Summary
A novel biosensor for l-ascorbic acid determination in different beverages was elaborated. The ascorbate oxidase enzyme (AAO) from Cucurbita sp., EC 1.10.3.3, was immobilized on a screen-printed carbon electrode with poly(ethylene glycol) (400) diglycidyl ether (PEGDGE) as a crosslinking agent. The standards and samples were measured first with a blank electrode. An inert protein, bovine serum albumin (BSA), was immobilized on the surface of this electrode with PEGDGE. The BSA mass was equivalent to the mass of 10 U of AAO enzyme immobilized on the electrodes (0.021 mg). The linear measuring range for l-ascorbic acid was between 5 and 150 µmol/L. As l-ascorbic acid is a vital vitamin and a common antioxidant used in food industry, fruit juices and vitamin C effervescent tablets were examined. The results were compared to HPLC measurements.
Key words: biosensor, l-ascorbic acid, ascorbate oxidase, screen-printed carbon electrodes
Introduction
l-Ascorbic acid (vitamin C) occurs naturally in many different fruits and vegetables. It is also widely used in the food industry as an antioxidant (1) and an efficient scavenger of free radicals. For this reason, the determination of l-ascorbic acid might provide information concerning the antioxidant properties of the given sample (2). There are various approaches utilized for l-ascorbic acid measurements: enzymatic methods, titration with oxidizing agents, HPLC with UV/Vis detection, gas chromatography, and spectrophotometry (1). A rapid, low-cost and reliable approach is the use of biosensors. The utilization of ascorbate oxidase enzyme (AAO, EC 1.10.3.3) for biosensor construction is one of the possible approaches. This protein belongs to the blue multicopper oxidase family, along with laccase, ceruloplasmin or plastocyanin. It catalyses the oxidation of ascorbic acid to dehydroascorbic acid; the reaction also results in the reduction of molecular oxygen to water (3). Several ascorbate oxidase enzyme-based biosensors have been reported for l-ascorbic acid determination.
In the work of Akyilmaz and Dinçkaya (4) AAO from cucumber (Cucumis sativus L.) was coupled with a dissolved oxygen probe. The enzyme was immobilized with gelatin via glutaraldehyde on a teflon membrane. The l- -ascorbic acid content of fruit juices and vitamin C tablets was measured in the range of 50–1200 µmol/L (4). Another publication (5) describes a micelle structure formed by binding AAO to polymaleimidostyrene in polystyrene membrane. l-Ascorbic acid was detected by amperometric measurements after coating the AAO membrane on gold and glassy carbon electrodes. The vitamin content of different fruit juices was examined in 5–400 µmol/L range. AAO was also covalently immobilized on a polycarbonate strip pre-activated by 1-fluoro-2-nitro-4-azidobenzene in photochemical reaction. l-Ascorbic acid content of fruit juices was determined in ppm using these AAO immobilized strips. The strips can be used ten times, maintaining approx. 75% of their initial activity (1). AAO isolated from Lagenaria siceraria was immobilized on an egg shell membrane. The vitamin C content of fruit juices, vitamin C tablets and serum ascorbate was determined with gold electrode in the range of 10–400 µmol/L (6). AAO from Cucumis sativus was immobilized on a graphite/epoxy electrode to form a potentiometric sensor. l- -Ascorbic acid content was measured in different pharmaceutical samples in the range between 8 and 450 µmol/L (7).
Another AAO sensor was developed earlier in our lab (8). AAO was immobilized on a natural protein membrane in thin-layer enzyme cell connected to the flow injection analysis system with an amperometric detector cell using glassy carbon working electrode. In this work, results of the above-mentioned research were utilized. The use of the versatile, robust and low-cost screen-printed electrodes (SPEs) (9) enabled us to further develop the method. SPEs enable the elaboration of miniaturized measuring systems, and enhance the possibility of on-site measurements. The main goal of our experiments is the development of a novel ascorbate oxidase-based biosensor for the determination of l-ascorbic acid in various fruit juices and vitamin C effervescent tablets, based on screen-printed carbon electrodes.
