Abstract
Vascular smooth muscle cells (VSMCs) play important roles in cardiovascular disorders and biology. Outlined in this paper is a step-by-step procedure for isolating aortic VSMCs from adult C57BL6J male mice by enzymatic digestion of the aorta using collagenase. The plating, culturing, and subculturing of the isolated cells are discussed in detail along with techniques to characterize VSMC phenotype by gene expression and immunofluorescence. Traction force microscopy was used to characterize contractility of single subcultured VSMCs at baseline.
Keywords: Vascular smooth muscle cell, Murine, Aorta, Subculture, Caldesmon 1, Calponin 1, Smooth muscle 22α, Smooth muscle α-actin, Smooth muscle myosin heavy chain, Smoothelin
Introduction
Vascular smooth muscle cells (VSMCs) constitute the major cellular component that provides structural and functional integrity to blood vessels. Characterization of VSMCs in vivo and in vitro for the past 40 years has proven challenging due to the ability of VSMCs to dedifferentiate and differentiate in response to multiple stimuli [1–6]. The plasticity of VSMCs in response to the extracellular matrix, cell/cell contact, influence of endothelial and cells and other cell types, lipids, inflammatory mediators, and stretch and hemodynamics alters proliferative, migratory, and contractile properties [6, 7].
Evidence to date suggests heterogeneity of VSMCs in the vessel wall with multiple subpopulations of VSMCs [8, 9]. Isolating and passaging of primary aortic VSMCs in culture is widely accepted as a strategy to identify mechanistic signaling pathways in VSMCs since 1913/1914 when Champy et al. published the original paper [10]. Previous studies have been published outlining the procedures for isolating murine aortic VSMCs [11]. However, characterization of VSMCs with passage has not been reported. This study outlines isolation, culture, and subculture protocols of murine adult aortic VSMC with associated phenotypes of proliferation and contractility.
Methods and Results
The detailed methodology is listed in the Online Supplemental Methods.
Animals
Adult 14- to 16-week male C57BL6J mice were purchased from Jackson Labs. All procedures were performed in compliance with the IACUC guidelines at the University of Minnesota. The animals were housed four to a cage and provided NIH chow and water ad libitum.
Materials for Isolation of VSMCs from Murine Aorta
Materials for VSMC isolation from the mouse aorta include a dissecting microscope, tabletop centrifuge, autoclaved surgical instruments including fine-tipped forceps (Fine Science Tools), surgical scissors (Fine Science Tools), and superfine curved micro-scissors (# VS1022, Academic Instruments), sterile alcohol prep pads (#MDS090735, Medline), polystyrene sterile tubes (#55476013, 5 mL, Sarstedt), sterile disposable vacuum filtration systems (#SCGP00525, Millipore), 6-well and 24-well tissue culture flat bottom plates (Falcon).
Solutions
Dulbecco’s modified Eagle’s medium (DMEM) (high glucose, # SH30243, Invitrogen), Fetal Bovine Serum (FBS, HyClone) Penicillin/streptomycin (#15140-122, GIBCO), Trypsin (0.25 %, #25-053-CI, Corning), phosphate buffered saline (PBS, #10010-023, Life Technologies), 70 % ethanol, Fungizone (#15290-018, Life Technologies), Collagenase Type 2 (# S8N10850, Worthington), Trypan blue (SV30084.01, HyClone).
Preparing Reagents
Prepare complete media by combining DMEM with 10 % FBS and 1 % penicillin/streptomycin and store at 4 °C until use. Prepare 70 % ethanol by mixing 70 mL of 100 % ethanol (Pharmaco-AAPR) and 30 mL of distilled water and store at room temperature. Prepare Fungizone by adding 10 μL of Fungizone to 10 mL of complete media and filter-sterilize using a sterile disposable vacuum filtration unit. Prepared Fungizone can be stored for a week at 4 °C. Prepare Collagenase Type 2 in complete media. Weigh and dissolve 14.2 mg of Collagenase Type 2 in 10 mL of complete media (final concentration 1.42 mg/mL) and filter-sterilize using sterile disposable vacuum filtration unit. The volume of collagenase prepared can be adjusted according to the number of mice to be used. Prepared collagenase type 2 can also be stored at 4 °C for a week.
