Abstract
All cells possess signaling pathways designed to trigger antiviral responses, notably characterized by type I interferon (IFN) production, upon recognition of invading viruses. Especially, host sensors recognize viral nucleic acids. Nonetheless, virtually all viruses have evolved potent strategies that preclude host responses within the infected cells. The plasmacytoid dendritic cell (pDC) is an immune cell type known as a robust type I IFN producer in response to viral infection. Evidence suggests that such functionality of the pDCs participates in viral clearance. Nonetheless, their contribution, which is likely complex and varies depending on the pathogen, is still enigmatic for many viruses. pDCs are not permissive to most viral infections, and consistently, recent examples suggest that pDCs respond to immunostimulatory viral RNA transferred via noninfectious and/or noncanonical viral/cellular carriers. Therefore, the pDC response likely bypasses innate signaling blockages induced by virus within infected cells. Importantly, the requirement for cell-cell contact is increasingly recognized as a hallmark of the pDC-mediated antiviral state, triggered by evolutionarily divergent RNA viruses.
INTRODUCTION
The innate immune response represents the first line of defense against many pathogens. This response is initiated by the recognition of pathogen-associated molecular patterns (PAMPs) by cellular pathogen recognition receptors (PRRs), including Toll-like receptors (TLRs). This leads to the production of antiviral molecules, including interferons (IFNs), a broad range of interferon-stimulated genes (ISGs), and inflammatory cytokines. This first line of host response suppresses viral spread and jump-starts the adaptive immune response.
Dendritic cells (DCs) serve as unique immune sentinels, surveying tissues, sensing infection and inflammation, sampling potential antigens, integrating these peripheral cues, and instructing both the innate and the adaptive immune system accordingly. Through this array of specialized functions, DCs orchestrate powerful pathogen-directed immunity and are pivotal in the regulation of viral pathogenesis. Different DC subsets respond in unique and specialized fashions to orchestrate antiviral responses. Among these, plasmacytoid dendritic cells (pDCs) are key players in the early antiviral responses, notably by their ability to produce a large amount of type I IFN (IFN-α and IFN-β) (i.e., 1,000-fold more than other cell types) and type III IFN (IFN-λ/interleukin-28 [IL-28]/IL-29) (reviewed in reference 1). Their response is rapid and triggered mainly by the endosomal sensors TLR7 and TLR9, which recognize viral nucleic acids (RNA and DNA, respectively).
The type I IFN response induced by pDCs is thought to be a key part of their role in the resolution of viral infections (1), especially at the acute phase. Direct evidence is still limited in human studies; nevertheless, an association between the resolution of viral infections and pDC functionality has been reported for certain viruses. For example, pDCs from elite controllers, a subset of human immunodeficiency virus type 1 (HIV-1)-infected patients who sustain undetectable viral loads in the absence of therapy, were found to induce notably greater production of IFN-α than pDCs from viremic patients (2). Similarly, a study conducted on dengue virus (DENV)-infected patients showed that the number of circulating pDCs and their attendant IFN responses were inversely associated with viral load and disease severity (3). Studies using mouse models also provide evidence for the role of pDCs in the clearance of viral infections (1). For example, the depletion of pDCs revealed that they are central for early IFN-α production in response to several systemic viral infections, as first reported for mouse hepatitis virus (MHV) (4) and later for, e.g., lymphocytic choriomeningitis virus (LCMV), respiratory syncytial virus (RSV), and herpes simplex virus 1/2 (HSV-1/2) (1, 5–8). Importantly, pDCs promoted virus control and host survival in some of these models (5, 6, 8).
This Gem highlights the current working models for the activation of an antiviral state by pDCs via cell-cell contacts with infected cells. We also discuss how the pDC response contributes to the control of viral infections, likely, at least in part, via their ability to produce large amounts of IFN-α.