Materials and Methods
Reagents
Ascorbate oxidase from Cucurbita sp. (EC 1.10.3.3), metaphosphoric acid (HPO3)n and bovine serum albumin (BSA) were obtained from Sigma-Aldrich (St Louis, MO, USA). Poly(ethylene glycol) (400) diglycidyl ether was purchased from Polysciences Inc. (Warrington, PA, USA). All other reagents were of analytical grade and double distilled water was used throughout our experiments. Different fruit juices and vitamin C effervescent tablets used for l-ascorbic acid content determination were purchased in local supermarkets (Eger, Hungary).
Instrumentation
The biosensor was integrated into a flow injection analysis (FIA) utilizing QuadStat EA164 potentiostat and e-corder A/D converter (eDAQ, Colorado Springs, CO, USA) coupled with a Dropsens flow cell (Llanera, Asturias, Spain) and screen-printed carbon electrodes (SPCE, Metrohm 61208110; Herisau, Switzerland). The diameter of the SPCEs carbon working electrode was 4 mm, the auxiliary electrode was also made of carbon, while the reference electrode was silver. Chart software (eDAQ) was used for data acquisition and elaboration. The constant buffer flow was maintained by a peristaltic pump Minipuls 4 (Gilson, Villiers le Bel, France) and the sample was injected with a Rheodyne 7725i type (Rohnert Park, CA, USA) manual injector using 20-µL sample loop.
HPLC method
The reference HPLC measurements were carried out with an Agilent 1200 Series device (Agilent Technologies, Santa Clara, CA, USA). The column used was an Agilent Eclipse Plus C18 (4.6 mm×250 mm, 5 µm). The measurements were carried out with the following eluents: A=0.1% trifluoroacetic acid and B=methanol, with an isocratic elution: 90% A and 10% B. The flow rate was 0.8 mL/min, the sample volume was 10 µL. The retention time was 3.85 min and sensitivity 38.625 (mAU·min)/ppm. l-Ascorbic acid was detected at 250 nm using diode array detector. The linear measuring range for l-ascorbic acid was between 40 and 500 ppm, with the limit of detection of 0.015 ppm.
Results and Discussion
Development of the ascorbate oxidase-based amperometric biosensor
The previously mentioned publication (8) presents extensive and successful experiments concerning the stability of the l-ascorbic acid content of the standards and samples. Preliminary examinations for buffer composition (results not shown) were carried out to determine the proper buffer composition for the measurements with screen-printed electrodes. These experiments proved that the buffer composition described in the previously mentioned article (8) was the most suitable for our experiments. Therefore, we also applied 200 mmol/L of sodium acetate buffer (pH=4.65) and 2 mmol/L of metaphosphoric acid to stabilize the ascorbic acid. Taking the characteristics of the screen-printed electrodes into consideration, 100 mmol/L of KCl were added to the buffer. Experiments were carried out to determine the appropriate polarization potential and the quantity of immobilized enzyme required for the measurements.
Polarization potential
The electrochemical signal of 1 µmol/L of l-ascorbic acid was studied as a function of the polarization potential. The ascorbic acid gave low response at 350 and 400 mV (approx. 1 and 3 nA). However, the signal was significantly higher at 450 mV (almost 13 nA). The response was again lower at 500 mV, further decreasing between 500 and 650 mV (6-2 nA). Based on these results, 450-mV potential was applied throughout the experiments.
Experiments with native enzyme
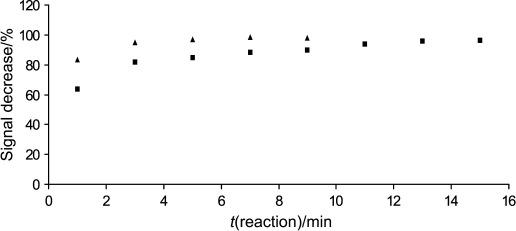
Dissolved ascorbate oxidase enzyme (AAO) was used to test the activity on l-ascorbic acid. Enzyme solutions of 4, 8 and 12 U of AAO were added to 2 mL of 100 µmol/L of l-ascorbic acid solutions. When using 4 U of AAO an adequate decrease of the l-ascorbic acid content in the samples was not observed, whereas 8 and 12 U of AAO reacted with almost 100% of the vitamin content in the solutions after 13 and 7 min respectively, meaning that the signal decreased by 96% (8 U) and 98% (12 U) (Fig. 1). Following further experiments with both native and immobilized enzyme (results not shown), 10 U of AAO was chosen for immobilization on the enzyme electrodes.
