Abstract
Objectives
We previously reported the efficacy of nab-paclitaxel added to cisplatin, 5-FU, and cetuximab (APF-C) followed by concurrent high dose bolus cisplatin and radiation therapy (CRT) in patients with locally advanced head and neck squamous cell carcinoma (HNSCC). In this phase II trial, we determined the efficacy of APF (without cetuximab) followed by CRT in similar patients.
Materials and methods
Eligible patients had stage III–IV oropharynx (OP), larynx, or hypopharynx SCC and adequate organ function and performance status. T1 tumors were excluded. Patients were treated with three cycles of APF followed by CRT. Efficacy endpoints included two-year disease-specific survival (DSS), progression-free survival (PFS), overall survival (OS), and relapse rate.
Results
Thirty patients were enrolled. Most patients were smokers (77%) with bulky T3/4 (73%) and N2/3 (83%) tumors. Analyses were stratified for human papilloma virus (HPV) status: HPV-related OPSCC (n = 17; 57%) and HPV-unrelated HNSCC (n = 13; 43%). With a minimum follow-up of 21 months, relapse occurred in 1 (3%) patient. Two-year DSS was 94% in HPV-related OPSCC and 100% in HPV-unrelated HNSCC. Two-year PFS was 94% in HPV-related OPSCC and 100% in HPV-unrelated HNSCC. Two-year OS was 94% in HPV-related OPSCC and 92% in HPV-unrelated HNSCC. Causes of death were relapse (1), treatment-related mortality (1), and co-morbidity (1). Two patients with HPV-unrelated HNSCC treated with APF declined CRT and remained free of relapse at 36 and 28 months of follow-up.
Conclusion
This phase II trial demonstrated favorable two-year DSS, PFS, and OS and a low relapse rate in HPV-unrelated HNSCC and HPV-related OPSCC treated with APF followed by CRT.
Keywords: Head and neck cancer, nab-Paclitaxel, Cisplatin, 5-Fluorouracil, Induction, Chemotherapy
Introduction
Induction chemotherapy followed by radiation therapy (RT) is an organ-sparing treatment approach for selected sub-sites of locally advanced head and neck squamous cell carcinoma (HNSCC) [1]. Induction regimens originally included cisplatin and 5-fluorouracil (PF) [2]. However, more recent phase III trials showed that docetaxel added to PF (TPF) resulted in superior efficacy in patients treated with RT [3] or carboplatin and RT [4]. After randomized trials showed improvement in overall survival (OS) when the epidermal growth factor receptor (EGFR) inhibitor cetuximab was added to definitive RT or palliative chemotherapy [5,6], cetuximab was added to induction regimens in hopes of improving outcomes. Clinical trials which added cetuximab to induction regimens followed by chemotherapy and RT showed favorable outcomes; however, none of these trials had controlled comparison groups to establish that the addition of cetuximab was beneficial [7–9].
We reported excellent efficacy of a novel induction regimen that included nab-paclitaxel followed by concurrent high dose bolus cisplatin and RT (CRT) administered to patients with HNSCC [10]. nab-paclitaxel, a nanoparticle albumin-bound taxane that may improve drug delivery to tumor tissue [11–18], was added to PF and cetuximab (APF-C) followed by CRT. Most patients were smokers (90%) with bulky T3/4 (73%) and N2/3 (80%) tumors. Analyses were stratified for human papilloma virus (HPV) status: HPV-related oropharyngeal (OP) SCC (n = 17; 57%) and HPV-unrelated HNSCC (n = 13; 43%). With a minimum follow-up of 25 months (median 59), relapse occurred in 5 (17%) patients. Two-year disease-specific survival (DSS) was 100% in HPV-related OPSCC and 91% in HPV-unrelated HNSCC. Two-year OS was 94% in HPV-related OPSCC and 83% in HPV-unrelated HNSCC. Efficacy outcomes of patients treated with APF-C followed by CRT were better than those of a historical group treated with TPF and cetuximab (TPF-C) followed by CRT [19].
Given the absence of randomized trials confirming a benefit of cetuximab added to induction regimens, we performed a phase II trial in a similar cohort of patients treated with APF (without cetuximab) followed by CRT. The trial objective was to determine the efficacy of APF followed by CRT as assessed by the following endpoints: DSS, PFS, OS, and relapse rates.