pDC ACTIVATION BY CELL-CELL CONTACT WITH VIRUS-INFECTED CELLS
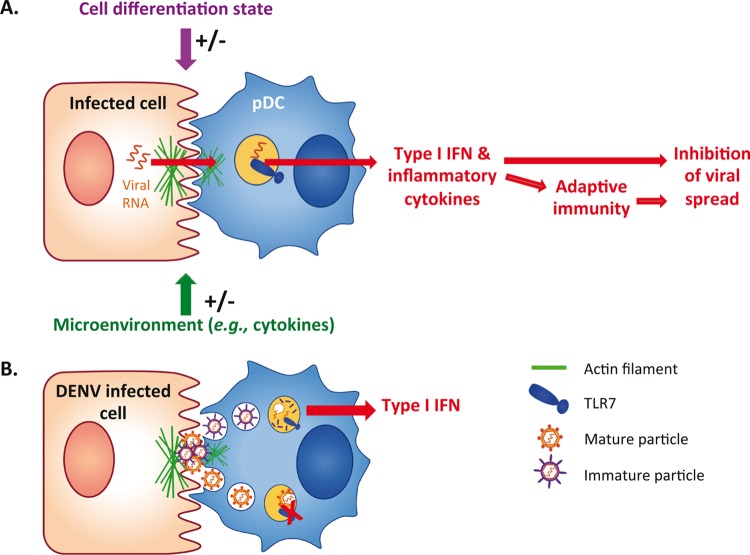
Recent studies revealed that pDCs sense viral infections when in physical contact with infected cells (reviewed in reference 9). This previously unsuspected feature of innate sensing is increasingly recognized as a hallmark of the pDC-mediated antiviral state, triggered by evolutionarily distant RNA viruses (i.e., Flaviviridae, Picornaviridae, Arenaviridae, and Retroviridae) and likely DNA viruses as well (9). Recently, we further revealed that key elements polarize at the contact point between pDCs and cells infected by DENV (i.e., surface glycoproteins and the actin cytoskeleton) (10) (Fig. 1). In this viral model, the actin network may act as a structural platform for the concentration of PAMP carriers at the contact and thereby for their efficient transmission to the pDCs, leading to TLR7-mediated activation of the IFN response (10). One might speculate that polarization of cellular components at the contacts is a primary feature of the pDC functionality to ensure an efficient antiviral response.
FIG 1.
Model of pDC activation by cell-cell contact with infected cells. (A) pDCs sense cells infected by evolutionarily distant viruses via physical contact with infected cells, thus representing a primary feature of pDC activation. Immunostimulatory viral RNAs are transmitted via various carriers, including exosome and immature virus, leading to robust production of type I IFN and other cytokines, likely modulated by the microenvironment and pDC differentiation state. (B) In the context of DENV-infected cells, pDCs are activated via immature particles, and the actin network acts as a structural platform at the cell-cell contact for efficient transmission of the PAMP carriers.
The activation of pDCs often involves clathrin-mediated endocytosis of secreted PAMPs, reflecting the involvement of a vesicle-mediated cell-to-cell transfer of the viral RNA to the pDCs (9). This cell type is known to be resistant to most viral infections, likely because they constitutively express interferon regulatory factor 7 (IRF7), a transcription factor downstream of TLRs, and rapidly produce large amounts of IFNs (1, 9). In accordance, recent reports highlighted that productive infection is dispensable for pDC activation (9). Importantly, the immunostimulatory RNA can be carried from infected cells to the pDCs by noncanonical and/or noninfectious viral particles. Notably, for evolutionarily distant viruses, including hepatitis C virus (HCV), LCMV, and hepatitis A virus (HAV), infected cells secrete exosome-like vesicles containing viral elements that efficiently trigger pDC IFN production (11–13). HCV-infected cells produce these vesicles via the cellular exosomal pathway and without the requirement of viral structural proteins (12). This process is presumably similar for LCMV (13). While HAV was originally classified as a nonenveloped virus, recent work demonstrated that viral capsids containing HAV genomes can acquire cell membranes via the exosomal pathway and are thus identified as an enveloped form of HAV (eHAV) (14). This form of viral particles may have evolved to evade the humoral immune response by preventing the recognition of viral structural components by neutralizing antibodies (14). Nonetheless, on the downside, this vesicular form of HAV is also an efficient PAMP carrier for the activation of the pDC response (11). For these various viruses, the exosomal transfer of the immunostimulatory RNA to pDC commonly requires cell-cell contact with infected cells (11–13). Importantly, we recently illustrated that the cell-cell sensing of infected cells by pDC might involve other types of PAMP carriers. The activation of pDCs by DENV-infected cells is mediated by noninfectious viral particles that nevertheless contain the envelope viral proteins at their surface (10). For many viruses, it is known that infected cells release a large proportion of viral particles that expose uncleaved envelope viral proteins and that are thus noninfectious (15). Especially, DENV-infected cells release vast amounts of noninfectious particles, called immature particles, that can bind to the cell surface but are not competent for membrane fusion. Importantly, DENV-infected cells impaired in the production of immature particles are poor inducers of the pDC response, suggesting that immature particles are the carriers of PAMPs to pDCs (10). Since PAMP recognition by TLR7 is known to occur in the endolysosome compartment, it is tempting to speculate that the immature virions (i.e., those incompetent for membrane fusion) are likely retained in this TLR7-containing endolysosome compartment, resulting in a potent TLR7-mediated IFN response by the pDCs, as opposed to mature particles, which likely escape TLR7 recognition by membrane fusion (10) (Fig. 1B).