Fig. 1.
Activity of dissolved AAO measured as a signal decrease caused by the addition of 8 (square) and 12 U (triangle) of enzyme to 100 µmol/L of l-ascorbic acid in 2 mL of buffer (working conditions: 100 mmol/L of phosphate buffer, pH=7.0, with 2 mmol/L of (HPO3)n and 100 mmol/L of KCl; flow rate of 2.0 mL/min; polarization potential of 450 mV)
Electrode set-up
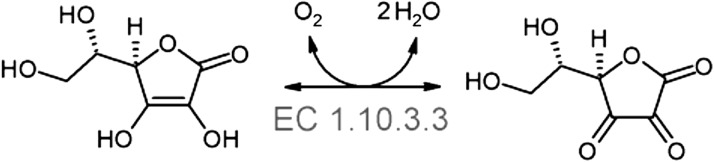
The oxidation of l-ascorbic acid is catalysed by AAO. Molecular oxygen is required for the following reaction (Scheme 1):
Scheme 1.
Oxidation of l-ascorbic acid
At oxidizing potential, the ascorbate present in the standard or sample changes the measured current (8). The AAO immobilized on a SPCE reduces the l-ascorbic acid concentration, resulting in a current decrease compared to the measurements carried out on a SPCE without the AAO. The l-ascorbic acid concentration in the standards and samples was calculated from two measurements. It also has to be taken into consideration that the samples normally contain a large scale of electrochemically active compounds, which can also influence the current measured by the amperometric cell. To eliminate these interferences, the samples were measured with an electrode without AAO, and an electrode with 10 U of AAO by replacing the screen-printed electrode in the flow through the cell between each set of measurements.
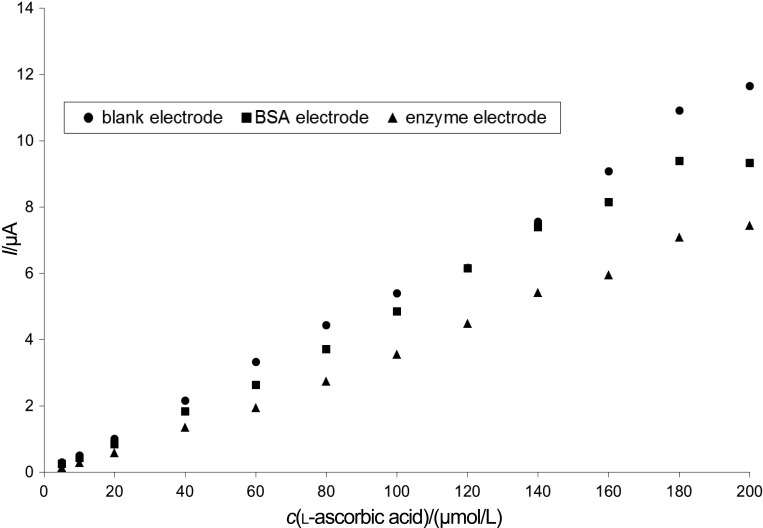
Experiments were carried out to create the adequate blank electrode. The amperometric response of the sensors was studied by measuring l-ascorbic acid with three differently prepared electrodes: (i) blank electrode, (ii) BSA electrode (0.021 mg of BSA was immobilized on the electrode with 4 mg/mL of PEGDGE; the protein mass was equivalent to the mass of 10 U of AAO immobilized on the enzyme electrode), and (iii) ascorbate oxidase enzyme electrode (10 U of AAO were immobilized on the electrode with 4 mg/mL of PEGDGE).