Methods
Patient selection criteria
Eligible patients were 18 years of age or older with untreated HNSCC stages III and IVa/b [20] (T1 excluded) originating in the oropharynx (OP), larynx, and hypopharynx. Other criteria included adequate performance status (ECOG 0–2) and vital organ function. Key exclusion criteria included ≥ Grade 2 peripheral neuropathy. The Washington University Human Research Protection Office approved the protocol and all study participants signed informed consent. The clinical trial was registered at ClinicalTrials.gov NCT01566435.
Study design
APF consisted of every three week cycles of intravenous nab-paclitaxel 100 mg/m2 weekly on days 1, 8, and 15, cisplatin 75 mg/m2 on day 1 and 5-FU 750 mg/m2 continuous infusion daily on days 1–3. After two cycles of APF, an assessment of tumor response at the primary site was performed by clinical examination, as previously described [10], using categorical outcomes [2,21]: complete response (CR) – no residual tumor; partial response (PR) – at least 50% reduction in tumor size; stable disease (SD)-less than 50% reduction, but no progression; and progressive disease (PD)-interval enlargement. Patients with favorable (CR, PR) tumor response at the primary site received a third cycle of APF before CRT whereas patients with an unfavorable (SD, PD) tumor response proceeded to CRT or to surgery followed by adjuvant therapy.
Definitive CRT began 22–56 days after initiation of cycle 3 of APF, and was given as previously described [10]. Intensity modulated RT was used in all patients. Patients with serum creatinine <2.0 mg/dL were scheduled to receive high dose bolus cisplatin (100 mg/m2 on days 1, 22 and 43) with RT. Patients who failed to meet this criteria received cetuximab (400 mg/m2 IV loading dose one week before RT, then 250 mg/m2 weekly × 7) as a single agent concurrent with RT.
Standard assessments
Baseline assessments included physical examination, laryngoscopy, computed tomography (CT) neck, and fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT. After two cycles of APF and at 10–12 weeks after RT, patients underwent tumor response assessment by clinical examination and CT neck. FDG-PET/CT was performed after two cycles of APF. RECIST version 1.0 was used to determine tumor response by CT [22]. After therapy, tumor status was assessed with physical examination and fiberoptic office laryngoscopy every 2–3 months for the 2 years, 4 months for years 3 and 4 and then every 6–12 months. A FDG-PET/CT was performed 3–4 months after therapy. CT neck/chest was performed at 6, 12, 20, 28 and 36 months.
Patient’s co-morbidities were quantified using the Adult Co-morbidity Evaluation (ACE)-27 index [23]. Adverse events (AE) were monitored weekly during therapy, and then every 1–6 months. AEs were graded using NCI-CTCAE version 3.0. Smokers were defined as current or former tobacco users.
Studies performed on archived tumor tissue
Archival tumor tissue obtained at diagnosis was used to assess p16 status, a surrogate marker of HPV. Methodology for p16 by immunohistochemistry (IHC) was previously described [24].
Objectives and statistical analyses
The primary objective of the analysis was to determine the DSS (stratified by HPV status) of patients treated APF followed by CRT. Secondary objectives were to determine PFS, OS, relapse rate, and AEs of patients treated with APF followed by CRT. Tumor response at the primary site after two cycles of APF was also determined. The sample size was calculated based on the proportion of patients with a CR at the primary tumor site based on visual examination after two cycles of APF. The historical rate CR rate at the primary tumor site after two cycles of APF-C was 53% [10]. A difference of as much as 23% was considered to be clinically acceptable, and resulted in a patient target number practically achievable at a single institution within a reasonable time frame. Under these conditions, a sample size of 30 patients had a power of 0.89 at a maximum significance level of 0.084 to conclude that the CR rate at the primary tumor site after two cycles of APF was not inferior to that expected with APF-C.
Efficacy endpoints were stratified by HPV status. OS (time from diagnosis to death or last follow-up alive), DSS (time from diagnosis to death due to disease progression or last follow-up alive), and PFS (time from diagnosis to death due to disease progression, to disease progression, or last follow-up alive) were estimated by the Kaplan-Meier (KM) method [25]. RT delivery (number of fractions, elapsed days, total dose) and chemotherapy administration (number of planned doses administered, total dose/m2) were summarized using proportions with medians and ranges.
