙ disappearance. Assuming that during the reaction all of the antioxidants present in the oil react with the DPPH˙ radical, constants k1 and k2 can represent the rate of antioxidant disappearance. Therefore, it can be concluded that the amount of disappeared DPPH˙ is equal to the capacity of the antioxidants present in the oil to scavenge radicals.
In order to determine the possible involvement of oil tocopherols and fatty acids in two steps of DPPH˙ disappearance and its rate, the rate constant values k1 and k2 were correlated with the tocopherol content (α-, γ- and δ-) and with oleic (C18:1) and linoleic (C18:2) acid content using a Kendall-Tau correlation test. Correlation analysis between k1 values and selected oil parameters showed that the first step of DPPH˙ disappearance in the experimental oil samples was positively influenced by γ- and δ-tocopherol content, with a stronger relationship between k1 and δ-tocopherol content (data not shown). Relations between k1 and other oil parameters were also positive, but insignificant at p<0.05 level. The same analysis test showed negative dependence between k2 values and α-tocopherol and C18:2 content, while C18:1 content positively influenced the reaction rate k2. Thus, these results suggest that the γ- and δ-tocopherols are antioxidants that are involved in the first step of DPPH˙ scavenging in the experimental oil samples and that they have a major influence on it. By contrast, in the second reaction of DPPH˙ disappearance, α-tocopherol, oleic (C18:1) and linoleic (C18:2) acids were predominantly involved. In addition to the nonparametric test, multiple linear regression analysis was used, which simultaneously compares various oil parameters and rate constants (k1 and k2) leading to a linear predictive model for rate constant values. This analysis resulted in the following correlations for k1 and k2:
Nonparametric regression analysis showed that γ- and δ-tocopherols affected DPPH˙ disappearance during the first step of the reaction, but the multiple linear regression equation suggested that the δ-tocopherol predominantly influenced DPPH˙ disappearance in the experimental oil samples. In the second step of the reaction, a very weak linear relationship between constant k2 and tested parameters (α-tocopherol, C18:1 and C18:2) was achieved, indicating that none of the tested oil parameters had a dominant effect on the DPPH˙ disappearance rate during the second step of reaction.
Besides, in this work an attempt has been made to analyse differences in the antioxidant activity of individual tocopherol isomers. In almost all oil samples γ-tocopherol was identified as the dominant isomer, except for sample S2 (salad oil mixture) where higher content of α-tocopherol was found (Table 1). In these samples the manufacturers added synthetic α-tocopherol and other supplements during the production process, in quantities known only to the producers, to enrich and improve the quality of oil. To determine the antioxidant capacity of tocopherols involved in both steps of DPPH˙ disappearance, experimental values were fitted to the equation of the first-order double-exponential model (Table 5).
Table 5. Parameters of DPPH˙ disappearance determined by first-order double-exponential model, total radical scavenging activity (RSAT), radical scavenging activity during the first and second steps of reaction (RSA1 and RSA2 respectively) and antioxidant activity of tocopherol isomers involved in different steps of the reaction.
| Oil sample |
DPPH˙1 | DPPH˙2 | DPPH˙r | RSAT | RSA1 | RSA2 | Activity | Ratio of activities (B/A) |
||
|---|---|---|---|---|---|---|---|---|---|---|
| % | A | B | ||||||||
| CP1 | 0.376 | 0.365 | 0.246 | 74.07 | 38.08 | 37.02 | 0.060 | 1.473 | 24.51 | |
| CP2 | 0.336 | 0.431 | 0.230 | 76.75 | 33.71 | 43.24 | 0.040 | 1.505 | 37.40 | |
| RS1 | 0.357 | 0.277 | 0.366 | 63.41 | 35.71 | 27.72 | 0.033 | 0.342 | 10.37 | |
| RS2 | 0.294 | 0.338 | 0.368 | 63.21 | 29.40 | 33.82 | 0.065 | 0.159 | 2.46 | |
| RS3 | 0.312 | 0.342 | 0.350 | 65.36 | 31.05 | 34.09 | 0.042 | 0.085 | 2.02 | |
| RS4 | 0.264 | 0.293 | 0.442 | 55.75 | 26.44 | 29.31 | 0.032 | 0.131 | 4.10 | |
| RS5 | 0.290 | 0.297 | 0.410 | 58.69 | 29.08 | 29.77 | 0.047 | 0.125 | 2.65 | |
| S1 | 0.659 | 0.124 | 0.222 | 78.32 | 65.59 | 12.34 | 0.101 | 0.025 | 0.25 | |
| S2 | 0.553 | 0.273 | 0.177 | 82.54 | 55.10 | 27.22 | 0.255 | 0.045 | 0.18 | |
A=activity of γ- and δ-tocopherols, calculated as (0.4·RSA1)/(w(γ-tocopherol)+w(δ-tocopherol)), B=activity of α-tocopherol, calculated as (0.4·RSA2)/w(α-tocopherol). DPPH˙0, DPPH˙t, DPPH˙1, DPPH˙2 and DPPH˙r are the ratios of absorbance at the beginning of the experiment (at time t=0), at time t, steps 1 and 2 and remaining value, respectively