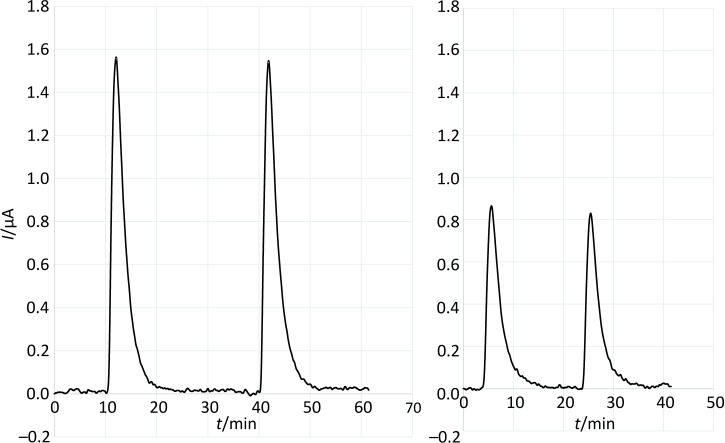
Measuring l-ascorbic acid standards in the concentration range of 5–200 µmol/L, it can be seen in Fig. 2 that immobilizing protein on the electrode caused some signal decrease, when compared to the measurements with the blank electrode. As samples should be measured under the same conditions to make sure that the only signal decrease occurring is caused by the AAO activity, further measurements were carried out with the BSA and the AAO electrodes. Thus, we were able to compensate for the signal of other electroactive compounds present in the samples (Fig. 2). Fig. 3 shows typical analytical signals when measured with the biosensor presented in this article.
Fig. 2.
Calibration of the AAO-based biosensor expressed as a signal response of three different electrodes (working conditions: 200 mmol/L of sodium acetate buffer with 100 mmol/L of KCl, pH=4.65, and 2 mmol/L of (HPO3)n; flow rate of 2.0 mL/min; polarization potential of 450 mV)
Fig. 3.
Analytical signals produced by ten times diluted 100% apple juice injected onto BSA electrode (left) and onto AAO electrode (right) (working conditions: 200 mmol/L of sodium acetate buffer with 100 mmol/L of KCl, pH=4.65, and 2 mmol/L of (HPO3)n; flow rate of 2.0 mL/min; polarization potential of 450 mV)
Parameters of l-ascorbic acid measurement
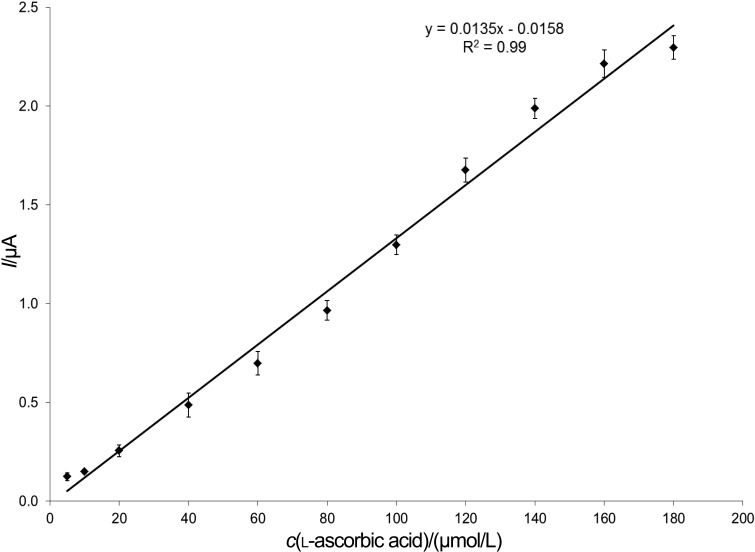
The biosensor was calibrated, and the limit of detection (3 µmol/L) and linear measuring range (5–150 µmol/L) for l-ascorbic acid were determined (Fig. 4). Our results are in good accordance with the results published earlier.
Fig. 4.