Results
Patient and tumor characteristics
Thirty patients were enrolled onto this phase II trial. Most patients were smokers (77%) with co-morbidities (77%) and had bulky T3/4 (73%) and N2/3 (83%) tumors (Table 1). Stage IV disease was present in 27 (90%) patients. Analyses were stratified for HPV status: HPV-related OPSCC (n = 17; 57%) and HPV-unrelated HNSCC (n = 13; 43%). Twelve patients with larynx or hypopharynx SCC were referred for an organ preservation approach to avoid total laryngectomy. Eighteen patients with OPSCC were referred with unresectable disease (n = 14; due to encasement of the carotid artery, skull base involvement, or extension to the nasopharynx) or to avoid open surgery and/or free flap reconstruction procedures (n = 4).
Table 1.
Patient and tumor characteristics.
| Characteristic | APF (n = 30) |
|---|---|
| Age (years) | |
| Median | 57 |
| Range | 43–75 |
| Gender | |
| Male | 25 |
| Female | 5 |
| Smoking history | |
| Yes | 23 |
| No | 7 |
| ACE score | |
| 0 (none) | 7 |
| 1 (mild) | 12 |
| 2–3 (moderate-severe) | 11 |
| T classification | |
| 2 | 8 |
| 3 | 13 |
| 4a | 8 |
| 4b | 1 |
| N classification | |
| 0–1 | 5 |
| 2a | 0 |
| 2b | 6 |
| 2c | 12 |
| 3 | 7 |
| Overall stage | |
| III | 3 |
| IVA | 18 |
| IVB | 9 |
| Primary site | |
| Oropharynx | 18 |
| Larynx | 9 |
| Hypopharynx | 3 |
| Oral Cavity | 0 |
| HPV status – oropharynx only | |
| p16+ | 17/18 |
Patient flow, treatment delivery, and median follow-up
All patients completed two cycles of APF. Twenty-eight of 30 patients completed three cycles of APF and were treated with RT and cisplatin (n = 25) or cetuximab (n = 1) and 2 declined CRT. Two of 30 patients experienced an unfavorable tumor response at the primary site after two cycles of APF. One patient with T4N2c laryngeal SCC had exam findings interpreted as stable disease and then underwent resection and pathology showed no cancer. The second patient with T4N2c HPV-related OPSCC experienced disease progression, received CRT, and expired due to progressive disease.
Delivery of APF and CRT was favorable (Table 2). Only one patient received cetuximab during RT, due to the interim development of renal dysfunction. The median follow-up of surviving patients was 29 (21–39) months.
Table 2.
Treatment delivery.
| Treatment | Chemotherapy | Radiation | |
|---|---|---|---|
| Mean % total dose (range) | Mean dose (range) in Gy | Mean (range) elapsed days | |
| Induction chemotherapy (n = 30) | |||
| nab-Paclitaxel | 89 (53–100) | – | – |
| Cisplatin | 95 (50–100) | – | – |
| 5-Fluorouracil | 95 (67–100) | – | – |
| Radiation therapy (n = 27) | – | 70 (64–70) | 53 (43–76) |
| Chemotherapy (with RT) | |||
| Cisplatin (n = 26) | 87 (67–100) | – | – |
| Cetuximab (n = 1) | 100 | – | – |
Adverse events during APF
AEs that occurred during APF are shown in Table 3. Notable grade 3–4 AEs included neutropenia (23%), febrile neutropenia (13%), diarrhea (10%), mucositis (3%), elevated AST and/or ALT (3%), and sensory neuropathy (3%).
Table 3.