Mouse Aortic VSMC Isolation Procedure
Prior to euthanizing the mice, warm complete DMEM, collagenase type 2, and Fungizone solutions to 37 °C in a water bath. Add 1 mL of Fungizone solution in each well of a 6-well tissue culture plate.
Euthanize mice with a continuous flow of carbon dioxide using a flow meter unit for 3–5 min at the flow rate of 2 L/min. Weigh the mouse and secure it to a flat moveable surface in supine position prior to viewing under a dissecting microscope. Wipe the abdomen and thorax regions with alcohol prep pads or 70 % alcohol, and open the skin to expose the abdomen and the thorax using surgical scissors. Remove the lungs, and expose the entire aorta until the renal bifurcation. Perfuse the aorta using a tuberculin syringe filled with sterile PBS to remove remaining blood. Working rapidly, the aorta is dissected out and placed in ~100 μL of Fungizone in a 6-well tissue culture plate and cleaned off of the surrounding fat and adventitial tissue with the help of fine-tipped forceps. At this point, the aorta will be a smooth tube. Cover the plate and transfer it to a biosafety tissue culture hood.
In a biosafety tissue culture hood, cut the aorta into 1- to 2-mm pieces using fine scissors and place into 5-mL tubes containing 0.1 mL of collagenase type 2 solution. Gently close the lid of the tube and place in a 37 °C incubator (with 5 % CO2) for 4–6 h. Incubating for 5–6 h gives better results than 4 h.
After 6 h, remove the 5-mL tubes, and tap to suspend the cells. In the hood, add 3 mL of complete DMEM in the tube and gently mix the cell suspension. Centrifuge at 300×g for 5 min at room temperature in a tabletop centrifuge to pellet the cells. In the hood, remove the medium and wash the pellet using 3 mL of complete DMEM and centrifuge again at 300×g for 5 min. Resuspend the resulting cell pellet in 0.5–1 mL of complete DMEM and plate cells in a 24-well plate.
Place cells in 37 °C incubator with 5 % carbon dioxide, undisturbed for 5 days. Do not change the media.
Subculturing of Murine Aortic VSMCs
When confluent, passage the cells to achieve confluency on the fifth day (1:2 dilutions have been used in our lab). First, wash all the wells using 500 μL 1X sterile PBS. Incubate cells in 200 μL of trypsin with 0.25 % EDTA for 3–4 min, and tap the plate gently to detach the cells. Do not mix vigorously. Neutralize trypsin by adding 500 μL of complete DMEM. Pellet the cells by centrifuging at 800×g for 10 min, remove the supernatant, and resuspend the cells in complete DMEM. In a separate tube, mix an aliquot of cell suspension and Trypan blue in a ratio of 1:1, and count the total number of viable cells per milliliter using a hemocytometer. Typically, 25–35×103 cells/mL cells are obtained after 5 days of undisturbed growth.
Morphology
The morphology of murine adult aortic P1 VSMCs grown undisturbed for 5 days on plastic is predominantly a spindle shape with an oval-shaped nucleus containing two or more nucleoli (Fig. 1a–c). The cytoplasm appears phase dense and contains few visible inclusions. After subculturing and growth for five additional days, the morphology of the P2 VSMCs (Fig. 1d–e) is a mixture of “cobblestone-like” and spindle-shape morphologies.
Fig. 1.
