ABSTRACT
Acute human immunodeficiency virus (HIV) infection represents a period of intense immune perturbation and activation of the host immune system. Study of the eclipse and viral expansion phases of infection is difficult in humans, but studies in nonprogressive and progressive nonhuman primate (NHP) infection models can provide significant insight into critical events occurring during this time. Cytokines, chemokines, and other soluble immune factors were measured in longitudinal samples from rhesus macaques infected with either SIVmac251 (progressive infection) or SIVmac239Δnef (attenuated/nonprogressive infection) and from African green monkeys infected with SIVsab9315BR (nonpathogenic infection). Levels of acute-phase peak viral replication were highest in SIVmac251 infection but correlated positively with viremia at 3 months postinfection in all three infection models. SIVmac251 infection was associated with stronger corresponding acute-phase cytokine/chemokine responses than the nonprogressive infections. The production of interleukin 15 (IL-15), IL-18, gamma interferon (IFN-γ), granulocyte colony-stimulating factor (G-CSF), monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1β (MIP-1β), and serum amyloid A protein (SAA) during acute SIVmac251 infection, but not during SIVmac239Δnef or SIVsab9315BR infection, correlated positively with chronic viremia at 3 months postinfection. Acute-phase production of MCP-1 correlated with viremia at 3 months postinfection in both nonprogressive infections. Finally, a positive correlation between the acute-phase area under the curve (AUC) for IL-6 and soluble CD40 ligand (sCD40L) and chronic viremia was observed only for the nonprogressive infection models. While we observed dynamic acute inflammatory immune responses in both progressive and nonprogressive SIV infections, the responses in the nonprogressive infections were not only lower in magnitude but also qualitatively different biomarkers of disease progression.
IMPORTANCE NHP models of HIV infection constitute a powerful tool with which to study viral pathogenesis in order to gain critical information for a better understanding of HIV infection in humans. Here we studied progressive and nonprogressive simian immunodeficiency virus (SIV) infection models in both natural and nonnatural host NHP species. Regardless of the pathogenicity of the virus infection and regardless of the NHP species studied, the magnitude of viremia, as measured by area under the curve, during the first 4 weeks of infection correlated positively with viremia in chronic infection. The magnitude of cytokine and chemokine responses during primary infection also correlated positively with both acute-phase and chronic viremia. However, the pattern and levels of specific cytokines and chemokines produced differed between nonprogressive and progressive SIV infection models. The qualitative differences in the early immune response in pathogenic and nonpathogenic infections identified here may be important determinants of the subsequent disease course.
INTRODUCTION
Acute human immunodeficiency virus (HIV) infection lasts only for a few weeks, but the dynamics of virus-host interactions during this period can have lasting consequences through the course of infection (1, 2). Understanding the early immune responses, including the identity, kinetics, and magnitude of production of soluble mediators of inflammation, may shed light on how these early responses can impact the subsequent course of the infection.
Although the identification of infected humans during the eclipse period of HIV infection is extremely difficult (3), a study conducted on plasma donor panels effectively described the kinetics of systemic cytokine immune responses during the days to weeks following HIV transmission (4). Rapid elevations in levels of cytokines, including alpha interferon (IFN-α) and interleukin 15 (IL-15), were reported prior to peak viremia in acute infection, while slightly slower, more-sustained elevations were observed for other analytes, including IL-6, IL-8, IL-18, and IFN-γ, along with a later-peaking increase in IL-10. There was also a biphasic increase in the levels of acute-phase proteins, including serum amyloid A protein (SAA), indicating an early response to infection followed by further acute-phase protein induction coinciding with the production of the proinflammatory cytokines tumor necrosis factor alpha (TNF-α), IL-6, and IL-22 (5). While these studies provide valuable insight into the chronology of immune induction during acute HIV infection, it is not known how these cytokine responses relate to the subsequent disease course, since long-term virologic and clinical follow up was not available for these individuals.
The potential functional effects of cytokines on the immune system and HIV replication are complex. Many cytokines are pleiotropic and can even have opposing effects, simultaneously promoting the suppression of virus replication and enabling virus replication by immune activation (e.g., IL-15 or TNF-α). Only very few cytokine responses are thought to have an overall beneficial effect for the HIV-infected individual (reviewed in reference 6). Nonhuman primate (NHP) models of HIV infection enable study of the relationship between viral replication and cytokine responses in controlled experimental settings (7). These models include simian immunodeficiency virus (SIV) infection of Asian macaques, which are nonnatural hosts of SIV, such as SIVmac infection of Indian rhesus macaques (RM), and infection of species that are natural hosts of endemic SIVs, such as SIVagm infection of African green monkeys (AGM) (8). The course of experimental SIV infection is variable in RM and depends on the particular virus strain used for infection. Like untreated HIV infection in humans, experimental SIVmac251 infection of RM is highly pathogenic, resulting in a rapid increase in viremia followed by partial containment but ongoing viral replication. This is associated with pathological immune activation and a progressive decline in CD4+ T cell populations, and eventually there is a complete loss of viral containment with the development of clinically apparent immunodeficiency, manifested by AIDS-defining conditions including opportunistic infections, wasting, and death (9). In contrast, infections of RM with live attenuated viruses, such as SIVmac239Δnef, are generally much less pathogenic (10). Viremia is more efficiently contained, resulting in a very low chronic-phase viremia that is maintained either indefinitely or for prolonged periods. In contrast to SIV infections of nonnatural hosts, SIV infections of natural NHP hosts, such as SIVagm infection of AGM, do not typically result in serious disease (8). Although chronic-phase viral replication and viremia are only partially contained, the loss of CD4+ T cells is transient, immune activation is limited to primary infection, and progression to AIDS does not occur.
Progressive SIV infection in RM results in increased expression of markers for cell activation and proliferation, along with sustained production of cytokines, including type I IFN (11, 12). Heightened T cell turnover occurs in response to viral infection and, in the presence of persistent proinflammatory cytokines, results in loss of memory T cells (13, 14). A number of similarities between nonprogressive and progressive SIV infections have been identified, including an initial type I IFN response accompanying the increase in acute-phase viremia (14). However, natural hosts are able to downmodulate the virus-associated immune activation, resolving type I IFN upregulation. Natural hosts are also able to maintain functional mucosal immunity, allowing for the preservation of T regulatory and Th17 cells, the maintenance of gut mucosal integrity with no increased microbial translocation, and limited systemic immune activation in chronic infection (15). A comparison of transcriptional profiles of nonprogressive and progressive SIV infections has revealed differences in transcriptional kinetics in lymphoid tissues (16). The gene expression patterns in progressive infection exhibit a shift toward general Th1 immune responses with strong and sustained IFN type I and II responses, loss of T regulatory cells, and loss of control of T cell activation (17–19). Although the magnitudes of systemic immune activation at the time of acute infection are comparable between nonprogressive and progressive SIV infections at the gene expression level, cytokine/chemokine protein levels have not been extensively tested (16).
Although individual cytokine signals in early SIV infection have been measured previously, the ability to measure multiple protein analytes simultaneously and sensitively by cytokine bead multiplex assay has allowed a more comprehensive approach to the study of inflammatory mediators in acute viral infections (4, 20–22). Cytokine multiplex assays have been developed using antibodies against human cytokines that were found to be cross-reactive with NHP analytes. In this study, we investigated the magnitudes of changes in the levels of 25 analytes in plasma following progressive infection of RM with SIVmac251, nonprogressive infection of RM with SIVmac239Δnef, and nonprogressive infection of AGM with SIVsab9315BR. Our analysis reveals differences in acute-phase levels of several cytokines in nonprogressive and progressive SIV infections and shows which patterns of cytokine production in acute infection correlate with chronic-phase viremia.
MATERIALS AND METHODS
Animals and viruses.
A total of 19 sabaeus AGM (Chlorocebus sabaeus) were studied for baseline cytokine/chemokine levels. The animals were either imported from St. Kitts in the Caribbean or were purchased from the New Iberia Research Center (New Iberia, LA). Six of these sabaeus AGM were studied after infection with an equivalent of 143 ng p27 tissue culture supernatant of Molt4(cl8) cells infected with SIVsab9315BR. Cell-free virus was originally isolated from the cerebrospinal fluid (CSF) and homogenized brain and lymph node (LN) tissues of AGM 9315 at the time of necropsy (23). All AGM were maintained in accordance with the guidelines of the Committee on the Care and Use of Laboratory Animals under a NIAID-approved animal study protocol, and all studies and procedures were reviewed and approved by the institutional animal care and use committees (IACUC) of the NIH and Harvard University. The 6 SIVsab9315BR-infected AGM were part of the control group for a study to compare B and T cell depletion during acute infection (24). These animals received intravenous (i.v.) IgG (Gammagard; NIH Nonhuman Primate Reagent Resource) at 50 mg/kg of body weight on the day of infection. The AGM infected with SIVsab9315BR were bled prior to infection and at multiple time points throughout the course of acute and chronic infection.