AE’s that occurred during APF.
| Toxicity | Grade 1–2 AEs |
Grade 3–4 AEs |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Hematological | ||||
| Neutropenia | 9 | 30 | 7 | 23 |
| Thrombocytopenia | 11 | 37 | 0 | 0 |
| Anemia | 24 | 80 | 2 | 7 |
| Auditory/ear | ||||
| Tinnitus | 3 | 10 | 1 | 3 |
| Dermatologic | ||||
| Rash | 5 | 17 | 0 | 0 |
| Gastrointestinal | ||||
| Diarrhea | 9 | 30 | 3 | 10 |
| Mucositis | 8 | 27 | 1 | 3 |
| Infection | ||||
| Febrile neutropenia | 0 | 0 | 4 | 13 |
| Lymphatics | ||||
| Edema: Limb | 5 | 17 | 0 | 0 |
| Metabolic/laboratory | ||||
| Creatinine | 7 | 23 | 0 | 0 |
| ALT | 4 | 13 | 1 | 3 |
| AST | 2 | 7 | 1 | 3 |
| Neurological | ||||
| Sensory neuropathy – peripheral | 4 | 13 | 1 | 3 |
| Constitutional | ||||
| Fatigue | 13 | 43 | 1 | 3 |
| Weight loss | 7 | 23 | 6 | 20 |
Tumor response at the primary and neck nodal sites after two cycles of APF
In HPV-unrelated HNSCC, the CR rate at the primary site after two cycles of APF was 77%, (10 of 13 patients). The PR rate at the primary site was 15% (2 patients). In HPV-related OPSCC, the CR rate at the primary site was 76% (13 out of 17 patients). The PR rate at the primary site was 18% (3 patients). Tumor responses at the primary and neck nodal sites and overall based on clinical examination, CT and FDGPET/CT are shown in Table 4.
Table 4.
Tumor response assessment at primary, neck nodal sites, and overall (both sites) by clinical exam, CT, and FDG-PET/CT after two cycles of APF.
| Assessment | Primary site | Neck nodes | Overall | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eval (#) | CR (%) | PR (%) | SD/PD (%) | Eval (#) | CR (%) | PR (%) | SD/PD (%) | Eval (#) | CR (%) | PR (%) | SD/PD (%) | |
| Clinical exam | 30 | 77 | 17 | 7 | 28 | 68 | 25 | 7 | 30 | 60 | 37 | 3 |
| CT scan | 30 | 50 | 33 | 17 | 26 | 42 | 27 | 31 | 30 | 30 | 60 | 10 |
| FDG-PET/CT | 28 | 32 | 64 | 4 | 27 | 33 | 56 | 11 | 29 | 10 | 86 | 3 |
Surgical treatment following APF or definitive therapy
One patient with T4N2c laryngeal SCC had stable disease after two cycles of APF underwent total laryngectomy with neck dissections and pathology showed no cancer. One patient with T2N3 HPV-related OPSCC had a residual neck mass after CRT and underwent a neck dissection, which showed no cancer.
Feeding tubes and tracheostomy
Percutaneous endoscopic gastrostomy (PEG) tube was placed in most patients (69%) during CRT but all were removed by year 1 after therapy (Table 5). Ten percent of patients had a tracheostomy tube placed. Peripheral neuropathy was present in 11 patients (39%) at one year after therapy.
Table 5.
Percutaneous endoscopic gastrostomy (PEG) tube, tracheostomy, weight, and neuropathy at key time points.
| Baseline | End of APF | End of CRT | Year 1 after CRT | |
|---|---|---|---|---|
| PEG # (%) |
3 (10%) |
5 (17%) |
20 (69%) |
0 (0%) |
| Tracheostomy # (%) |
3 (10%) |
2 (7%) |
1 (3%) |
2 (7%) |
| Weight (kg) Mean (range) |
85 (52–125) |
83 (56–128) |
77 (50–113) |
75 (51–110) |
| Neuropathy (all grades) |
0 (0%) |
5 (16.7%) |
7 (24.1%) |
11 (39.3%) |
Relapse events, DSS, PFS, and OS
Relapse of disease occurred in 1 patient (3%). Relapse occurred at local-regional and distant sites in a patient with T4N2c HPV-related OPSCC.