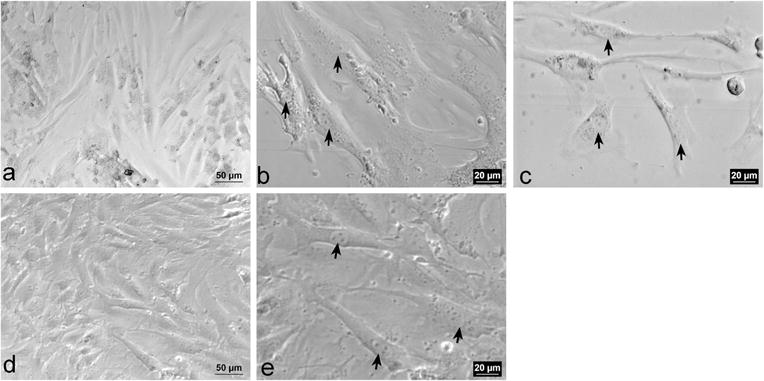
Representative phase-contrast images of P1 VSMCs. a–c Represent P1 VSMCs a Confluent monolayer of P1 VSMCs (×20). b and c Spindle-shaped VSMCs with phase dense cytoplasm and oval nuclei with two nucleoli (arrows, ×40). d–e Represent P2 VSMCs. d Confluent monolayer of P2 VSMCs (×20). e Spindle-shaped VSMCs with oval nuclei containing 3–5 nucleoli (arrows, ×40)
Purity of VSMCs
To determine the purity of the isolation procedure, we stained the VSMCs with sm22α and Acta2, two smooth muscle cell specific markers [6]. Figure 2a–f shows representative images of sm22α and Acta2 staining in P1 and P2 VSMCs. A total of 97–99 % of VSMCs stained positive for both sm22α and Acta2.
Fig. 2.
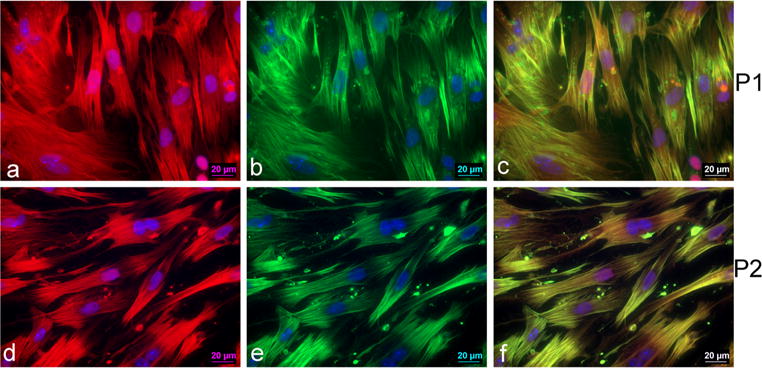
Representative micrographs of P1 VSMCs (×63). a Sm22α expression in P1 VSMCs, b Acta2 expression in P1 VSMCs, and c co-localization of sm22α and smooth muscle α-actin expression in P1 VSMCs. Representative micrographs of P2 VSMCs (×63). d Sm22α expression in P2 VSMCs. e Acta2 expression in P2 VSMCs. f Co-localization of sm22α and Acta2 expression in P2 VSMCs
Gene and Protein Expression
To assess gene expression and heterogeneity within and between passages, we assessed single cell gene expression of the smooth muscle specific markers caldesmon1 (Cald1), Cnn1, Transgelin (Tagln or sm22α), Acta2, smooth muscle myosin heavy chain (Myh11), and smoothelin (Smtn) (see Fig. 3a). The violin plots in Fig. 3a show the distribution of expression of six genes in P1 (n=22) and in P2 (n=45) cells. Cnn1 and Cald1 have a relatively tight expression pattern that is uniform between passages. Myh11 has a low level of expression in P1 that becomes more widely distributed in P2. Sm22α and Acta2 have a wide distribution pattern in P1 that is tighter and higher in P2, and Smtn has a wide distribution pattern in P1 and in P2. Western blotting showed an increased protein expression of sm22α at P2 compare to P1 (p<0.043, n=3) (Fig. 3b, c).
Fig. 3.