Interestingly, these examples revealed that aspects of the molecular basis of the transfer of PAMPs to pDCs differ from aspects of the known transmission of viral infectivity. The identification of the underlying mechanisms, including attachment to the cell surface of the PAMP carriers, is thus of great interest. Indirect evidence suggested that pDC activation by eHAV involves phosphatidylserine (PS), a lipid present in the membranes of exosomes, which might be recognized by a PS receptor expressed by the pDCs (11). In the context of HIV, pDC activation involves the viral envelope glycoproteins (16), in accordance with the modulation of pDC activation by its receptor, CD4 (17). These examples illustrate the existence of different modes of PAMP carrier transmission from infected cells to pDCs. Nonetheless, as the requirement for cell-cell contact with infected cells is a widespread feature of pDC activation observed for evolutionarily distant viruses and in different species (i.e., humans, mice, and pigs [9]), one might speculate that the establishment of these contacts is a primary functionality of the pDCs that likely involve similar cellular machinery and/or surface molecules for the sensing of cells infected by different viruses.
IN VIVO FUNCTIONS OF pDC, A MASTER IFN-PRODUCING CELL TYPE
The regulatory functions of pDCs are likely varied and complex, notably as a reflection of the myriad of immune responses controlled by their main effector, type I IFN, including, e.g., the activation of T cells and natural killer (NK) cells and maturation of antigen-presenting cells (1). For example, studies using a mouse model genetically modified for specific depletion of pDCs revealed that pDCs regulate the anti-LCMV helper T cell responses, which critically depend on the regulation of CD4+ T cell function by type I IFNs (6). pDCs also respond in an additional fashion(s) to viral infections, including through the secretion of other proinflammatory cytokines, e.g., tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and IL-8. Nonetheless, the importance of those cytokine secretions relative to the production of type I IFNs on the pDC-mediated modulation of the immunity is still elusive and likely depends on the type and stage of infection.
Furthermore, recent evidence illustrates the dynamics of pDC responsiveness during the course of viral infection. For example, in a macaque model of simian immunodeficiency virus (SIV) infection, IFN-α production is temporally related to pDC activation, with IFN-α induction detected only in pDCs, indicating that pDCs are the main IFN-α producer at early stages of SIV infection (18). Nonetheless, at the acute- to chronic-phase transition, the pDC response vanishes, likely due to the renewal of pDC precursors with limited IFN production ability (18). Additionally, one might speculate that the microenvironment also modulates pDC responsiveness in the course of viral infections (Fig. 1A).
FUTURE DIRECTIONS
The concept that type I IFNs are antiviral per se has been challenged, as recent evidence implied that prolonged type I IFN secretion can suppress the immune system, therefore promoting viral persistence (19, 20). For instance, in a mouse model of chronic LCMV infection, long-term type I IFN-mediated immunosuppression was found to be associated with the induction of inhibitory signals that switch the antiviral regulation by type I IFNs during the acute phase of infection to a proviral function at the chronic stage (19, 20). Thus, the regulatory functions of the pDCs in viral infection, notably via the ability to produce type I IFNs, are likely highly complex.