A total of 74 RM were studied for baseline cytokine and chemokine calculations. Ten RM were infected with SIVmac251 by the i.v. route at a dose equivalent of 0.15 ng SIV p27 Gag per RM. Ten additional RM were infected with SIVmac239Δnef by the i.v. route at a dose equivalent of 50 ng SIV p27 Gag per animal. The infected RM were bled prior to infection and at multiple time points throughout the course of acute and chronic infection. The SIVmac251-infected RM received monoclonal i.v. IgG 1 week before infection (50 mg human immunoglobulin; NIH Nonhuman Primate Reagent Resource), since these animals were part of control groups in experimental studies (J. E. Schmitz, unpublished observations). The SIVmac239Δnef-infected RM did not receive an antibody injection until the end of the observation period described here. All RM were maintained in accordance with the guidance of the Standing Committee on Animals for the Harvard Medical School (HMS) and the Guide for the Care and Use of Laboratory Animals (25). Although the SIVmac239Δnef-infected RM involved in this study were maintained with standard research and veterinary practices, additional aspects of the SIVmac239Δnef experiment that are not pertinent for this report were not included in the protocol and thus were not contemporaneously approved by the HMS IACUC. When the investigators and the IACUC realized this protocol error, the IACUC determined that the unapproved procedures in question in all likelihood would have been approved if they had been described in the protocol.
Cell stimulation.
Peripheral blood mononuclear cells (PBMC) from HIV-negative humans (n = 8), SIV-negative RM (n = 11), and AGM (n = 6) were cultured in RPMI medium with 10% fetal calf serum (FCS) alone or with phytohemagglutinin M (PHA-M; Sigma, Atlanta, GA) at 5 μg/ml, lipopolysaccharide (LPS; Sigma) at 1 ng/ml, phorbol 12-myristate 13-acetate (PMA; Sigma) (10 ng/ml)–ionomycin (calcium ionophore; Sigma) (1 μg/ml), or the imidazoquinoline Toll-like receptor 7 (TLR-7)/TLR-8 agonist CLO-97 (Invitrogen, San Diego, CA) (2.5 μg/ml). The supernatants from unstimulated cells and from cells stimulated with PHA-M, LPS, or PMA-ionomycin were harvested after 24 h of incubation, and CLO-97-stimulated cells were harvested after 18 h. The human samples were collected with informed consent under a protocol approved by the University of California—San Francisco (UCSF) Committee on Human Research.
Determination of plasma virus levels.
Levels of SIVmac251 and SIVmac239Δnef in plasma were determined by a real-time reverse transcription-PCR assay, essentially as described previously (Quantitative Molecular Diagnostic Section, AIDS Vaccine Program, NCI—Frederick, Frederick, MD) (the limit of detection for use in the present studies was 60 RNA copies/ml) (26). Plasma SIVsab9315BR RNA levels in sabaeus AGM were quantified by the UltraSense One-Step Quantitative RT-PCR system (Invitrogen Corp., Carlsbad, CA) as described previously (24).
Cytokine/chemokine analysis.
Twenty-three cytokines/chemokines were measured using the nonhuman primate cytokine Milliplex kit (Millipore, Billerica, MA) according to the manufacturer's instructions. This panel measured IL-1β, IL-1 receptor antagonist (IL-1ra), IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12/23 (p40), IL-13, IL-15, IL-17, IL-18, IFN-γ, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1α (MIP-1α), MIP-1β, TNF-α, transforming growth factor α (TGF-α), soluble CD40 ligand (sCD40L), and vascular endothelial growth factor (VEGF). Analyte detection ranged from 0.64 to 10,000 pg/ml. Plasma or PBMC supernatants were incubated overnight with antibody-coupled beads, followed by incubation with a biotinylated detection antibody and, finally, incubation with phycoerythrin (PE)-conjugated streptavidin. Each sample was assayed in duplicate, and cytokine standards and controls supplied by the manufacturer were run on each plate. Multianalyte profiling was performed using a Luminex-100 system; data were analyzed using Bio-Plex Manager software (Bio-Rad, Hercules, CA). Analytes determined by Bio-Plex to be out of range below the standard were assigned values of half the difference between the lowest standard and zero. Human IFN-α was measured by an enzyme-linked immunosorbent assay (ELISA) (PBL Interferon Source, Piscataway, NJ) detecting 11 of the 14 known types of IFN-α. Levels of SAA were measured using a colorimetric ELISA (Multispecies SAA ELISA kit; Tridelta Development Ltd., Maynooth, County Kildare, Ireland). Diluted plasma samples were assayed in duplicate; concentrations were calculated from the standard curve obtained with a mixture of standards from different species, such as bovine, porcine, canine, feline, and equine species, provided with the kit.
Statistical analysis.
In the validation experiment, supernatants from PBMC that were stimulated in culture were compared for differences in analyte levels by Kruskal-Wallis analysis of variance (ANOVA) with Dunn's multiple-comparison tests. Increased expression of cytokines/chemokines after infection was identified by establishing a threshold of reactivity. To determine this threshold, baseline levels of all analytes in plasma were measured in uninfected animals; the upper 95% confidence limit of the mean analyte value for uninfected animals was determined. This criterion, in addition to a value that was 2-fold the baseline value and greater than 5 pg/ml, was used to establish the threshold of response. In the cytokine/chemokine comparative analysis, only analytes that were elevated in 50% or more of the animals in a group were considered positive for that group. In individual cytokine/chemokine analyses, comparisons were made between analyte and viremia for each animal. Analyte levels were compared between two groups by the Mann-Whitney test or between multiple groups by one-way ANOVA followed by Tukey's honestly significant difference (HSD) test. Linear regression was performed on all animals and cytokines by the Spearman test to compare peak plasma viremia or the area under the curve (AUC) for the first 4 weeks with chronic day 98 or day 100 viremia (grouped as 3 months). Linear regression by the Spearman test was also performed to compare analyte concentrations (measured as the AUC or the peak concentration) over the first 4 weeks with viremia in chronic infection. Throughout the analyses, P values were computed and were then adjusted for false discovery rates (FDR) by use of the Benjamini-Hochberg controlling procedure (27). Statistical significance was indicated by a P value of <0.05 and an FDR of <0.1. The statistical software R/Bioconductor (version 2.15.1 with gdata, gee, ggplot2, grid, lattice, lme4, multtest, npmc, outliers, pwr, and stats) and GraphPad Prism, version 6.0, were utilized for analyses.
RESULTS
Recognition of RM and AGM analytes by cytokine/chemokine assays.
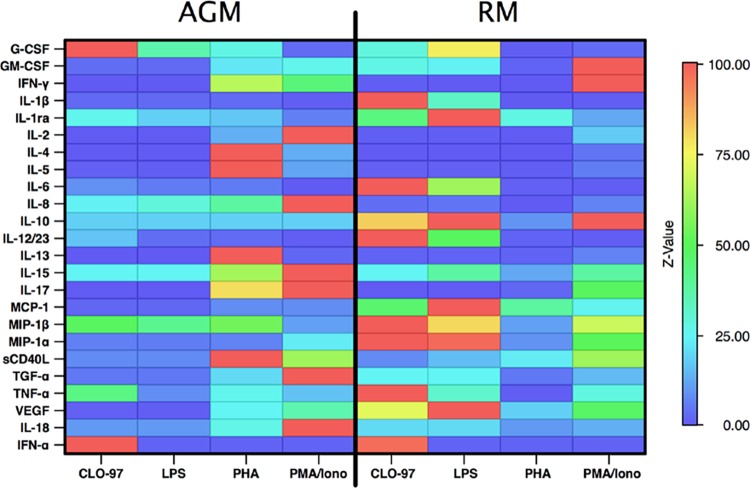
To evaluate the consistency of recognition of each analyte across primate species in the assays, we first validated the assays. Because no gold-standard primate-specific cytokines are currently available, we performed an in-house validation of the assays using supernatants from human and NHP PBMC stimulated in vitro with compounds that elicit a broad spectrum of cytokines/chemokines. We assessed the abilities of the Millipore 23-plex NHP cytokine panel and a human IFN-α ELISA to recognize RM and AGM cytokines and chemokines. Assay validation using supernatants from two PBMC samples from each of seven different primate species stimulated with LPS and PHA-M to signal through TLR-4 and CD3, respectively, had been performed previously by Millipore according to the user guide provided for this assay. The current validation expanded on the prior work and included the additional stimulants PMA-ionomycin and CLO-97 to signal via protein kinase C and TLR-7/8, respectively, to maximize the diversity of cytokines/chemokines produced. The Milliplex NHP panel identified elevations in every analyte in response to at least one of the stimuli employed in the supernatants from human PBMCs; for the vast majority of the analytes, this was also the case for both RM and AGM (Table 1). The heat map shown in Fig. 1 summarizes the responses across the primate species and the stimulants. Although the Luminex assay and the ELISA were able to detect most cytokines and chemokines in the panel in NHP species and humans, in vitro stimulation of PBMC did not lead to detection of a significant increase in the levels of some analytes over those for unstimulated controls in one or both of the NHP species. Measurements of IL-10, IL-15, MCP-1, and TGF-α in supernatants from in vitro-activated AGM PBMC failed to show any significant increase. In RM, levels of all chemokines/cytokines except for IL-15 (Luminex assay) and IFN-α (ELISA) rose significantly after in vitro stimulation of PBMC relative to levels for unstimulated controls, although the differences from baseline levels were not statistically significant, because only two samples were available for testing. Failure to detect elevations in the level of a particular analyte in this experiment may indicate that the analyte was not detected by the assay but could, alternatively, indicate that the analyte was not produced after in vitro PBMC stimulation in the species concerned. Notably, some of the analytes for which elevations were not detected following in vitro stimulation of PBMC were subsequently detected in NHP plasma samples, showing that the assay was able to detect them but suggesting that they were not substantially upregulated after in vitro stimulation; this was observed for MCP-1, IL-10, and IL-15, possibly because the in vitro stimulation conditions selected did not reflect in vivo immune induction. Since the majority of cytokines and chemokines examined were detected in both RM and AGM, we included all analytes in our subsequent analysis. SAA was not evaluated in the validation experiments. To minimize species-related differences in the detection of SAA levels, we used an ELISA in which the data were normalized against a standard mixture containing SAA derived from multiple species.