An evident difference in the antioxidant activity between the two steps was found only in salad oil mixtures (Table 5). Assuming that tocopherols are responsible for scavenging about 40% of DPPH˙ (11) and taking into account the mass fraction of each tocopherol isomer as well as their contribution to DPPH˙ scavenging (RS1 and RS2), it was found that they possess different efficacy as antioxidants. Comparing the antioxidant activity of γ- and δ-tocopherols in the first step of the DPPH˙ disappearance reaction with the antioxidant activity of α-tocopherol, involved in the second step of reaction, there was approx. 30 times more activity of α-tocopherol in the cold-pressed oil samples. Very similar antioxidant tocopherol behaviour was observed in the oil samples from roasted seed paste, where the ability of α-tocopherol to scavenge DPPH˙ radical was 5 times higher compared to γ- and δ-tocopherols. Therefore, all these facts may indicate that the biphasic double-exponential behaviour of DPPH˙ disappearance in oil samples is a result of the presence of different tocopherol isomers that scavenged DPPH˙ with different intensity. The obtained results indicate the strongest antioxidant activity of the α-tocopherol isomer, which is in agreement with the results of the in vitro study of the reactivity of tocopherols reported by Burton and Ingold (35). They found that the observed discrepancy between tocopherol reactivity was due to two effects, the inductive and the stereoelectronic. More specifically, the inductive effect arises as a consequence of the presence of electron donating methyl groups in the ortho or para position in the hydroxyl group on the aromatic ring of tocopherol. Such positioning of methyl groups increases the electron density of the active centres, allowing homolytic cleavage of O-H bond and increasing the stability of the chromanoxyl radical and its reactivity with a peroxy radical (17). Since the α-tocopherol ring is substituted with two methyl groups in the ortho position of the hydroxyl group on the aromatic ring of the tocopherol molecule, it will easily release the hydrogen ion compared to the γ- and δ-tocopherols. Another effect, the stereoelectronic, indicates that individual tocopherol isomers showed differences in their reactivity due to the heterocyclic ring with an oxygen atom in the para position of the hydroxyl group on the aromatic ring of the tocopherol molecule. This oxygen atom possesses a single electron pair whose p-type orbitals overlap with the semi-occupied molecular orbitals of the tocopherol molecule, making it more stable because of the conjugated electron delocalization. If the overlapping of orbitals is of higher intensity, the antioxidant activity is stronger. Concerning these effects, α-tocopherol is structurally the most potent as a hydrogen donor compared to other isomers and it was found that it is mainly α-tocopherols that regenerate β-, γ- and δ-tocopherols from their radicals (17). It should not be overlooked that some authors advocate the coantioxidant activity of tocopherols and other antioxidants present in food, i.e. ascorbate or flavonoids and that partial regeneration between tocopherols (particularly δ-isomer) and flavonoids occurs (36).
Conclusions
In order to predict the antioxidant activity of oil and give a more comprehensive understanding of kinetic reaction mechanisms, a combination of different oil composition parameters, i.e. their mutual analyses and kinetic evaluation, is necessary. Thus, we recommend the use of a first-order double-exponential model in describing biphasic DPPH˙ disappearance, providing immediate and detailed information on antioxidant potential linked to the differences in the chemical composition of the oil. This model also suggests that tocopherol isomers found in the oil possess different radical suppressing ability and that they react at different rates with radicals, thereby causing two steps of reaction. For a deeper insight into the mechanisms involving the DPPH˙ and oil antioxidants, it is necessary to characterize the oil in more detail with regard to the presented antioxidants, the reaction intermediates and the products exhibiting different efficiencies towards radicals, as well as to develop a mathematical model that will link together all the parameters. This will be included in our future research.
Acknowledgements
This work was supported by the Croatian Ministry of Science, Education and Sports (grant number 062-0621341- 0061).