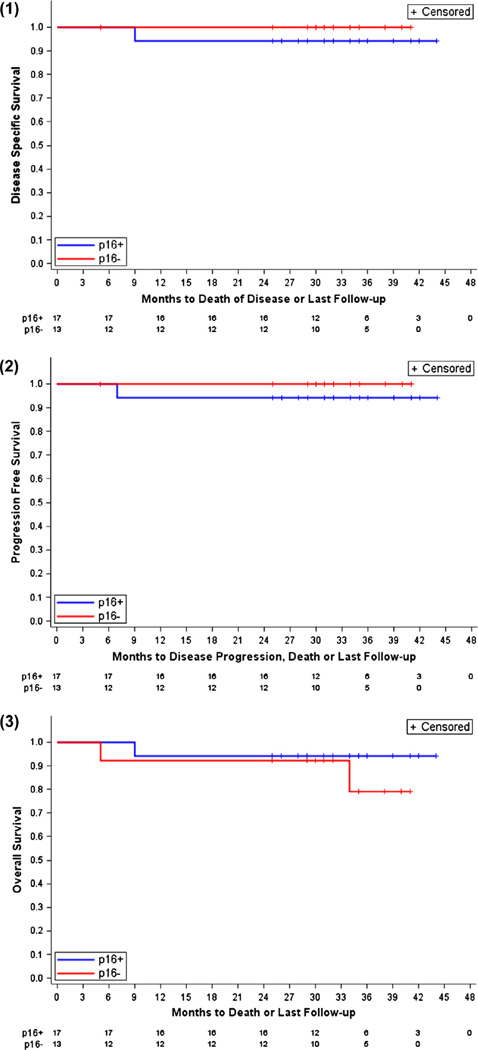
The two-year DSS was 97%, two-year PFS was 97%, and the two-year OS was 93%. In HPV-related OPSCC (n = 17), two-year DSS was 94%, two-year PFS was 94%, and two-year OS was 94%. In HPV-unrelated HNSCC (n = 13), two-year DSS was 100%, two-year PFS was 100%, and two-year OS was 92% (Fig. 1). Causes of death (n = 3) were relapsed disease, treatment-related mortality (TRM), and co-morbidity. The TRM event occurred in the patient who had stable disease after two cycles of APF, underwent resection and expired after surgery due to bacterial sepsis. The co-morbidity death occurred 29 months after CRT.
Fig. 1.
Survival curves: 1. DSS, stratified by p16 (HPV) status. 2. PFS, stratified by p16 (HPV) status. 3. OS, stratified by p16 (HPV) status.
Outcome of two patients treated with APF alone (no CRT and no surgery)
Interestingly, two patients treated with three cycles of APF declined CRT and received no further therapy. Both patients remained free of disease relapse at 36 and 28 months of follow-up. One patient, a 70 year old who smoked, presented with T3N1M0 (Stage III) SCC of the supraglottic larynx. Tracheostomy and a PEG tube were placed for airway management and nutritional support. The patient declined total laryngectomy/neck dissections, and was referred for larynx preservation approach. After two cycles of APF, video laryngoscopy showed a CR at the primary site, CT scan and FDG-PET/CT showed CR at the primary site and neck node. After cycle three of APF, the patient declined CRT. After APF, laryngoscopy and CT scans were performed every 3–6 months with no signs of recurrent cancer detected. The tracheostomy was decannulated and the PEG tube removed two months after therapy. At 36 months of follow-up, the patient’s voice quality and swallowing function were normal, and weight was 5 kg over pretreatment weight.
The second patient, a 52 year old who smoked, presented with a T4aN0M0 SCC (Stage IVa) of the glottic and supraglottic larynx. The patient declined total laryngectomy/neck dissections, and was referred for larynx preservation approach. After two cycles of APF, laryngoscopy, CT scan, and FDG-PET/CT showed CR at the primary site. Biopsies of the primary site showed no cancer. After cycle three of APF, the patient declined CRT. After APF, clinical examinations and CT scans were performed at regular intervals and showed no signs of recurrent cancer. At 28 months of follow-up, the patient’s voice quality and swallowing function were normal, and weight was 6 kg over pre-treatment weight.
Discussion
This analysis included 30 patients with HNSCC treated on a phase II trial with nab-paclitaxel-based induction chemotherapy (APF) followed by CRT. Survival outcomes were very favorable with two-year DSS of 97%, two-year PFS of 97%, and two-year OS of 93%. Nearly all patients (96.7%) had one or more poor risk features including bulky primary site and/or neck nodal disease (defined as T3–4 and/or N2b/3 classifications) and a smoking history. Survival outcomes were also favorable when stratified by HPV-related OPSCC (two-year DSS 94%, two-year PFS 94%, and OS 94%) and HPV-unrelated HNSCC (two-year DSS 100%, two-year PFS 100%, and OS 92%). Prior studies showed that the interval in which the majority (>90%) of relapse events occur in both HPV-related OPSCC and HPV-unrelated HNSCC is eighteen months [2,4,26]. The minimum follow-up for our patients was 21 months.