TABLE 1.
Multiplex kit cross-species reactivity
| Analyte | Median fold change in analyte levels in PBMC exposed to the indicated stimulanta |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human |
AGM |
RM |
||||||||||
| CLO-97 | LPS | PHA | PMA/Iono | CLO-97 | LPS | PHA | PMA/Ionob | CLO-97 | LPS | PHA | PMA/Iono | |
| G-CSF | 2,961c | 5,363 | 26 | 15 | 162 | 58 | 44 | 5 | 46 | 124 | 1 | 5 |
| GM-CSF | 155 | 182 | 77 | 2,673 | 14 | 9 | 90 | 108 | 112 | 98 | 6 | 410 |
| IFN-γ | 100 | 34 | 38 | 4,993 | 7 | 11 | 3,110 | 2,115 | 23 | 12 | 14 | 4,776 |
| IL-1β | 8,856 | 2,910 | 10 | 260 | 73 | 84 | 97 | 14 | 3,637 | 1,172 | 7 | 18 |
| IL-1ra | 106 | 123 | 13 | 31 | 17 | 12 | 11 | 4 | 28 | 65 | 18 | 8 |
| IL-2 | 7 | 7 | 9 | 444 | 11 | 15 | 1,116 | 8,514 | 31 | 40 | 21 | 1,481 |
| IL-4 | 13 | 23 | 225 | 769 | 1 | 2 | 2,266 | 286 | 1 | 1 | 7 | 107 |
| IL-5 | 6 | 7 | 103 | 596 | 2 | 1 | 198 | 24 | 2 | 2 | 1 | 11 |
| IL-6 | 7,610 | 7,751 | 1,124 | 188 | 253 | 157 | 160 | 4 | 3,015 | 1,861 | 28 | 19 |
| IL-8 | 106 | 106 | 86 | 106 | 756 | 925 | 1,205 | 3,199 | 104 | 146 | 9 | 204 |
| IL-10 | 180 | 607 | 48 | 26 | 2 | 2 | 2 | 2 | 9 | 11 | 1 | 11 |
| IL-12/23 | 1,701 | 855 | 9 | 16 | 297 | 57 | 42 | 8 | 1,783 | 867 | 27 | 13 |
| IL-13 | 22 | 26 | 79 | 288 | 1 | 1 | 639 | 12 | 9 | 9 | 9 | 42 |
| IL-15 | 5 | 7 | 3 | 6 | 2 | 2 | 5 | 8 | 2 | 3 | 1 | 3 |
| IL-17 | 0 | 1 | 12 | 20 | 1 | 1 | 497 | 627 | 0 | 0 | 11 | 305 |
| MCP-1 | 12 | 12 | 12 | 12 | 1 | 1 | 5 | 5 | 29 | 63 | 24 | 16 |
| MIP-1β | 390 | 535 | 137 | 192 | 185 | 157 | 206 | 41 | 382 | 305 | 41 | 262 |
| MIP-1α | 3,693 | 4,592 | 262 | 4,561 | 281 | 276 | 300 | 1,104 | 4,770 | 4,666 | 415 | 2,410 |
| sCD40L | 2 | 3 | 6 | 6 | 1 | 1 | 13 | 8 | 1 | 2 | 3 | 8 |
| TGF-α | 28 | 43 | 14 | 2 | 1 | 1 | 4 | 20 | 5 | 5 | 1 | 3 |
| TNF-α | 7,770 | 2,265 | 290 | 3,167 | 1,281 | 241 | 787 | 488 | 3,006 | 925 | 18 | 846 |
| VEGF | 20 | 23 | 4 | 6 | 5 | 2 | 114 | 164 | 335 | 464 | 84 | 219 |
| IL-18 | 8 | 3 | 2 | 3 | 3 | 3 | 8 | 30 | 6 | 6 | 3 | 4 |
| IFN-αc | 51 | 1 | 1 | 1 | 77 | 1 | 1 | 1 | 75 | 1 | 1 | 1 |
Human (n = 8), AGM (n = 6), and RM (n = 11) PBMC either were cultured in RPMI medium only or were stimulated with CLO-97, LPS, PHA, or PMA-ionomycin (PMA/Iono), and the fold change in the analyte level was calculated as the level in stimulated PBMC divided by the level in unstimulated PBMC. Boldface values represent significant increases (P < 0.05) over baseline values for unstimulated PBMC (by the Kruskal-Wallis test with Dunn's multiple-comparison tests).
Three AGM samples stimulated with PMA-ionomycin were tested.
For IFN-α, 2 human samples, 3 AGM samples, and 2 RM samples were tested.
FIG 1.
Heat map illustrating normalized data from the validation study. PBMC from naïve RM (n = 11) and naïve AGM (n = 6) were cultured in RPMI medium alone or stimulated with CLO-97, LPS, PHA, or PMA-ionomycin. The median fold change for each analyte was calculated as the stimulated level divided by the unstimulated level and was normalized to the highest response across both species and stimulation conditions. In the spectrum of median fold change, red represents the maximum response, and blue represents the minimum response, across both types of NHP for each analyte.
Comparison of viral dynamics in SIVmac251- and SIVmac239Δnef-infected RM and SIVsab9315BR-infected AGM.
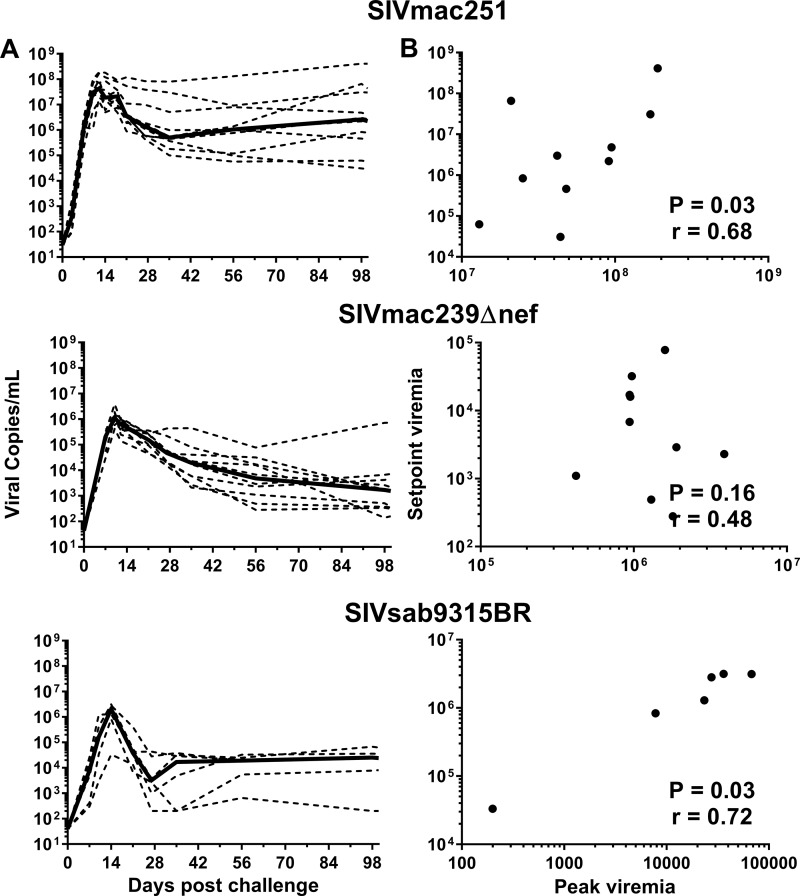
Previous investigations have shown a significant correlation between the magnitude of peak viremia and viremia at 3 months postinfection for SIVmac251-infected RM (28); similarly, our data also indicated a significant correlation (P = 0.03). Notably, we observed that the AUC for viremia during the first 4 weeks following SIV infection was significantly correlated with viremia at 3 months postinfection in SIVmac251-infected RM (P = 0.03) (Fig. 2A and B). A comparison of RM infected with SIVmac251 to RM infected with SIVmac239Δnef or SIVsab9315BR-infected AGM revealed similar dynamic responses, with peak viremia at 2 weeks followed by a steady decline to a set point viremia in the nonprogressive infections, although both nonprogressive infections were characterized by somewhat lower median peak viremia and viremia at 3 months postinfection than the pathogenic infection (Fig. 2A). Despite the nonpathogenic nature of SIVsab9315BR in AGM, a significant correlation was observed between the first-4-week AUC and viremia at 3 months postinfection in SIVsab9315BR-infected AGM (P = 0.03) (Fig. 2A and B). In contrast to the other two infections, there was not a significant correlation between the first-4-week AUC and viremia at 3 months postinfection in SIVmac239Δnef-infected RM, but there was potentially a trend for a positive correlation (P = 0.16) (Fig. 2A and B).