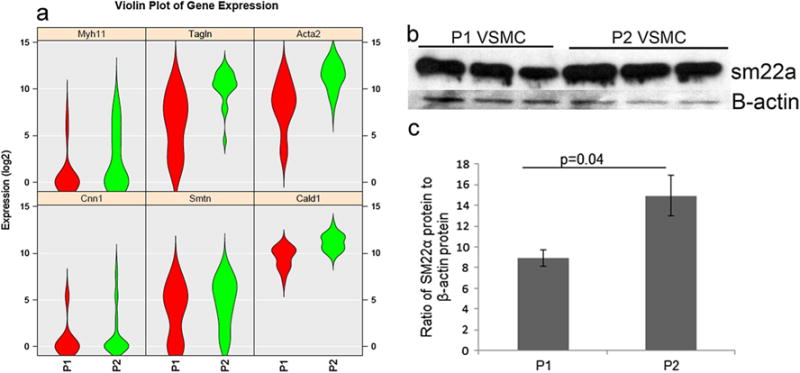
a Violin plots depicting single VSMC gene expression in P1 (red, n=22 cells) and P2 (green, n=45 cells). Genes include Myh11, Tagln (Sm22α), Acta2, Cnn1, Smtn, and Cald1. b Western blot analysis of SM22α and b-actin protein expression in P1 and P2 VSMC. c Densitometry analysis (n=3, p=0.04)
Proliferation
After 5 days of undisturbed growth, 61±1 % of VSMCs at P1 were proliferating compared to 53±1 % of VSMCs at P2 (n=3, p=0.001).
Single-Cell Contractility by Traction Force Microscopy (TFM)
Basal cell contractility of P1 and P2 VSMCs was assessed with TFM. Representative images are provided for bright-field image of a single cell, substrate with beads, and bead displacement after chemical relaxation (Fig. 4a–c). Basal tone, measured by strain energy, between P1 and P2 was not different (Fig. 4d). However, the distribution of strain energies for the P1 cell population was much larger compared to P2, indicating greater heterogeneity in the P1 population compared to P2 (Fig. 4d).
Fig. 4.
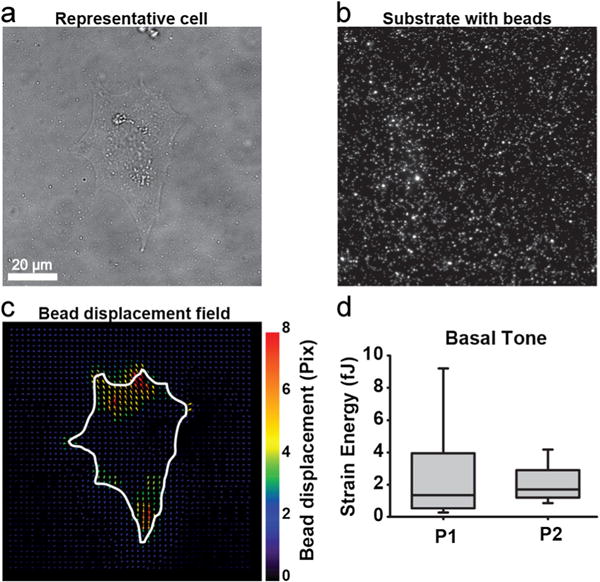
VSMC contractility at baseline using TFM. Representative cell traction force microscopy. White line, cell outline. a Bright-field image of cell. b Polyacrylamide substrate with 0.2 μm beads. c Bead displacements after cell is relaxed with HA-1077. d Basal tone measured by strain energy comparing P1 and P2 (n=24–25 in each cohort)
Discussion
This study provides guidelines for the isolation and subculturing of murine adult aortic VSMCs along with characterization profiles. These guidelines highlight the following major findings. The morphology of PI cells is spindle shaped compared to P2 which are a mixture of cobblestone and spindle shapes. Sm22α protein expression was increased in the P2 VSMCs compared to P1. Proliferation was greater in the P1 cells. Finally, RNA expression profiles of single VSMCs revealed heterogeneity in a wide number of RNAs including sm22α, Cnn1, Cald1, Myh11, and Acta2.