It is important to place these data in the context of current nonsurgical therapy. Focusing first on HPV-related OPSCC treated with CRT, Ang et al. stratified patients into high, intermediate and low risk disease with three-year OS of 46%, 71% and 93%, respectively [27]. Using the Ang et al. risk stratification model, 17 of our 18 patients with HPV-related OPSCC treated with APF and CRT would be placed in intermediate (n = 9) and low (n = 8) risk groups with three-year OS of 88.9% and 100%, respectively. Only one patient would be in the high risk group and that patient remained disease-free at 21 months of follow-up. The small number of patients in each risk group limits the comparison; however, OS of patients with HPV-related OPSCC treated with APF followed by CRT was very favorable across these intermediate and low risk groups.
The DeCIDE and Paradigm trials showed that TPF followed by CRT or CRT alone resulted in similar OS [28,29]. In the DeCIDE trial, three-year OS was approximately 80% in HPV-related HNSCC and 75% in non-oropharyngeal (presumably HPV-unrelated) HNSCC [28]. In the Paradigm trial, two-year OS was 73–78% for all patients [29]. DeCIDE and Paradigm did not prospectively stratify for HPV status and both included patients with more favorable characteristics (T1 and/or higher proportion with stage III disease) than in the APF trial. Relapse risks were 24% in DeCIDE and Paradigm trials, but only 3% in the APF trial. In sum, APF followed by CRT is a highly effective therapy for HPV-related OPSCC and HPV-unrelated HNSCC.
We previously reported the efficacy outcomes in patients with HNSCC treated with APF-C followed by CRT [10]. With a minimum follow-up of 25 months, relapse occurred in 17%. Two-year DSS was 100% in HPV-related OPSCC and 91% in HPV-unrelated HNSCC. In the current trial, a cohort with similar patient and tumor characteristics were treated with APF (no cetuximab) followed by CRT. With a minimum follow-up of 21 months, relapse occurred in only 3%. Two-year DSS was 94% in HPV-related OPSCC and 100% in HPV-unrelated HNSCC. The favorable efficacy outcomes of both cohorts prompted us to move forward with nab-paclitaxel-based induction regimens that do not include cetuximab. Our next generation study is evaluating the efficacy of “AP” (eliminating 5-FU) followed by CRT in one group of patients (ECOG PS 0–1; no major co-morbidities) and nab-paclitaxel (“A”) monotherapy (eliminating 5-FU and cisplatin) followed by concurrent cetuximab and RT in another group (ECOG PS 2–3 and/or major co-morbidities) [“The APA Trial: NCT02573493”].
Tumor response at the primary site to induction PF was predictive of relapse after RT and is used as a decision point to direct subsequent treatment: RT for favorably responsive tumors or salvage surgery for poorly responsive tumors [2,21,30]. The high CR rate (77%) at the primary site following two cycles of APF in combination with the favorable DSS are evidence that APF is ideal to select patients for de-escalation CRT. A de-escalation strategy was tested in the ECOG-1308 trial in which patients with HPV-related OPSCC were treated with induction cisplatin, paclitaxel, and cetuximab followed by cetuximab with low dose (54 Gy) RT for patients with CR after induction or full dose (69.3 Gy) RT for all other patients [31]. To be most effective, this strategy must maximize the proportion of patients directed to de-escalation CRT by utilization of an induction regimen that results in the highest CR rate. APF resulted in a CR at the primary site in 76% of patients with HPV-related OPSCC and in 77% with HPV-unrelated HNSCC. Given these high CR rates and favorable DSS observed with APF followed by CRT, an equal argument can be made to investigate de-escalation of CRT in HPV-unrelated HNSCC as well as HPV-related OPSCC.