FIG 2.
Dynamics of SIV viremia and correlation of peak and set point viremias. RM were infected with either SIVmac251 (n = 10) or SIVmac239Δnef (n = 10); AGM were infected with SIVsab9315BR (n = 6). (A) Magnitude of SIV viremia in the first 3 months postchallenge. Broken lines represent results for individual animals; solid lines represent median values. (B) Pearson correlation of the AUC for viremia during the first 4 weeks following SIV infection versus the set point viremia at 3 months after infection.
Elevations in the levels of more cytokines and chemokines in plasma are detected after progressive SIV infection.
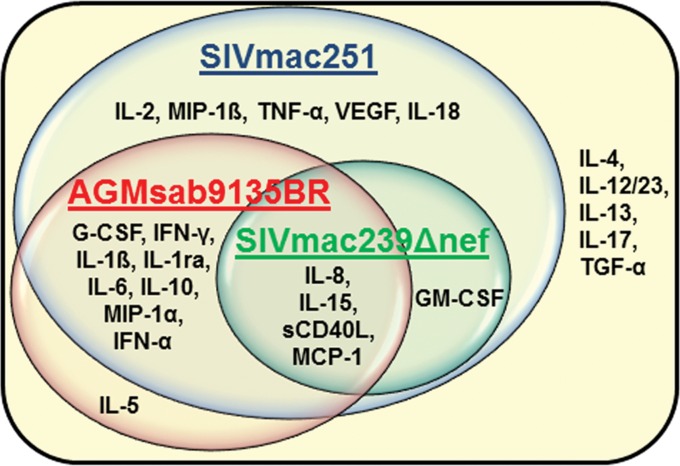
To determine the baseline levels of our cytokines/chemokines in plasma, we measured plasma analyte levels in 74 RM and 19 AGM that were all SIV negative. Elevations in the level of each analyte above the species baseline were then evaluated over time in each infection model. Of the 25 analytes tested, 19, 5, and 13 analytes were elevated in SIVmac251-infected RM, SIVmac239Δnef-infected RM, and SIVsab9315BR-infected AGM at any time point, respectively. More analytes showed detectable elevations in SIVmac251-infected RM than in SIVmac239Δnef-infected RM or SIVsab9315BR-infected AGM (Table 2) (P < 0.0001 and P = 0.004, respectively). The cytokines and chemokines exhibiting elevated levels in each animal model at serial time points after infection are shown in Fig. 3. Of note, the analytes that were elevated in both SIVmac251- and SIVsab9315BR-infected animals were G-CSF, IFN-γ, IL-1β, IL-1ra, IL-6, IL-8, IL-10, IL-15, MCP-1, MIP-1α, sCD40L, and IFN-α. GM-CSF, IL-8, IL-15, MCP-1, and sCD40L were elevated in SIVmac239Δnef infection, and IL-2, MIP-1β, TNF-α, VEGF, and IL-18 were uniquely recognized in SIVmac251 infection, while IL-5 was recognized only in SIVsab9315BR infection.
TABLE 2.
Percentages of animals with cytokine responses after SIV infection
| Analyte | Upper 95% CIa for baseline analyte level in: |
% responders after infectionb |
|||
|---|---|---|---|---|---|
| RM (n = 74) | AGM (n = 19) | RMinfected with: |
AGM infected with SIVsab9315BR (n = 6) | ||
| SIVmac251 (n = 10) | SIVmac239Δnef (n = 10) | ||||
| G-CSF | 1.3 | 2.6 | 60 | 30 | 50 |
| GM-CSF | 17.0 | 6.5 | 80 | 50 | 17 |
| IFN-γ | 11.0 | 5.2 | 100 | 30 | 83 |
| IL-1β | 12.0 | 2.1 | 100 | 0 | 50 |
| IL-1ra | 40.0 | 22.0 | 100 | 0 | 50 |
| IL-2 | 20.0 | 49.0 | 50 | 40 | 33 |
| IL-4 | 0.7 | 1.6 | 0 | 10 | 17 |
| IL-5 | 1.7 | 3.3 | 10 | 30 | 50 |
| IL-6 | 1.9 | 18.0 | 60 | 10 | 50 |
| IL-8 | 164.0 | 20.0 | 60 | 90 | 50 |
| IL-10 | 2.5 | 2.6 | 100 | 0 | 50 |
| IL-12/23 | 152.0 | 62.0 | 30 | 0 | 0 |
| IL-13 | 0.6 | 1.2 | 30 | 0 | 17 |
| IL-15 | 4.3 | 2.2 | 100 | 50 | 67 |
| IL-17 | 0.9 | 0.9 | 40 | 0 | 17 |
| MCP-1 | 153.0 | 408.0 | 100 | 50 | 67 |
| MIP-1β | 2.5 | 8.5 | 50 | 0 | 0 |
| MIP-1α | 21.0 | 18.0 | 70 | 20 | 67 |
| sCD40L | 318.0 | 1,395.0 | 60 | 80 | 83 |
| TGF-α | 59.0 | 4.0 | 0 | 0 | 0 |
| TNF-α | 19.0 | 2.3 | 50 | 0 | 33 |
| VEGF | 43.0 | 60.0 | 100 | 0 | 33 |
| IL-18 | 38.0 | 1.8 | 90 | 0 | 17 |
| IFN-α | 1.7 | 4.1 | 100 | 0 | 50 |
| SAAc | 146.7 | 256.5 | 90 | 44 | 67 |
The upper 95% confidence interval (CI) (expressed in picograms per milliliter) was calculated by testing the baseline analyte levels in 74 untreated RM and 19 untreated AGM.
Percentage of animals whose analyte levels at any time point were above the upper 95% confidence interval of the group baseline, 2-fold elevated over the individual baseline, and >5 pg/ml. Boldface values indicate positive responses for the group (>50% response for the cytokine in the group).
For the SAA evaluation, 19 RM and 6 AGM were included in the baseline analysis. One of the SIVmac239Δnef-infected RM was not included in the SAA evaluation due to insufficient sample volume.
FIG 3.
Graphic representation of the analytes upregulated in the different SIV infection models. Positive cytokine and chemokine responses were defined in each animal as levels at a >95% confidence interval above the baseline mean for each species tested, twice the baseline value for each individual animal, and >5 pg/ml. If more than 50% of animals in a group exhibited a positive response for a given analyte, that analyte was determined to be upregulated for the group. The positive responses observed in each SIV model are indicated in the circles for AGM sab9135BR infection (red), RM SIVmac251 infection (blue), and RM SIVmac239Δnef infection (green). The common responses are represented in the intersection of the SIV model circles. The cytokines that were not found to be upregulated in any of the infection models are outside of all three circles.
Many cytokines were not elevated in acute SIV infection.
A number of analytes never crossed the threshold used to define a positive response during the course of acute infection. If fewer than 50% of animals in a group exhibited a positive response for a given analyte, upregulation of that analyte was determined to be negative for the group. Increases were not detected for 6 of 25 analytes (IL-4, IL-5, IL-12, IL-13, IL-17, and TGF-α) following SIVmac251 infection of RM, for 20 of 25 analytes (G-CSF, IFN-γ, IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, IL-17, MIP-1β, MIP-1α, TGF-α, TNF-α, VEGF, IL-18, IFN-α, and SAA) following SIVmac239Δnef infection of RM, and for 11 of 25 analytes (GM-CSF, IL-2, IL-4, IL-12, IL-13, IL-17, MIP-1β, TGF-α, TNF-α, VEGF, and IL-18) following SIVsab9315BR infection of AGM. Regardless of the NHP species or virus infection, we did not detect significant elevations in IL-4, IL-12, IL-13, IL-17, or TGF-α levels. Of note, each of these analytes except for TGF-α was detectable in both NHP species in our assay validation work (Table 1), demonstrating that apart from TGF-α, the lack of detectable elevation was not due to an inability of the assay to detect the analyte. MIP-1β, VEGF, and IL-18 levels did not increase after SIVsab9315BR infection of AGM or SIVmac239Δnef infection of RM. The only cytokines that were not detected in the validation studies and did not have detectably increased levels in SIV-infected NHP were GM-CSF and TGF-α in AGM.
Peak cytokine responses are of higher magnitude after SIVmac251 infection.