Generally, VSMCs isolated from adult aorta that have been grown between 5 and 7 days exhibit a spindle shape with an oval-shaped nucleus containing two or more nucleoli, and the cytoplasm is phase dense [11, 12]. Previously, the expression of Acta2 was used exclusively to verify the smooth muscle phenotype. However, it is recommended that multiple differentiation markers should be used to characterize the specific phenotype of cultured VSMC as no single marker may identify VSMCs exclusively at any passage or from other cell types [2, 6].
Our data in mouse VSMCs is similar to a comparative study of VSMC specific markers and morphology in rat aortic VSMCs [2]. Specifically, Thyberg and colleagues [13] demonstrated that rat adult aortic VSMCs in culture undergo structural changes within 2–4 days in culture including a transition from cytoplasmic microfilament bundles to an extensive rough endoplasmic reticulum and a large Golgi complex. Frid and colleagues demonstrated four different subpopulations of VSMCs within the medial wall of the bovine pulmonary artery and the aortic arch [9]. These four distinct subpopulations were identified in layers of the medial wall (lumen side, middle layer, and outer layers) and exhibited different staining patterns of VSMC specific markers smooth muscle α-actin (Acta2), smooth muscle myosin, calponin1 (Cnn1), and desmin, different morphology (small, irregular, thick, and thin spindle), and altered cell orientation (circumferential or longitudinal). Within the layers of the medial wall, VSMCs were found in clusters [9]. Our single cell RNA sequencing data supports the heterogeneity in VSMCs in the vessel wall. We identified a wide range of many RNAs in P1 and P2 cells including the genes known as smooth muscle cell markers.
P1 VSMCs showed greater proliferative capacity compared to P2. Chamley-Campbell et al. (1979) [12] reported that contractile VSMCs and subcultured VSMCs readily undergo cell division. Adult VSMCs are able to proliferate while in the “contractile state” although proliferation appears to be more prevalent in dedifferentiated VSMCs [12]. Our data supports this previous work.
We found no difference in basal contractility between P1 and P2. Chamley-Campbell, (1979) [12] reported that only a very small portion of primary VSMCs from rat, rabbit, monkey aorta, and human saphenous vein contract spontaneously or in response to chemical or electrical or mechanical stimulation while subcultured VSMC do not contract in response to any stimuli [1, 12]. However, several reports over the years have shown contraction of subcultured VSMCs from species including rat, bovine, pig, and human aorta, both in intact aortic strips and in culture, in response to stimuli like angiotensin II [14, 15], endothelin-1 and vasopressin [15–17], serotonin [12, 18, 19], and epidermal growth factor [20].
We show spontaneous contraction of single subcultured aortic VSMCs at P1 and P2 as measured by TFM. This method has been used to measure tractional stresses in cultured single VSMCs isolated from the pulmonary artery of newborn calves [21] to measure TGF-b induced contraction in primary human bladder smooth muscle cells [22], in isolated renal VSMCs [23], and in corneal fibroblasts [24].
In conclusion, we provide guidelines for isolation of mouse adult VSMC and characterization. We show that P1 VSMCs exhibit increased proliferation and a greater heterogeneity in the expression of VSMC marker genes, sm22α, Acta2, and Smtn. P2 VSMCs show increased protein expression of sm22α and a tighter distribution of sm22α RNA expression as well as Acta2 expression. These findings suggest that P2 VSMCs may provide more consistent data for experiments conducted in vitro.
Supplementary Material
Acknowledgments
The authors wish to thank the University of Minnesota Genomics Core for single-cell RNA sequencing.
Source of Funding The sources of funding are from DK54733 (LW), DK60521 (LW), LHI Innovation Grant (LW, JLH), and the Lindahl Foundation (JLH).
Abbreviations
- Acta2
Smooth muscle α-actin
- APS
Ammonium persulfate
- Cald1
Caldesmon1
- Cnn1
Calponin1
- EdU
5-Ethynyl-2′-deoxyuridine
- FN
Fibronectin
- Myh11
Smooth muscle myosin heavy chain
- P1
Passage 1
- P2
Passage 2
- PA
Polyacrylamide
- PDMS
Polydimethylsiloxane
- sm22α
(Tagln) Smooth muscle22α (also known as Transgelin (Tagln))
- Smtn
Smoothelin
- TFM
Traction force microscopy
- TEMED
Tetramethylethylenediamine
- VSMC
Vascular smooth muscle cell
Footnotes
Associate Editor Lorrie Kirshenbaum oversaw the review of this article
Electronic supplementary material The online version of this article (doi:10.1007/s12265-015-9616-6) contains supplementary material, which is available to authorized users.