References
- 1.Vujasinovic V, Djilas S, Dimic E, Romanic R, Takaci A. Shelf life of cold-pressed pumpkin (Cucurbita pepo L.) seed oil obtained with a screw press. J Am Oil Chem Soc. 2010;872:1497–505. 10.1007/s11746-010-1630-x [DOI] [Google Scholar]
- 2.Neđeral Nakić S, Rade D, Škevin D, Štrucelj D, Mokrovčak Z, Bartolić M. Chemical characteristics of oils from naked and husk seeds of Cucurbita pepo L. Eur J Lipid Sci Technol. 2006;108:936–43. 10.1002/ejlt.200600161 [DOI] [Google Scholar]
- 3.Gohari Ardabili A, Farhoosh R, Haddad Khodaparast MH. Chemical composition and physicochemical properties of pumpkin seeds (Cucurbita pepo subsp. pepo var. styriaka) grown in Iran. J Agr Sci Tech. 2011;13:1053–63. [Google Scholar]
- 4.Andjelkovic M, Van Camp J, Trawka A, Verhé R. Phenolic compounds and some quality parameters of pumpkin seed oil. Eur J Lipid Sci Technol. 2010;112:208–17. 10.1002/ejlt.200900021 [DOI] [Google Scholar]
- 5.Ryan E, Galvin K, O’Connor TP, Maguire AR, O’Brien NM. Phytosterol, squalene, tocopherol content and fatty acid profile of selected seeds, grains, and legumes. Plant Foods Hum Nutr. 2007;62:85–91. 10.1007/s11130-007-0046-8 [DOI] [PubMed] [Google Scholar]
- 6.Fu C, Shi H, Li Q. A review on pharmacological activities and utilization technologies of pumpkin. Plant Foods Hum Nutr. 2006;61:73–80. 10.1007/s11130-006-0016-6 [DOI] [PubMed] [Google Scholar]
- 7.Nyam KL, Tan CP, Lai OM, Long K, Che Man YB. Physicochemical properties and bioactive compounds of selected seed oils. LWT –. Food Sci Technol (Campinas). 2009;42:1396–403. 10.1016/j.lwt.2009.03.006 [DOI] [Google Scholar]
- 8.Murkovic M, Hillebrand A, Winkler J, Leitner E, Pfannhauser W. Variability of fatty acid content in pumpkin seeds (Cucurbita pepo L). Z Lebensm Unters Forsch. 1996;203:216–9. 10.1007/BF01192866 [DOI] [PubMed] [Google Scholar]
- 9.Shahidi F. Antioxidants in food and food antioxidants. Nahrung. 2000;44:158–63. [DOI] [PubMed] [Google Scholar]
- 10.Murkovic M, Pfannhauser W. Stability of pumpkin seed oil. Eur J Lipid Sci Techol. 2000;102:607–11. http://dx.doi.org/ 10.1002/1438-9312(200010)102:10<607::AID EJLT607>3.0.CO;2-E [DOI]
- 11.Fruhwirth GO, Wenzl T, El-Toukhy R, Wagner FS, Hermetter A. Fluorescence screening of antioxidant capacity in a pumpkin seed oils and other natural oils. Eur J Lipid Sci Technol. 2003;105:266–74. 10.1002/ejlt.200390055 [DOI] [Google Scholar]
- 12.Butinar B, Bučar-Miklavčič M, Mariani C, Raspor P. New vitamin E isomers (gamma-tocomonoenol and alpha-tocomonoenol) in seeds, roasted seeds and roasted seed oil from the Slovenian pumpkin variety ‘Slovenska golica’. Food Chem. 2011;128:505–12. 10.1016/j.foodchem.2011.03.072 [DOI] [PubMed] [Google Scholar]
- 13.Neđeral S, Škevin D, Kraljić K, Obranović M, Papeša S, Bataljaku A. Chemical composition and oxidative stability of roasted and cold pressed pumpkin seed oils. J Am Oil Chem Soc. 2012;89:1763–70. 10.1007/s11746-012-2076-0 [DOI] [Google Scholar]
- 14.Schneider C. Chemistry and biology of vitamin E. Mol Nutr Food Res. 2005;49:7–30. 10.1002/mnfr.200400049 [DOI] [PubMed] [Google Scholar]
- 15.Schoof A, Grünert J, Ritter S, Hemmerich A. Reducing the linewidth of a diode laser below 30 Hz by stabilization to a reference cavity with a finesse above 105. Opt Lett. 2001;26:1562–4. 10.1364/OL.26.001562 [DOI] [PubMed] [Google Scholar]
- 16.Grusak MA, DellaPenna D. Improving the nutrient composition of plants to enhance human nutrition and health. Annu Rev Plant Physiol. 1999;50:133–61. 10.1146/annurev.arplant.50.1.133 [DOI] [PubMed] [Google Scholar]
- 17.Kamal-Eldin A, Appelqvist LA. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids. 1996;31:671–701. 10.1007/BF02522884 [DOI] [PubMed] [Google Scholar]
- 18.Bondet V, Brand-Williams W, Berset C. Kinetics and mechanisms of antioxidant activity using the DPPH˙ free radical method. LWT –. Food Sci Technol (Campinas). 1997;30:609–15. 10.1016/fslt.1997.0240 [DOI] [Google Scholar]