The calibration curve of the AAO-based biosensor, defined as the difference between the signals obtained using the BSA and the enzyme electrode (working conditions: 200 mmol/L of sodium acetate buffer with 100 mmol/L of KCl, pH=4.65, and 2 mmol/L of (HPO3)n; flow rate of 2.0 mL/min; polarization potential of 450 mV)
An amperometric sensor based on AAO immobilized on egg shell, with a limit of detection (LOD) of 10 µmol/L, and measuring range of 10–400 µmol/L (R2=0.99) was reported (6). A carbon nanotube-based amperometric sensor with LOD=0.7 µmol/L and measuring range of 1 µmol/L to 18 mmol/L was also developed (R2=0.99) (10). A vitamin C biosensor with AAO immobilized in a polystyrene membrane with LOD=2–4 µmol/L and measuring range of 5–400 µmol/L (R2=0.99) was also elaborated (5). Another amperometric sensor with AAO immobilized on graphite electrode modified with gold nanoparticles (11) was able to determine l-ascorbic acid in the concentration range between 2 µmol/L and 3.3 mmol/L (LOD=1.5 µmol/L, R2=0.99). AAO was also immobilized on poly(3,4-ethylenedioxythiophene)/multi-walled carbon nanotube composite films. l-Ascorbic acid was determined amperometrically (LOD=15 µmol/L, linear measuring range of 10–400 µmol/L, R2=0.99) (12). AAO immobilization was also carried out in the biocompatible conducting poly(3,4-ethylenedioxythiophene)-lauroylsarcosinate (PEDOT-SL) film. l-Ascorbic acid was measured amperometrically (LOD= 0.464 µmol/L with linear measuring range of 2 µmol/L to 14 mmol/L, R2=0.99) (13). Compared to the results found in the literature, it can be stated that the sensor presented herein operates within similar detection limit.
The advantages of our system are the high sample frequency (30 samples per hour), good reusability (approx. 200 samples can be measured with one enzyme electrode without significant signal decrease) and simple sample preparation. The electrodes were stored in refrigerator at 4 °C.
Method validation with standard addition
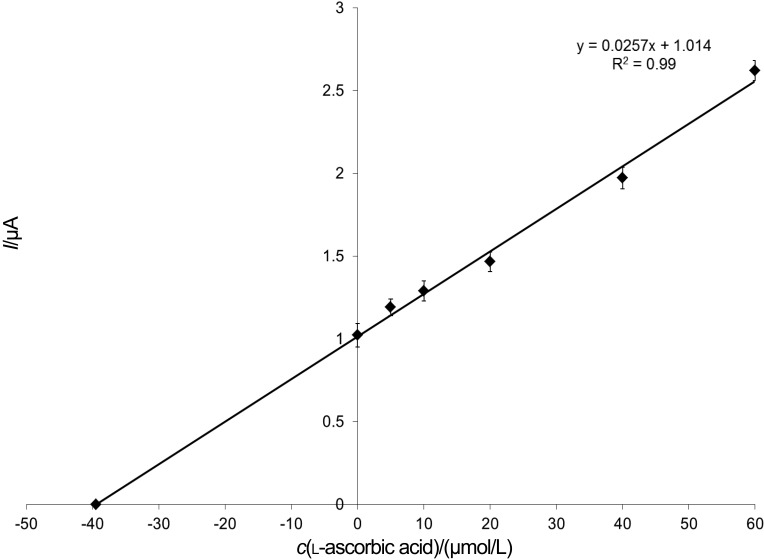
The vitamin C content of an effervescent tablet (80 mg per tablet) was examined with spiking method. The tablet was dissolved in buffer solution (one tablet in 100 mL) and 20, 30 or 40 µmol/L of ascorbic acid standard solutions were added. The solution was then degassed and diluted 50 times. The concentration determined with the biosensor ((73.0±3.7) mg per tablet) correlated significantly with the data provided by the producer (Fig. 5). These results were in good accordance with the vitamin C content written on the package of the product, and also with the HPLC reference measurements.
Fig. 5.
Validation with standard addition method (working conditions: 200 mmol/L of sodium acetate buffer with 100 mmol/L of KCl, pH=4.65, and 2 mmol/L of (HPO3)n; flow rate of 2.0 mL/min; polarization potential of 450 mV)
Vitamin C content in fruit juices and effervescent tablets
Various fruit juices and dissolved vitamin C effervescent tablets were examined with the novel biosensor. There was no need for tedious sample preparation, as the beverages were only centrifuged (12 000×g, 10 min, 4 °C). The juices were diluted with respect to their probable vitamin C content (5–40× dilutions with 200 mmol/L of sodium acetate buffer with 100 mmol/L of KCl, pH=4.65, and 2 mmol/L of metaphosphoric acid). Approximately 15 different samples were measured using the biosensor. Their l-ascorbic acid content was between 10 and 400 mg/L.