The Use of Laboratory Animals All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the appropriate institutional committees.
Contributor Information
Neeta Adhikari, Division of Cardiology, Lillehei Heart Institute, Department of Medicine, University of Minnesota, 4-138 CCRB, 2231 6th Street SE, Minneapolis, MN 55455, USA.
Kadambari Chandra Shekar, Division of Cardiology, Lillehei Heart Institute, Department of Medicine, University of Minnesota, 4-138 CCRB, 2231 6th Street SE, Minneapolis, MN 55455, USA.
Rodney Staggs, Division of Cardiology, Lillehei Heart Institute, Department of Medicine, University of Minnesota, 4-138 CCRB, 2231 6th Street SE, Minneapolis, MN 55455, USA.
Zaw Win, Department of Bioengineering, University of Minnesota, Minneapolis, MN, USA.
Kerianne Steucke, Department of Bioengineering, University of Minnesota, Minneapolis, MN, USA.
Yi-Wei Lin, Department of Pharmacology, University of Minnesota, Minneapolis, MN, USA.
Li-Na Wei, Department of Pharmacology, University of Minnesota, Minneapolis, MN, USA.
Patrick Alford, Department of Bioengineering, University of Minnesota, Minneapolis, MN, USA.
Jennifer L. Hall, Email: jlhall@umn.edu, Division of Cardiology, Lillehei Heart Institute, Department of Medicine, University of Minnesota, 4-138 CCRB, 2231 6th Street SE, Minneapolis, MN 55455, USA.
References
- 1.Chamley JH, Campbell GR, McConnell JD, Groschel-Stewart U. Comparison of vascular smooth muscle cells from adult human, monkey and rabbit in primary culture and in subculture. Cell and Tissue Research. 1977;177(4):503–522. doi: 10.1007/BF00220611. [DOI] [PubMed] [Google Scholar]
- 2.Firulli AB, Han D, Kelly-Roloff L, Koteliansky VE, Schwartz SM, Olson EN, Miano JM. A comparative molecular analysis of four rat smooth muscle cell lines. In Vitro Cellular and Developmental Biology - Animal. 1998;34(3):217–226. doi: 10.1007/s11626-998-0127-5. [DOI] [PubMed] [Google Scholar]
- 3.Lemire JM, Covin CW, White S, Giachelli CM, Schwartz SM. Characterization of cloned aortic smooth muscle cells from young rats. American Journal of Pathology. 1994;144(5):1068–1081. [PMC free article] [PubMed] [Google Scholar]
- 4.Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. Journal of Cell Biology. 1971;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thyberg J. Differentiated properties and proliferation of arterial smooth muscle cells in culture. International Review of Cytology. 1996;169:183–265. doi: 10.1016/s0074-7696(08)61987-7. [DOI] [PubMed] [Google Scholar]
- 6.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiological Reviews. 2004;84(3):767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 7.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiological Reviews. 2003;83(4):1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 8.Thomas WA, Florentin RA, Reiner JM, Lee WM, Lee KT. Alterations in population dynamics of arterial smooth muscle cells during atherogenesis. IV. Evidence for a polyclonal origin of hypercholesterolemic diet-induced atherosclerotic lesions in young swine. Experimental and Molecular Pathology. 1976;24(2):244–260. doi: 10.1016/0014-4800(76)90009-5. [DOI] [PubMed] [Google Scholar]
- 9.Frid MG, Moiseeva EP, Stenmark KR. Multiple phenotypically distinct smooth muscle cell populations exist in the adult and developing bovine pulmonary arterial media in vivo. Circulation Research. 1994;75(4):669–681. doi: 10.1161/01.res.75.4.669. [DOI] [PubMed] [Google Scholar]
- 10.Champy C. Quelques resultats de la methode de culture des tissus. I. Generalities. II. Le muscle lisse. Arch Zool Exp Gen. 1913;53:42–51. [Google Scholar]