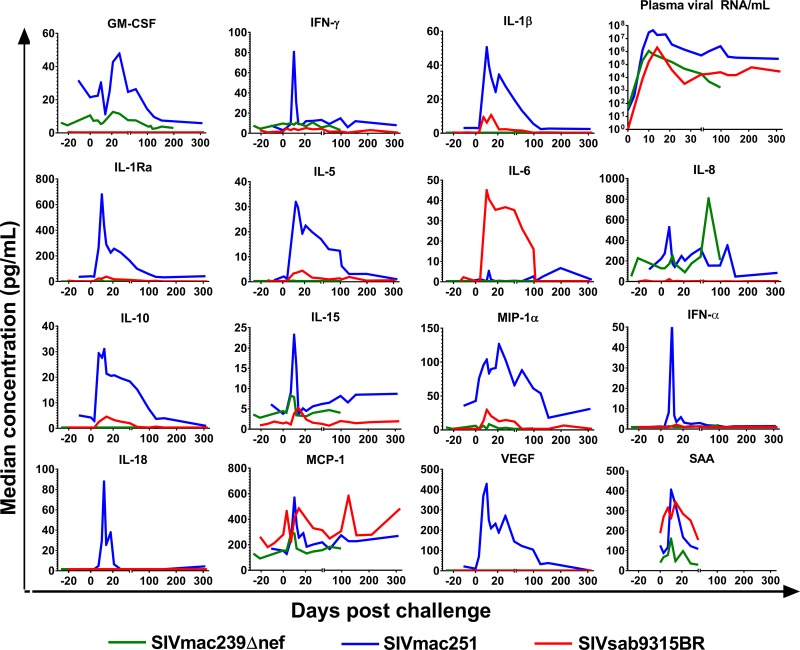
In addition to differences in the breadth of responses detected, progressive versus nonprogressive infections might differ in peak magnitude or the timing of responses. To compare peak analyte levels, we focused on factors that were upregulated after infection and did not significantly differ between the groups at baseline. Analyte levels in the SIVmac251- and SIVmac239Δnef-infected RM groups were not significantly different at baseline (Fig. 4). However, IL-5 and MCP-1 were expressed at significantly higher baseline levels, while IL-8 and IL-15 were expressed at significantly lower baseline levels, in AGM than in RM and were thus excluded from comparative analysis. We first compared increases in analyte levels in RM SIVmac251 infection and AGM SIVsab9315BR infection. Among the factors that were elevated in both comparison groups (Table 3), we found higher peak levels of IFN-γ, IL-1β, IL-1ra, IL-5, IL-8, IL-10, MIP-1α, and IFN-α after SIVmac251 infection than after SIVsab9315BR infection. SAA, IL-2, and IL-6 did not significantly differ in their peak responses. G-CSF, GM-CSF, IL-2, IL-4, IL-12, IL-13, IL-17, MIP-1β, TGF-α, TNF-α, VEGF, IL-18, and sCD40L were not compared due to low or no responses in SIVsab9315BR- and/or RM SIVmac251-infected animals. Among the factors elevated in RM, GM-CSF, IFN-γ, IL-1β, IL-1ra, IL-5, IL-6, IL-10, IL-15, MCP-1, MIP-1α, MIP-1β, TNF-α, VEGF, IFN-α, and SAA peak levels were higher in SIVmac251-infected RM. Interestingly, a higher peak concentration of IL-8 in SIVmac239Δnef-infected animals occurred at day 56, after the peak of most other cytokine responses. Finally, sCD40L levels were higher in SIVsab9315BR than in SIVmac239Δnef infection. Within and across NHP species, peak levels of a number of analytes showing elevated levels were notably higher in the progressive infection than in nonprogressive infections, attesting to the robustness of this observation.
FIG 4.
Magnitude of analyte elevations over time after SIV infection. Median concentrations of 15 of the 25 analytes measured in plasma for the various groups are shown over time. Blue, RM infected with SIVmac251; green, RM infected with SIVmac239Δnef; red, AGM infected with SIVsab9315BR. Analytes whose concentrations did not rise above 5 pg/ml and twice the median baseline at any time point in any group are not shown. Serum amyloid A levels were measured during the first 5 weeks of infection in RM infected with SIVmac251 (n = 10), RM infected with SIVmac239Δnef (n = 9), and AGM infected with SIVsab9315BR (n = 6). One of the SIVmac239Δnef-infected RM was not included in the SAA evaluation due to insufficient sample volume.
TABLE 3.
Cytokine responses after pathogenic and nonpathogenic SIV infections in RM and AGM
| Cytokinea | Rhesus macaques |
African green monkeys |
|||
|---|---|---|---|---|---|
| Median (IQR) baseline responseb | Median peak responsec (day postinfectiond) after infection with: |
Median (IQR) baseline responseb | Median peak responsec (day postinfectiond) after infection with SIVsab9315BR | ||
| SIVmac251 | SIVmac239Δnef | ||||
| G-CSF | 0.3 (0.3–0.7) | 1.8 (12) | 2.3 (21) | 0.3 (0.3–2) | 4.5 (10) |
| GM-CSFc | 3.4 (0.3–15) | 47.8 (27) | 12.6 (21) | 0.6 (0.3–7) | 0.5 (NR) |
| IFN-γ | 6.2 (3–11) | 80.5 (10) | 10.8 (27) | 2 (1–5) | 5.2 (21) |
| IL-1β | 0.3 (0.3–3.5) | 50.5 (10) | 0.3 (NR) | 0.3 (0.3–3) | 10.8 (14) |
| IL-1ra | 8.0 (0.3–37) | 678.5 (10) | 7.2 (NR) | 4 (2–18) | 38.4 (14) |
| IL-2 | 14.7 (4.3–23) | 25.9 (154) | 23.3 (56) | 24 (4–59) | 23.6 (21) |
| IL-5 | 0.3 (0.1–0.3) | 31.9 (10) | 0.5 (7) | 0.6 (0.3–3) | 4.4 (14) |
| IL-6 | 0.3 (0.3–0.3) | 6.8 (154) | 0.3 (NR) | 0.3 (0.3–9) | 45.0 (7) |
| IL-8 | 98.2 (61–189) | 526.4 (7) | 807.5 (56) | 2 (0.3–9) | 22.4 (7) |
| IL-10 | 0.3 (0.2–0.3) | 31.0 (12) | 0.3 (NR) | 0.3 (0.3–0.6) | 4.6 (14) |
| IL-15 | 4.0 (2.4–5.5) | 23.2 (10) | 8.3 (7) | 0.8 (0.3–2) | 5.2 (14) |
| MCP-1 | 130.4 (102–168) | 568.4 (10) | 291.9 (7) | 316 (188–378) | 582.6 (125) |
| MIP-1α | 7.0 (0.3–19) | 126.4 (21) | 8.5 (NR) | 2 (2–13) | 29.8 (10) |
| MIP-1β | 0.3 (0.3–0.8) | 6.4 (154) | 0.3 (ND) | 0.3 (0.3–0.3) | 0.3 (ND) |
| sCD40L | 209.1 (119–393) | 269.7 (NR) | 348.5 (56) | 392 (149–1,047) | 551.5 (21) |
| TNF-α | 3.4 (0.3–13) | 7.0 (NR) | 0.9 (NR) | 0.3 (0.3–1) | 1.8 (NR) |
| VEGF | 0.3 (0.3–7.5) | 427.3 (10) | 1.6 (NR) | 2 (2–56) | 1.6 (NR) |
| IL-18 | 0.3 (0.3–9.6) | 87.8 (12) | 1.6 (NR) | 2 (2–2) | 1.6 (NR) |
| IFN-α | 1.0 (0.6–2.1) | 63.8 (10) | 1.9 (NR) | 1 (1–2) | 3.4 (12) |
| SAA | 40 (20–87) | 452 (7) | 80 (7) | 39 (19–65.5) | 316.6 (7) |
Cytokines with significant differences (determined by ANOVA followed by Tukey's HSD test) in peak levels between the three different infection models are shown in boldface. (IL-4, IL-12, IL-13, IL-17, and TGF-α were not found to be upregulated in >50% of animals and are not included in this table.)
Median and lower and upper quartiles (interquartile range [IQR]) for the baseline response. Values are reported in picograms per milliliter.
Median peak response for each group (in picograms per milliliter), which differed between animals, analytes, and groups.
NR, no response. The analyte level did not reach the upper 95% confidence interval of the group baseline, did not exhibit a 2-fold change over the individual baseline at any time point, or did not exceed 5 pg/ml—the criteria for response upregulation. ND, not detected.
Relationship between peak viremia and peak cytokine responses during acute infection.
We hypothesized that acute viral replication could be associated with the strongest cytokine responses, so we examined the correlation between peak cytokine levels and peak viremia during acute infection. No significant correlations were observed between peak cytokine responses and peak viremia in SIVmac251- and SIVmac239Δnef-infected RM (data not shown). In contrast, SIVsab9315BR infection of AGM was associated with a significant correlation between peak MCP-1 responses and peak viremia (P = 0.006). To determine the relationship between the general cytokine response over the first 4 weeks and peak viremia, we performed a correlation analysis between the 4-week cytokine AUC and peak viremia. We found a significant positive correlation in SIVmac239Δnef-infected animals for IL-8 (P = 0.04), a trend toward positive correlation for MCP-1 (P = 0.06), and a trend toward negative correlation for GM-CSF (P = 0.05). However, this analysis did not result in significant correlations in the SIVmac251- or SIVsab9315BR-infected animals.