- 19.Brand-Williams W, Cuvelier ME, Berset C. Use of a free-radical method to evaluate antioxidant activity. LWT –. Food Sci Technol (Campinas). 1995;28:25–30. 10.1016/S0023-6438(95)80008-5 [DOI] [Google Scholar]
- 20.Zheng H, Lu H. Use of kinetic, Weibull and PLSR models to predict the retention of ascorbic acid, total phenols and antioxidant activity during storage of pasteurized pineapple juice. LWT –. Food Sci Technol (Campinas). 2011;44:1273–81. 10.1016/j.lwt.2010.12.023 [DOI] [Google Scholar]
- 21.Maskan M. Production of pomegranate (Punica granatum L.) juice concentrate by various heating methods: colour degradation and kinetics. J Food Eng. 2006;72:218–24. 10.1016/j.jfoodeng.2004.11.012 [DOI] [Google Scholar]
- 22.Rufino MSM, Alves RE, Fernandes FAN, Brito ES. Free radical scavenging behaviour of ten exotic tropical fruits extracts. Food Res Int. 2011;44:2072–5. 10.1016/j.foodres.2010.07.002 [DOI] [Google Scholar]
- 23.Mishra K, Ojha H, Chaudhury NK. Estimation of antiradical properties of antioxidants using DPPH˙ center dot assay: a critical review and results. Food Chem. 2012;130:1036–43. 10.1016/j.foodchem.2011.07.127 [DOI] [Google Scholar]
- 24.Espin JC, Soler-Rivas C, Wichers HJ. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J Agric Food Chem. 2000;48:648–56. 10.1021/jf9908188 [DOI] [PubMed] [Google Scholar]
- 25.Odriozola-Serrano I, Soliva-Fortuny R, Martin-Belloso O. Influence of storage temperature on the kinetics of the changes in anthocyanins, vitamin C, and antioxidant capacity in fresh-cut strawberries stored under high-oxygen atmospheres. J Food Sci. 2009;74:C184–91. 10.1111/j.1750-3841.2009.01075.x [DOI] [PubMed] [Google Scholar]
- 26.Boesten JJTI, Aden K, Beigel C, Beulke S, Dust M, Dyson JS, et al. Guidance document on estimating persistance and degradation kinetics from environmental fate studies on pesticides in EU registration. The final report of the work group on degradation kinetics of FOCUS. Sanco/10058/2005, v. 2.0. 2006. Available from: http:/focus.jrc.ec.europa.eu/dk/docs/finalreportFODCDegKin04June06linked.pdf.
- 27.ISO 660:2009. Animal and vegetable fats and oils – determination of acid value and acidity. Geneva, Switzerland: International Organization for Standardization (ISO); 2009.
- 28.ISO 3960:2007. Animals and vegetable fats and oils – Determination of peroxide value – Iodometric (visual) endpoint determination. Geneva, Switzerland: International Organization for Standardization (ISO); 2007.
- 29.ISO 12966-2:2011. Animal and vegetable fats and oils – Gas chromatography of fatty acid methyl esters – Part 2: Preparation of methyl esters of fatty acids. Geneva, Switzerland: International Organization for Standardization (ISO); 2011.
- 30.Xu Z. Analysis of tocopherols and tocotrienols. Current Protocols in Food Analytical Chemistry. 2002;D:D1:D1.5. http://dx.doi.org/ 10.1002/0471142913.fad0105s04 [DOI]
- 31.Kalantzakis G, Blekas G, Pegklidou K, Boskou D. Stability and radical-scavenging activity of heated olive oil and other vegetable oils. Eur J Lipid Sci Technol. 2006;108:329–35. 10.1002/ejlt.200500314 [DOI] [Google Scholar]
- 32.Wolfram Research Mathematica®, v. 8.0, Champaign, IL, USA: WolframResearch Co. 2009. Available from: http://www.wolfram.com/mathematica/.
- 33.STATISTICA, v. 8, StatSoft, Inc, Tulsa, OK, USA; 2007. Available from: http://www.statsoft.com.
- 34.Butinar B, Bučar-Miklavčič M, Valenčič V, Raspor P. Stereospecific analysis of triacylglycerols as a useful means to evaluate genuineness of pumpkin seed oils: lesson from virgin olive oil analyses. J Agric Food Chem. 2010;58:5227–34. 10.1021/jf904542z [DOI] [PubMed] [Google Scholar]
- 35.Burton GW, Ingold KU. Autoxidation of biological molecules. 1. The antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vitro. J Am Chem Soc. 1981;103:6472–7. 10.1021/ja00411a035 [DOI] [Google Scholar]
- 36.Kadoma Y, Ishihara M, Okada N, Fujisawa S. Free radical interaction between vitamin E (alpha-, beta-, gamma- and delta-tocopherol), ascorbate and flavonoids. In Vivo. 2006;20:823–8. [PubMed] [Google Scholar]