- 11.Ray JL, Leach R, Herbert JM, Benson M. Isolation of vascular smooth muscle cells from a single murine aorta. Methods in Cell Science. 2001;23(4):185–188. doi: 10.1023/a:1016357510143. [DOI] [PubMed] [Google Scholar]
- 12.Chamley-Campbell J, Campbell GR, Ross R. The smooth muscle cell in culture. Physiological Reviews. 1979;59(1):1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Thyber J, Palmberg L, Nilsson J, Ksiazek T, Sjolund M. Phenotype modulation in primary cultures of arterial smooth muscle cells. On the role of platelet-derived growth factor. Differentiation. 1983;25(2):156–167. doi: 10.1111/j.1432-0436.1984.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 14.Do KH, et al. Angiotensin II-induced aortic ring constriction is mediated by phosphatidylinositol 3-kinase/L-type calcium channel signaling pathway. Experimental and Molecular Medicine. 2009;41:569–576. doi: 10.3858/emm.2009.41.8.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gohla A, Schultz G, Offermanns S. Role for G12/G13 in agonist-induced vascular smooth muscle cell contraction. Circulation Research. 2000;87(3):221–227. doi: 10.1161/01.res.87.3.221. [DOI] [PubMed] [Google Scholar]
- 16.Cain AE, Tanner DM, Khalil RA. Endothelin-1-induced enhancement of coronary smooth muscle contraction via MAPK-dependent and MAPK-independent [Ca2+]i sensitization pathways. Hypertension. 2002;39(2):543–549. doi: 10.1161/hy0202.103129. [DOI] [PubMed] [Google Scholar]
- 17.Wynne BM, Chiao CW, Webb RC. Vascular smooth muscle cell signaling mechanisms for contraction to angiotensin II and endothelin-1. Journal of the American Society of Hypertension. 2009;3(2):84–95. doi: 10.1016/j.jash.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Nueten JM, Janssens WJ, Vanhoutte PM. Serotonin and vascular reactivity. Pharmacological Research Communications. 1985;17(7):585–608. doi: 10.1016/0031-6989(85)90067-0. [DOI] [PubMed] [Google Scholar]
- 19.Watts SW. Serotonin activates the mitogen-activated protein kinase pathway in vascular smooth muscle: use of the mitogen-activated protein kinase kinase inhibitor PD098059. Journal of Pharmacology and Experimental Therapeutics. 1996;279(3):1541–1550. [PubMed] [Google Scholar]
- 20.Berk BC, Brock TA, Webb RC, Taubman MB, Atkinson WJ, Gimbrone MA, Alexander RW. Epidermal growth factor, a vascular smooth muscle mitogen, induces rat aortic contraction. Journal of Clinical Investigation. 1985;75(3):1083–1086. doi: 10.1172/JCI111772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polte TR, Eichler GS, Wang N, Ingber DE. Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. American Journal of Physiology Cell Physiology. 2004;286:C518–C528. doi: 10.1152/ajpcell.00280.2003. [DOI] [PubMed] [Google Scholar]
- 22.Ramachandran A, et al. JunB mediates basal- and TGFβ1-induced smooth muscle cell contractility. PloS One. 2013;8(1):e53430. doi: 10.1371/journal.pone.0053430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balasubramanian L, Lo CM, Sham JSK, Yip KP. Remanent cell traction force in renal vascular smooth muscle cells induced by integrin-mediated mechanotransduction. American Journal of Physiology Cell Physiology. 2013;304:C382–C391. doi: 10.1152/ajpcell.00234.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Li H, SundarRaj N, Wang JHC. Alpha-smooth muscle actin expression enhances cell traction force. Cell Motility and the Cytoskeleton. 2007;64(4):248–257. doi: 10.1002/cm.20178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