Early cytokine responses correlate with chronic viremia at 3 months.
Early cytokine responses were analyzed in order to identify a potential association with chronic viremia. The magnitude of the cytokine AUC responses of each individual animal over the first 4 weeks of infection was compared with the magnitude of chronic viremia at 3 months (Table 4). A number of individual cytokine responses in SIVmac251-infected RM correlated significantly with chronic viremia, including G-CSF, IFN-γ, IL-15, MIP-1β, and IL-18. In contrast, in the same analysis, MCP-1 and sCD40L showed a significant correlation in SIVmac239Δnef-infected RM, and IL-6 and MCP-1 showed a significant correlation in AGM infected with SIVsab9315BR. Additionally, a number of other cytokine responses during acute SIV infection were associated with viremia. A trend (P values between 0.05 and 0.2) was detected for the following analytes: sCD40L, IL-1β, and IL-8 in SIVsab9315BR-infected AGM; VEGF, IL-10, IL-6, and MIP-1α in SIVmac251-infected RM (Table 4). Of note, all correlations detected were positive, i.e., a relatively higher magnitude of cytokine responses during early infection correlated with a higher magnitude of viremia at 3 months.
TABLE 4.
Cytokine responses during early infection that correlate with the viral set point
| Virus and cytokine | R2 | P value | FDR |
|---|---|---|---|
| SIVsab9315BR | |||
| IL-6 | 0.85a | 0.009 | 0.07 |
| MCP-1 | 0.85 | 0.009 | 0.07 |
| sCD40L | 0.64 | 0.057 | 0.24 |
| IL-1β | 0.61 | 0.068 | 0.24 |
| IL-8 | 0.54 | 0.095 | 0.27 |
| SIVmac239Δnef | |||
| MCP-1 | 0.75 | 0.001 | 0.003 |
| sCD40L | 0.76 | 0.001 | 0.003 |
| IL-8 | 0.221 | 0.17 | 0.28 |
| SIVmac251 | |||
| IFN-γ | 0.83 | 0.0003 | 0.004 |
| IL-18 | 0.81 | 0.0004 | 0.004 |
| G-CSF | 0.64 | 0.006 | 0.04 |
| IL-15 | 0.59 | 0.009 | 0.05 |
| MIP-1β | 0.55 | 0.015 | 0.06 |
| SAA | 0.421 | 0.04 | 0.11 |
| MCP-1 | 0.405 | 0.048 | 0.11 |
| VEGF | 0.41 | 0.05 | 0.11 |
| IL-10 | 0.287 | 0.11 | 0.23 |
| IL-6 | 0.257 | 0.13 | 0.25 |
| MIP-1α | 0.246 | 0.145 | 0.25 |
For each analyte, the AUC was calculated for the first 4 weeks of infection, and a regression analysis was done comparing this value with set point viremia. Cytokines with nonsignificant P values (P values between 0.05 and 0.2, possibly representing a trend) and their associated values are italicized.
DISCUSSION
Cytokines/chemokines were used as biomarkers of the systemic immune response in order to investigate their association with establishing virus-host balance. We used a cross-species application of human- and primate-specific ELISAs and multiplex assays to study the induction of soluble immune mediators in acute and early SIV infection in models that do (SIVmac251-infected RM) or do not (SIVsab9315BR-infected AGM or SIVmac239Δnef-infected RM) typically show progressive disease leading to AIDS. A significantly higher magnitude and breadth of upregulation of soluble immune factors was detected in RM infected with SIVmac251 than in RM infected with SVImac239Δnef or AGM infected with SIVsab9315BR. In all three SIV infection models, the magnitude of peak viremia did not correlate with the levels of soluble immune markers investigated during the first 4 weeks after infection. However, we observed in all three infection models that chronic viremia was correlated with the levels of several soluble immune markers detected during the first 4 weeks of infection. A predominantly proinflammatory signature profile was observed in progressive infection; this was in contrast to the monocyte chemotactic cytokine signature detected in the nonprogressive SIV infection models.
During this study, all AGM were infused with 50 mg/kg of i.v. IgG on the same day as SIV infection, and the RM challenged with SIVmac251 received 50 mg/kg of i.v. IgG 1 week prior to infection. Clinical studies in humans have found modest cytokine changes to occur within hours to days after i.v. IgG infusion but have used doses an order of magnitude higher (400 to 600 mg/kg) than those administered here (29). In a study of HIV-infected individuals, TNF-α levels were found to decline by 5 days after i.v. IgG infusion (30). In our study, we found no significant difference in the profile of preinfection cytokines before and after i.v. IgG infusion for all cytokines analyzed here. Despite i.v. IgG infusion, cytokine responses increased after infection in parallel with viremia and had similar kinetics between animal groups, albeit slightly delayed in the AGM. A similar postinfection cytokine peak has also been seen in other studies in RM that did not receive i.v. IgG (20). In our study, the SIV-induced peak of cytokine response is substantially longer than the timing of cytokine changes after i.v. IgG infusion in previous studies in humans (31). Taken together, there is no evidence that i.v. IgG administration to AGM or RM had any confounding effect, likely due to the significantly lower dose of i.v. IgG administered here.
For each animal, only a single preinfection time point was available, which provided only limited information about the steady-state cytokine/chemokine levels for baseline determination. This was overcome by measuring analytes in a large number of uninfected animals and using the data to establish a baseline analyte level for each animal species. Before comparing the different infection models in two NHP species, we needed to confirm that the assays could detect these analytes across species. During the validation study, we established assay detection efficacy and found that the majority of the detection reagents were species cross-reactive. Nevertheless, it was difficult to determine if antibody-analyte binding was better in one species than in another or whether differences in results were due to differences in levels of analyte production and/or reabsorption after PBMC stimulation. This reveals a need for species-specific monoclonal antibodies and cytokine standards to be made to correctly quantify analyte concentrations for each species. Several analytes that showed better detection in the validation study in AGM were in fact expressed at higher levels in RM after SIV infection, including IL-5, IL-8, IL-1ra, IL-15, IL-18, and IFN-α, strongly suggesting that these analytes were indeed produced in greater quantities during pathogenic infection.
As expected, the dynamics of the three viral infections were similar, while the progressive SIVmac251 infection model resulted in higher peak and chronic viremias than the two nonprogressive infection models. As reported previously, SIVmac251 peak viremia was correlated with chronic viremia (28). This was also true for the nonprogressive SIV infection in AGM. While not statistically significant, our data suggested a trend for a positive correlation between peak viremia and viremia at 3 months in SIVmac239Δnef-infected RM. Thus, the magnitude of primary viremia appears to be associated with the magnitude of chronic viremia, regardless of the relative pathogenicity of SIV or the NHP species investigated.
Although we hypothesized that the inflammatory cytokine response would coincide and correlate with peak viremia, we did not find this to be true in RM, and we found that only MCP-1 correlated with SIVsab9315BR peak viremia in AGM (P = 0.006). In addition, peak viremia of SIVmac239Δnef showed weak and borderline positive correlations with the 4-week AUCs for MCP-1 and IL-8 and a negative correlation with the 4 week AUC for GM-CSF. One of the main objectives of this study was to determine if any acute cytokine profiles were associated with chronic levels of viral replication. Comparing 4-week cytokine AUCs in acute infection with set point levels of persisting viremia, we found that the inflammatory cytokines IL-15, IL-18, and IFN-γ positively correlated with the viral set point in RM SIVmac251 infection. IL-15 and IL-18 promote the activation of innate subsets and adaptive responses, indirectly enhancing virus control while simultaneously driving CD4+ T cell activation that promotes the stimulation and expansion of memory subsets of CD4+ T cells, providing potential reservoirs for viral expansion (32, 33); this may explain their positive correlation with chronic viremia. IFN-γ is a key component in the maturation of the T cell response during acute infection and is associated with the activation of CD8+ T cells and the antiviral NK cell response (34, 35). Several studies have shown that higher levels of IFN-γ-secreting antigen-specific T cells were associated with better control of SIV infection in RM (36), and IFN-γ may be important in enhancing the expansion of memory populations of immune cells for aiding long-term viral control in HIV infection (32). Given these potentially beneficial effects, the positive correlation of IFN-γ with the viral set point was unexpected. In humans, IFN-γ, IL-12p40, and IL-12p70 responses were associated with a lower viral set point, while IL-7 and IL-15 were associated with a higher set point (32, 36).
The only analyte showing increases during in the acute phase of infection and a correlation with set point viremia in all three SIV-infection models was MCP-1, a marker of systemic monocyte macrophage trafficking. When the virus targets the central nervous system (CNS) within days of infection, SIV-infected astrocytes produce the chemokine MCP-1, which is responsible for recruiting monocytes in SIV neuroinvasion and establishing the infected SIV reservoir in the brain (37). Suppressing monocyte activation in an acute infection model would be an attractive target in determining the relationship between monocyte activation and the subsequent viral set point.