Systemic and massive production of IFNs and inflammatory cytokines is known to be detrimental to the host in different contexts, as they correlate with many systemic homeostatic dysfunctions, including tissue damage and increased vascular permeability (e.g., via TNF-α in the context of DENV) (21). Importantly, recent in vitro studies uncovered that pDCs produce large amounts of type I IFNs only when in physical contact with cells infected by different viruses (9). Therefore, one might speculate that cell-cell contact as a primary requirement for pDC activation evolved in favor of the host's fitness to locally respond at the infected sites and thereby to thwart the otherwise harmful systemic IFN and inflammatory responses.
Recent works uncovered a compartmentalization in different organs of pDC subsets with varied abilities to produce IFNs (7, 22). Furthermore, pDC precursors mobilized in the course of viral infection can be less competent for IFN production than circulating pDCs (18). Therefore, the control of viral infections by pDC IFN responses may be modulated by the local environment and/or the route of infection. In this regard, in a mouse model of HSV-1 infection, pDCs contribute primarily to the induction of the type I IFN response and host survival in systemic infections, compared to local vaginal infections (7), yet the determinant(s) for such a difference is still elusive. It is thus conceivable that the response of pDCs to contact with virally infected cells might be greatly regulated by the local microenvironment, the route of infection, and/or the pDC differentiation/maturation state.
This newly uncovered regulation of the innate immunity via cell-cell contact might feature similarities with other types of cell-cell communications, including virological and immunological synapses (IS) that serve as platforms for viral transmission and activation of adaptive immunity, respectively. Interestingly, the first insights on the structure present at the contacts between pDCs and virus-infected cells highlighted a local accumulation of the actin network (10), a hallmark of previously described synapses (23). As the pDCs can establish IS in the context of antigen presentation (24), one might hypothesize that the structural components involved in this synapse can also be mobilized and/or reused by the pDCs to establish contact and sense virus-infected cells. Further work will decipher how pDCs establish functional contacts leading to potent innate responses, thus potentially representing an “innate immunological synapse.”
ACKNOWLEDGMENTS
We apologize to the individuals whose work was not specifically cited in this article due to length limitations. We are grateful to Marc Dalod (CIML, Marseille, France), Eliane Meurs (Institut Pasteur, Paris, France), and Nathalie Bendriss-Vermare (CRCL, Lyon, France) for critical reviews of the manuscript and for helpful discussions.
Funding Statement
B.W.'s postdoc fellowship is sponsored by EMBO and S.A.'s Ph.D. fellowship is sponsored by the French Ministry and by La Ligue contre le Cancer.
REFERENCES
- 1.Swiecki M, Colonna M. 2015. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol 15:471–485. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machmach K, Leal M, Gras C, Viciana P, Genebat M, Franco E, Boufassa F, Lambotte O, Herbeuval JP, Ruiz-Mateos E. 2012. Plasmacytoid dendritic cells reduce HIV production in elite controllers. J Virol 86:4245–4252. doi: 10.1128/JVI.07114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pichyangkul S, Endy TP, Kalayanarooj S, Nisalak A, Yongvanitchit K, Green S, Rothman AL, Ennis FA, Libraty DH. 2003. A blunted blood plasmacytoid dendritic cell response to an acute systemic viral infection is associated with increased disease severity. J Immunol 171:5571–5578. doi: 10.4049/jimmunol.171.10.5571. [DOI] [PubMed] [Google Scholar]
- 4.Dalod M, Salazar-Mather TP, Malmgaard L, Lewis C, Asselin-Paturel C, Briere F, Trinchieri G, Biron CA. 2002. Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J Exp Med 195:517–528. doi: 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swiecki M, Gilfillan S, Vermi W, Wang YM, Colonna M. 2010. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity 33:955–966. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cervantes-Barragan L, Lewis KL, Firner S, Thiel V, Hugues S, Reith W, Ludewig B, Reizis B. 2012. Plasmacytoid dendritic cells control T-cell response to chronic viral infection. Proc Natl Acad Sci U S A 109:3012–3017. doi: 10.1073/pnas.1117359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swiecki M, Wang YM, Gilfillan S, Colonna M. 2013. Plasmacytoid dendritic cells contribute to systemic but not local antiviral responses to HSV infections. PLoS Pathog 9:e1003728. doi: 10.1371/journal.ppat.1003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smit JJ, Rudd BD, Lukacs NW. 2006. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J Exp Med 203:1153–1159. doi: 10.1084/jem.20052359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Assil S, Webster B, Dreux M. 2015. Regulation of the host antiviral state by intercellular communication. Viruses 7:4707–4733. doi: 10.3390/v7082840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Décembre E, Assil S, Hillaire ML, Dejnirattisai W, Mongkolsapaya J, Screaton GR, Davidson AD, Dreux M. 2014. Sensing of immature particles produced by dengue virus infected cells induces an antiviral response by plasmacytoid dendritic cells. PLoS Pathog 10:e1004434. doi: 10.1371/journal.ppat.1004434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng Z, Li Y, McKnight KL, Hensley L, Lanford RE, Walker CM, Lemon SM. 2015. Human pDCs preferentially sense enveloped hepatitis A virions. J Clin Invest 125:169–176. doi: 10.1172/JCI77527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dreux M, Garaigorta U, Boyd B, Decembre E, Chung J, Whitten-Bauer C, Wieland S, Chisari FV. 2012. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe 12:558–570. doi: 10.1016/j.chom.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wieland SF, Takahashi K, Boyd B, Whitten-Bauer C, Ngo N, de la Torre JC, Chisari FV. 2014. Human plasmacytoid dendritic cells sense lymphocytic choriomeningitis virus-infected cells in vitro. J Virol 88:752–757. doi: 10.1128/JVI.01714-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Z, Hensley L, McKnight KL, Hu F, Madden V, Ping L, Jeong SH, Walker C, Lanford RE, Lemon SM. 2013. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature 496:367–371. doi: 10.1038/nature12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasquato A, Ramos da Palma J, Galan C, Seidah NG, Kunz S. 2013. Viral envelope glycoprotein processing by proprotein convertases. Antiviral Res 99:49–60. doi: 10.1016/j.antiviral.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Lepelley A, Louis S, Sourisseau M, Law HK, Pothlichet J, Schilte C, Chaperot L, Plumas J, Randall RE, Si-Tahar M, Mammano F, Albert ML, Schwartz O. 2011. Innate sensing of HIV-infected cells. PLoS Pathog 7:e1001284. doi: 10.1371/journal.ppat.1001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Brien M, Manches O, Wilen C, Gopal R, Huq R, Wu V, Sunseri N, Bhardwaj N. 2016. CD4 receptor is a key determinant of divergent HIV-1 sensing by plasmacytoid dendritic cells. PLoS Pathog 12:e1005553. doi: 10.1371/journal.ppat.1005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruel T, Dupuy S, Demoulins T, Rogez-Kreuz C, Dutrieux J, Corneau A, Cosma A, Cheynier R, Dereuddre-Bosquet N, Le Grand R, Vaslin B. 2014. Plasmacytoid dendritic cell dynamics tune interferon-alfa production in SIV-infected cynomolgus macaques. PLoS Pathog 10:e1003915. doi: 10.1371/journal.ppat.1003915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, Schreiber RD, de la Torre JC, Oldstone MB. 2013. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 340:207–211. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks DG. 2013. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 340:202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa VV, Fagundes CT, Souza DG, Teixeira MM. 2013. Inflammatory and innate immune responses in dengue infection: protection versus disease induction. Am J Pathol 182:1950–1961. doi: 10.1016/j.ajpath.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 22.Zucchini N, Bessou G, Robbins SH, Chasson L, Raper A, Crocker PR, Dalod M. 2008. Individual plasmacytoid dendritic cells are major contributors to the production of multiple innate cytokines in an organ-specific manner during viral infection. Int Immunol 20:45–56. doi: 10.1093/intimm/dxm119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritter AT, Angus KL, Griffiths GM. 2013. The role of the cytoskeleton at the immunological synapse. Immunol Rev 256:107–117. doi: 10.1111/imr.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mittelbrunn M, Martinez del Hoyo G, Lopez-Bravo M, Martin-Cofreces NB, Scholer A, Hugues S, Fetler L, Amigorena S, Ardavin C, Sanchez-Madrid F. 2009. Imaging of plasmacytoid dendritic cell interactions with T cells. Blood 113:75–84. doi: 10.1182/blood-2008-02-139865. [DOI] [PubMed] [Google Scholar]