HPLC reference measurements
The investigated biosensor was also validated with HPLC reference measurements determining the vitamin C content of the same samples as described in Materials and Methods section.
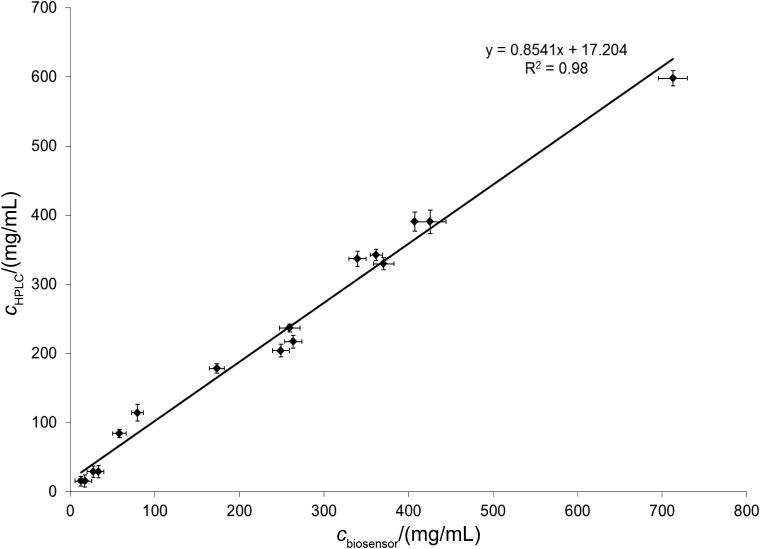
The results (Fig. 6) show that there is a satisfactory correlation (R2=0.98) between the two methods, confirming the suitability of the novel biosensor for l-ascorbic acid determination in fruit juice samples and effervescent tablets. Fig. 6 demonstrates that the biosensor method detailed above is accurate and reliable.
Fig. 6.
Comparison of the l-ascorbic acid content of fruit juices and effervescent tablets measured with the biosensor with the l-ascorbic acid content of the same products measured with HPLC
Conclusions
The successful elaboration of a novel amperometric biosensor for the measurement of l-ascorbic acid in fruit juices and effervescent tablets based on ascorbate oxidase enzyme-modified screen-printed carbon electrodes (SPCE) was presented in this paper. The biosensor worked in a flow injection analysis (FIA) system. The ascorbate oxidase enzyme (AAO) from Cucurbita sp., EC 1.10.3.3, was immobilized on an SPCE. The enzyme reduced the l-ascorbic acid concentration, resulting in an electrochemical signal decrease compared to the measurements carried out on a SPCE without ascorbate oxidase. For this reason, the l-ascorbic acid concentration of the standards and samples was calculated from two measurements. The samples were measured with a bovine serum albumin (BSA) electrode (0.021 mg of BSA was immobilized on the electrode with poly(ethylene glycol) (400) diglycidyl ether (PEGDGE)), and an AAO electrode (10 U of AAO were immobilized on the electrode with PEGDGE). Measurements were carried out at polarization potential of 450 mV and flow rate of 2 mL/min using 200 mmol/L of sodium acetate buffer with 100 mmol/L of KCl (pH=4.65) and 2 mmol/L of metaphosphoric acid. The limit of detection of l-ascorbic acid was 3 µmol/L, and the linear measuring range was between 5 and 150 µmol/L. Fruit juices and vitamin C effervescent tablets were measured with the biosensor. The results were compared with HPLC measurements, and good correlation was found between them. It can be concluded that the developed sensor can be applied for monitoring the l-ascorbic acid content of various products.
Acknowledgements
Financial support from TÁMOP-4.2.2.A-11/1/KONV-2012-0008 and AGR_PIAC-13-1-2013-0084 is greatly acknowledged. Special thanks to Péter Forgó for the HPLC measurements.