Another inflammatory cytokine, IL-6, uniquely correlated with the viral set point in AGM, while sCD40L uniquely correlated with the viral set point in SIVmac239Δnef-infected RM. Interestingly, IL-6 production is associated with severe pathogenicity in HIV infection in humans and SIV infection in RM (38–41). For example, SIVsmmPBj8 infection of RM results in death within a few weeks and induces significant IL-6 production, which may be a cause or effect of the pathogenic course of infection (42). It is currently unknown why IL-6 production in AGM does not lead to pathogenicity. One can only speculate whether IL-6 production may in fact be beneficial for AGM. Given its pleotropic effects, IL-6 may be involved in the induction of regulatory B cells and may thus restrain excessive immune activation, as recently described by Rosser et al. (43).
Acute SIV infection in both natural and nonnatural hosts elicits massive depletion of mucosal CD4+ T cells. There is only a transient increase in inflammatory responses coinciding with acute viremia, and eventually CD4+ T cell levels return to normal (44). Although nonprogressive SIV infection does not exhibit the same mucosal inflammation as progressive infection, recent work has shown that this nonprogressive equilibrium can be disrupted in AGM by use of an experimental model of colitis resulting in gut-associated microbial translocation, macrophage accumulation, immune activation, significantly elevated viral loads, and continued decline of CD4+ T cells, a pattern similar to that of progressive infection (45, 46). Treatment with IL-21 has been shown to reduce inflammation and support the maintenance of mucosal Th17 cells, preventing microbial translocation and inflammation in progressive infection (47). The initial inflammatory responses in both groups and the differences in outcome could be related to how the infection seeds at the mucosa and the immune responses present that induce or prevent immune dysfunction; this may be an innate immune difference between the animal species. Monocyte chemotactic factors (MCP-1 and IL-8) and inflammatory cytokine production (i.e., IL-1β, IL-6, TNF-α, and IL-18) in progressive infections could stem from this initial inflammatory cascade at the mucosal level; investigation into the mucosal immune response of nonprogressive hosts may shed light on some of these differences.
In summary, we observed higher levels of virus replication associated with a higher-magnitude of cytokine/chemokine response in progressive infection, and we hypothesize that excessive early cytokine release may contribute to further immune activation and virus replication, setting the stage for subsequent disease progression. However, since we only performed correlation analyses of the magnitude of cytokine responses and viremia in the three SIV infection models, the mechanistic basis for the difference between progressive and nonprogressive disease courses still remains elusive. In the future, interventional in vivo studies will have to be performed to determine whether the qualitative and quantitative differences in cytokine/chemokine responses contribute to the nonpathogenic nature of nonprogressive SIV infection.
ACKNOWLEDGMENTS
This work was supported by NIH, NIAID, Division of AIDS, grants AI0678501 (CHAVI, Center for HIV AIDS Vaccine Immunology) (B.M.K., P.B., N.L.L., P.J.N., J.E.S.) and AI065335 (J.E.S.) and in part with federal funds from the NCI/NIH under contract HHSN261200800001E. This publication was made possible with help from the Harvard University Center for AIDS Research (CFAR), an NIH-funded program (P30-AI060354), which is supported by the following NIH cofunding and participating institutes and centers: NIAID, NCI, NICHD, NHLBI, NIDA, NIMH, NIA, NIDDK, NIGMS, FIC, and OAR. P.B. is a Jenner Institute Investigator.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Weissman D, Barker TD, Fauci AS. 1996. The efficiency of acute infection of CD4+ T cells is markedly enhanced in the setting of antigen-specific immune activation. J Exp Med 183:687–692. doi: 10.1084/jem.183.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. 2010. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol 10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, Heldebrant C, Smith R, Conrad A, Kleinman SH, Busch MP. 2003. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 17:1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 4.Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, Lebedeva M, DeCamp A, Li D, Grove D, Self SG, Borrow P. 2009. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol 83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramer HB, Lavender KJ, Qin L, Stacey AR, Liu MK, di Gleria K, Simmons A, Gasper-Smith N, Haynes BF, McMichael AJ, Borrow P, Kessler BM. 2010. Elevation of intact and proteolytic fragments of acute phase proteins constitutes the earliest systemic antiviral response in HIV-1 infection. PLoS Pathog 6:e1000893. doi: 10.1371/journal.ppat.1000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katsikis PD, Mueller YM, Villinger F. 2011. The cytokine network of acute HIV infection: a promising target for vaccines and therapy to reduce viral set-point? PLoS Pathog 7:e1002055. doi: 10.1371/journal.ppat.1002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desrosiers RC, Letvin NL. 1987. Animal models for acquired immunodeficiency syndrome. Rev Infect Dis 9:438–446. doi: 10.1093/clinids/9.3.438. [DOI] [PubMed] [Google Scholar]
- 8.Sodora DL, Allan JS, Apetrei C, Brenchley JM, Douek DC, Else JG, Estes JD, Hahn BH, Hirsch VM, Kaur A, Kirchhoff F, Muller-Trutwin M, Pandrea I, Schmitz JE, Silvestri G. 2009. Toward an AIDS vaccine: lessons from natural simian immunodeficiency virus infections of African nonhuman primate hosts. Nat Med 15:861–865. doi: 10.1038/nm.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lifson JD, Nowak MA, Goldstein S, Rossio JL, Kinter A, Vasquez G, Wiltrout TA, Brown C, Schneider D, Wahl L, Lloyd AL, Williams J, Elkins WR, Fauci AS, Hirsch VM. 1997. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J Virol 71:9508–9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniel MD, Kirchhoff F, Czajak SC, Sehgal PK, Desrosiers RC. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science 258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 11.Mohri H, Bonhoeffer S, Monard S, Perelson AS, Ho DD. 1998. Rapid turnover of T lymphocytes in SIV-infected rhesus macaques. Science 279:1223–1227. doi: 10.1126/science.279.5354.1223. [DOI] [PubMed] [Google Scholar]
- 12.Rosenzweig M, DeMaria MA, Harper DM, Friedrich S, Jain RK, Johnson RP. 1998. Increased rates of CD4+ and CD8+ T lymphocyte turnover in simian immunodeficiency virus-infected macaques. Proc Natl Acad Sci U S A 95:6388–6393. doi: 10.1073/pnas.95.11.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Picker LJ, Hagen SI, Lum R, Reed-Inderbitzin EF, Daly LM, Sylwester AW, Walker JM, Siess DC, Piatak M Jr, Wang C, Allison DB, Maino VC, Lifson JD, Kodama T, Axthelm MK. 2004. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J Exp Med 200:1299–1314. doi: 10.1084/jem.20041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okoye A, Park H, Rohankhedkar M, Coyne-Johnson L, Lum R, Walker JM, Planer SL, Legasse AW, Sylwester AW, Piatak M Jr, Lifson JD, Sodora DL, Villinger F, Axthelm MK, Schmitz JE, Picker LJ. 2009. Profound CD4+/CCR5+ T cell expansion is induced by CD8+ lymphocyte depletion but does not account for accelerated SIV pathogenesis. J Exp Med 206:1575–1588. doi: 10.1084/jem.20090356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandrea I, Apetrei C. 2010. Where the wild things are: pathogenesis of SIV infection in African nonhuman primate hosts. Curr HIV/AIDS Rep 7:28–36. doi: 10.1007/s11904-009-0034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lederer S, Favre D, Walters KA, Proll S, Kanwar B, Kasakow Z, Baskin CR, Palermo R, McCune JM, Katze MG. 2009. Transcriptional profiling in pathogenic and non-pathogenic SIV infections reveals significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog 5:e1000296. doi: 10.1371/journal.ppat.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cumont MC, Diop O, Vaslin B, Elbim C, Viollet L, Monceaux V, Lay S, Silvestri G, Le Grand R, Muller-Trutwin M, Hurtrel B, Estaquier J. 2008. Early divergence in lymphoid tissue apoptosis between pathogenic and nonpathogenic simian immunodeficiency virus infections of nonhuman primates. J Virol 82:1175–1184. doi: 10.1128/JVI.00450-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosinger SE, Hosiawa KA, Cameron MJ, Persad D, Ran L, Xu L, Boulassel MR, Parenteau M, Fournier J, Rud EW, Kelvin DJ. 2004. Gene expression profiling of host response in models of acute HIV infection. J Immunol 173:6858–6863. doi: 10.4049/jimmunol.173.11.6858. [DOI] [PubMed] [Google Scholar]
- 19.Kornfeld C, Ploquin MJ, Pandrea I, Faye A, Onanga R, Apetrei C, Poaty-Mavoungou V, Rouquet P, Estaquier J, Mortara L, Desoutter JF, Butor C, Le Grand R, Roques P, Simon F, Barre-Sinoussi F, Diop OM, Muller-Trutwin MC. 2005. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J Clin Invest 115:1082–1091. doi: 10.1172/JCI23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abel K, Rocke DM, Chohan B, Fritts L, Miller CJ. 2005. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J Virol 79:12164–12172. doi: 10.1128/JVI.79.19.12164-12172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta M, MacNeil A, Reed ZD, Rollin PE, Spiropoulou CF. 2012. Serology and cytokine profiles in patients infected with the newly discovered Bundibugyo ebolavirus. Virology 423:119–124. doi: 10.1016/j.virol.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 22.Almansa R, Sanchez-Garcia M, Herrero A, Calzada S, Roig V, Barbado J, Rico L, Bobillo F, Eiros JM, Iglesias V, de Lejarazu RO, Bermejo-Martin JF. 2011. Host response cytokine signatures in viral and nonviral acute exacerbations of chronic obstructive pulmonary disease. J Interferon Cytokine Res 31:409–413. doi: 10.1089/jir.2010.0131. [DOI] [PubMed] [Google Scholar]
- 23.Broussard SR, Staprans SI, White R, Whitehead EM, Feinberg MB, Allan JS. 2001. Simian immunodeficiency virus replicates to high levels in naturally infected African green monkeys without inducing immunologic or neurologic disease. J Virol 75:2262–2275. doi: 10.1128/JVI.75.5.2262-2275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zahn RC, Rett MD, Li M, Tang H, Korioth-Schmitz B, Balachandran H, White R, Pryputniewicz S, Letvin NL, Kaur A, Montefiori DC, Carville A, Hirsch VM, Allan JS, Schmitz JE. 2010. Suppression of adaptive immune responses during primary SIV infection of sabaeus African green monkeys delays partial containment of viremia but does not induce disease. Blood 115:3070–3078. doi: 10.1182/blood-2009-10-245225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 26.Cline AN, Bess JW, Piatak M Jr., Lifson JD. 2005. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J Med Primatol 34:303–312. doi: 10.1111/j.1600-0684.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 27.Benjamini Y. 2010. Discovering the false discovery rate. J R Stat Soc Ser B 72:405–416. doi: 10.1111/j.1467-9868.2010.00746.x. [DOI] [Google Scholar]
- 28.Staprans SI, Dailey PJ, Rosenthal A, Horton C, Grant RM, Lerche N, Feinberg MB. 1999. Simian immunodeficiency virus disease course is predicted by the extent of virus replication during primary infection. J Virol 73:4829–4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ling ZD, Yeoh E, Webb BT, Farrell K, Doucette J, Matheson DS. 1993. Intravenous immunoglobulin induces interferon-gamma and interleukin-6 in vivo. J Clin Immunol 13:302–309. doi: 10.1007/BF00920238. [DOI] [PubMed] [Google Scholar]
- 30.Aukrust P, Hestdal K, Lien E, Bjerkeli V, Nordoy I, Espevik T, Muller F, Froland SS. 1997. Effects of intravenous immunoglobulin in vivo on abnormally increased tumor necrosis factor-alpha activity in human immunodeficiency virus type 1 infection. J Infect Dis 176:913–923. doi: 10.1086/516510. [DOI] [PubMed] [Google Scholar]
- 31.Aukrust P, Froland SS, Liabakk NB, Muller F, Nordoy I, Haug C, Espevik T. 1994. Release of cytokines, soluble cytokine receptors, and interleukin-1 receptor antagonist after intravenous immunoglobulin administration in vivo. Blood 84:2136–2143. [PubMed] [Google Scholar]
- 32.Roberts L, Passmore JA, Williamson C, Little F, Bebell LM, Mlisana K, Burgers WA, van Loggerenberg F, Walzl G, Djoba Siawaya JF, Karim QA, Karim SS. 2010. Plasma cytokine levels during acute HIV-1 infection predict HIV disease progression. AIDS 24:819–831. doi: 10.1097/QAD.0b013e3283367836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mueller YM, Do DH, Altork SR, Artlett CM, Gracely EJ, Katsetos CD, Legido A, Villinger F, Altman JD, Brown CR, Lewis MG, Katsikis PD. 2008. IL-15 treatment during acute simian immunodeficiency virus (SIV) infection increases viral set point and accelerates disease progression despite the induction of stronger SIV-specific CD8+ T cell responses. J Immunol 180:350–360. doi: 10.4049/jimmunol.180.1.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manetti R, Gerosa F, Giudizi MG, Biagiotti R, Parronchi P, Piccinni MP, Sampognaro S, Maggi E, Romagnani S, Trinchieri G. 1994. Interleukin 12 induces stable priming for interferon gamma (IFN-γ) production during differentiation of human T helper (Th) cells and transient IFN-γ production in established Th2 cell clones. J Exp Med 179:1273–1283. doi: 10.1084/jem.179.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G, Romagnani S. 1993. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med 177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyer JD, Maciag PC, Parkinson R, Wu L, Lewis MG, Weiner DB, Paterson Y. 2006. Rhesus macaques with high levels of vaccine induced IFN-γ producing cells better control viral set-point following challenge with SIV239. Vaccine 24:4498–4502. doi: 10.1016/j.vaccine.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Clements JE, Mankowski JL, Gama L, Zink MC. 2008. The accelerated simian immunodeficiency virus macaque model of human immunodeficiency virus-associated neurological disease: from mechanism to treatment. J Neurovirol 14:309–317. doi: 10.1080/13550280802132832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breen EC, Rezai AR, Nakajima K, Beall GN, Mitsuyasu RT, Hirano T, Kishimoto T, Martinez-Maza O. 1990. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol 144:480–484. [PubMed] [Google Scholar]
- 39.Ramesh G, Alvarez X, Borda JT, Aye PP, Lackner AA, Sestak K. 2005. Visualizing cytokine-secreting cells in situ in the rhesus macaque model of chronic gut inflammation. Clin Diagn Lab Immunol 12:192–197. doi: 10.1128/CDLI.12.1.192-197.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.French MA, Cozzi-Lepri A, Arduino RC, Johnson M, Achhra AC, Landay A, INSIGHT SMART Study Group. 2015. Plasma levels of cytokines and chemokines and the risk of mortality in HIV-infected individuals: a case-control analysis nested in a large clinical trial. AIDS 29:847–851. doi: 10.1097/QAD.0000000000000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDonald B, Moyo S, Gabaitiri L, Gaseitsiwe S, Bussmann H, Koethe JR, Musonda R, Makhema J, Novitsky V, Marlink RG, Wester CW, Essex M. 2013. Persistently elevated serum interleukin-6 predicts mortality among adults receiving combination antiretroviral therapy in Botswana: results from a clinical trial. AIDS Res Hum Retroviruses 29:993–999. doi: 10.1089/aid.2012.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tao B, Fultz PN. 1999. Pathogenicity and comparative evolution in vivo of the transitional quasispecies SIVsmmPBj8. Virology 259:166–175. doi: 10.1006/viro.1999.9759. [DOI] [PubMed] [Google Scholar]
- 43.Rosser EC, Oleinika K, Tonon S, Doyle R, Bosma A, Carter NA, Harris KA, Jones SA, Klein N, Mauri C. 2014. Regulatory B cells are induced by gut microbiota-driven interleukin-1β and interleukin-6 production. Nat Med 20:1334–1339. doi: 10.1038/nm.3680. [DOI] [PubMed] [Google Scholar]
- 44.Gordon SN, Klatt NR, Bosinger SE, Brenchley JM, Milush JM, Engram JC, Dunham RM, Paiardini M, Klucking S, Danesh A, Strobert EA, Apetrei C, Pandrea IV, Kelvin D, Douek DC, Staprans SI, Sodora DL, Silvestri G. 2007. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J Immunol 179:3026–3034. doi: 10.4049/jimmunol.179.5.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hao XP, Lucero CM, Turkbey B, Bernardo ML, Morcock DR, Deleage C, Trubey CM, Smedley J, Klatt NR, Giavedoni LD, Kristoff J, Xu A, Del Prete GQ, Keele BF, Rao SS, Alvord WG, Choyke PL, Lifson JD, Brenchley JM, Apetrei C, Pandrea I, Estes JD. 2015. Experimental colitis in SIV-uninfected rhesus macaques recapitulates important features of pathogenic SIV infection. Nat Commun 6:8020. doi: 10.1038/ncomms9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swan ZD, Wonderlich ER, Barratt-Boyes SM. 2016. Macrophage accumulation in gut mucosa differentiates AIDS from chronic SIV infection in rhesus macaques. Eur J Immunol 46:446–454. doi: 10.1002/eji.201545738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Micci L, Ryan ES, Fromentin R, Bosinger SE, Harper JL, He T, Paganini S, Easley KA, Chahroudi A, Benne C, Gumber S, McGary CS, Rogers KA, Deleage C, Lucero C, Byrareddy SN, Apetrei C, Estes JD, Lifson JD, Piatak M Jr, Chomont N, Villinger F, Silvestri G, Brenchley JM, Paiardini M. 2015. Interleukin-21 combined with ART reduces inflammation and viral reservoir in SIV-infected macaques. J Clin Invest 125:4497–4513. doi: 10.1172/JCI81400. [DOI] [PMC free article] [PubMed] [Google Scholar]