References
- 1.Kannoujia DK, Kumar S, Nahar P. Covalent immobilization of ascorbate oxidase onto polycarbonate strip for l-ascorbic acid detection. J Biosci Bioeng. 2012;114:402–4. 10.1016/j.jbiosc.2012.05.019 [DOI] [PubMed] [Google Scholar]
- 2.Mello LD, Kubota LT. Biosensors as a tool for the antioxidant status evaluation. Talanta. 2007;72:335–48. 10.1016/j.talanta.2006.11.041 [DOI] [PubMed] [Google Scholar]
- 3.Al-Madhoun AS, Sanmartin M, Kanellis AK. Expression of ascorbate oxidase isoenzymes in cucurbits and during development and ripening of melon fruit. Postharvest Biol Technol. 2003;27:137–46. 10.1016/S0925-5214(02)00090-X [DOI] [Google Scholar]
- 4.Akyilmaz E, Dinçkaya E. A new enzyme electrode based on ascorbate oxidase immobilized in gelatin for specific determination of l-ascorbic acid. Talanta. 1999;50:87–93. 10.1016/S0039-9140(99)00107-1 [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Watanabe H, Uchiyama S. Amperometric l-ascorbic acid biosensors equipped with enzyme micelle membrane. Talanta. 2008;74:1681–5. 10.1016/j.talanta.2007.09.008 [DOI] [PubMed] [Google Scholar]
- 6.Chauhan N, Dahiya T. Priyanka, Pundir CS. Fabrication of an amperometric ascorbate biosensor using egg shell membrane bound Lagenaria siceraria fruit ascorbate oxidase. J Mol Catal, B Enzym. 2010;67:66–71. 10.1016/j.molcatb.2010.07.007 [DOI] [Google Scholar]
- 7.Fernandes JCB, Kubota LT, de Oliveira Neto G. Potentiometric biosensor for l-ascorbic acid based on ascorbate oxidase of natural source immobilized on ethylene-vinylacetate membrane. Anal Chim Acta. 1999;385:3–12. 10.1016/S0003-2670(98)00327-4 [DOI] [Google Scholar]
- 8.Vig A, Igloi A, Adanyi N, Gyemant G, Csutoras C, Kiss A. Development and characterization of a FIA system for selective assay of l-ascorbic acid in food samples. Bioprocess Biosyst Eng. 2010;33:947–52. 10.1007/s00449-010-0418-6 [DOI] [PubMed] [Google Scholar]
- 9.Renedo OD, Alonso-Lomillo MA, Martínez MJA. Recent developments in the field of screen-printed electrodes and their related applications. Talanta. 2007;73:202–19. 10.1016/j.talanta.2007.03.050 [DOI] [PubMed] [Google Scholar]
- 10.Liu M, Wen Y, Li D, Yue R, Xu J, He H. A stable sandwich-type amperometric biosensor based on poly(3,4-ethylenedioxythiophene)–single walled carbon nanotubes/ascorbate oxidase/nafion films for detection of l-ascorbic acid. Sens Actuators B Chem. 2011;159:277–85. 10.1016/j.snb.2011.07.005 [DOI] [PubMed] [Google Scholar]
- 11.Dodevska T, Horozova E, Dimcheva N. Electrochemical behavior of ascorbate oxidase immobilized on graphite electrode modified with Au-nanoparticles. Mater Sci Eng B. 2013;178:1497–502. 10.1016/j.mseb.2013.08.012 [DOI] [Google Scholar]
- 12.Liu M, Wen Y, Xu J, He H, Li D, Yue R, et al. An amperometric biosensor based on ascorbate oxidase immobilized in poly(3,4-ethylenedioxythiophene)/multi-walled carbon nanotubes composite films for the determination of l-ascorbic acid. Anal Sci. 2011;27:477–82. 10.2116/analsci.27.477 [DOI] [PubMed] [Google Scholar]
- 13.Wen Y, Xu J, Liu M, Li D, Lu L, Yue R, et al. A vitamin C electrochemical biosensor based on one-step immobilization of ascorbate oxidase in the biocompatible conducting poly(3,4-ethylenedioxythiophene)-lauroylsarcosinate film for agricultural application in crops. J Electroanal Chem. 2012;674:71–82. 10.1016/j.jelechem.2012.03.021 [DOI] [Google Scholar]