Two of the patients in this trial received only APF as treatment because they declined CRT. Neither patient underwent surgery. At 36 and 28 months of follow-up, disease relapse has not occurred. Both patients were heavy smokers with T3 or T4 laryngeal SCC. CR at the primary site and neck nodes was documented in each patient as assessed by laryngoscopy (with biopsy in one patient), CT scans, and FDG-PET/CT. At last follow-up, both patients retained a functional larynx with normal voice and swallowing function. Given the absence of recurrence with long term follow-up, these two patients were likely cured of laryngeal SCC with chemotherapy alone. A major benefit to these patients was that they did not experience the serious acute or long term toxicities of CRT. The striking observation that locally advanced HNSCC may be cured with chemotherapy alone, without RT or surgery, is particularly interesting in the current era of de-escalation of CRT. All current de-escalation trials are evaluating dose or volume reduction of RT or dose reduction or elimination of concurrent chemotherapy. De-escalation trials are only being performed in patients with HPV-related OPSCC and usually in the most favorable groups (limited smoking history; T4 and N3 excluded). Our observation supports that some patients with HNSCC may be cured with APF alone, even those with T3 or T4 HPV-unrelated (laryngeal) HNSCC.
The role of induction chemotherapy is controversial [32,33]. The DeCIDE and Paradigm trials failed to show an OS benefit with induction TPF added to CRT [28,29]. Challenges in interpreting the data from these trials include the unexpected epidemic of good prognosis HPV-related OPSCC and accrual issues [33]. Nevertheless, the results of these trials slowed the application and investigation of induction chemotherapy. In this context, it is important to reflect on why one should continue to investigate induction chemotherapy and specifically why investigate APF. The answer to the first question can be found by stating the weaknesses of the CRT paradigm: acute toxicity burden limiting broad application, high rates of disease recurrence in HPV-unrelated HNSCC, long term toxicities, and the increasing range of targeted therapeutic agents under evaluation for HNSCC. To improve the efficacy of the CRT paradigm, the induction window is the ideal time to add treatment since doing so during or after CRT is prohibitive in most patients. To answer the second question, the results of APF followed by CRT are informative: relapse rate of 3% and two-year OS rates of 94% in HPV-related OPSCC and 92% in HPV-unrelated HNSCC. It could be argued that the outcomes with APF and CRT are no better than CRT alone in patients with HPV-related OPSCC. However, in patients with HPV-unrelated HNSCC, the outcomes with APF are favorable compared to reports with CRT alone [28,29]. Also, APF is an ideal regimen to use to select patients for de-escalation approaches because the tumor response rate is very high. For these reasons, continued investigation of nab-paclitaxel-based induction chemotherapy and CRT in patients with HPV-unrelated HNSCC is reasonable.
There are limitations of this study. Most patients had oropharynx and larynx SCC; therefore, these results cannot be extrapolated to other HNSCC sub-sites. Although the efficacy analysis was stratified for HPV status, there is some heterogeneity in each of the subgroups that is difficult to control for in a small sample size of 30 patients. This trial was performed at a single academic institution with a large volume practice and excellent multidisciplinary subspecialty physicians and supportive care, so the results should be validated across diverse programs.
This analysis demonstrated very favorable two-year DSS, PFS, and OS and a very low relapse rate in HPV-unrelated HNSCC and in HPV-related OPSCC treated with APF followed by CRT. Based on these data, current multi-center studies are utilizing the tumor response to nab-paclitaxel-based induction regimens to direct de-escalation RT and surgery-based algorithms in patients with HPV-unrelated HNSCC and HPV-related OPSCC.
Acknowledgments
Disclosures statement
Research Support – Celgene.
The Siteman Cancer Center is supported in part by NCI Cancer Center Support Grant#P30CA91842. We would like to thank Celgene for financial support of this trial.
Dr. Wildes’ research is supported by Grant Number 1K12CA167540 through the National Cancer Institute (NCI) at the National Institutes of Health (NIH) and Grant Number UL1TR000448 through the Clinical and Translational Science Award (CTSA) program of the National Center for Advancing Translational Sciences (NCATS) at the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCI, NCATS, or NIH.
Footnotes
Presented in abstract form at the 2014 and 2015 Annual Meetings of the American Society of Clinical Oncology
References
- 1.National Comprehensive Cancer Network. [accessed April 11, 2016];Head and Neck Cancers (Version 1.2015) doi: 10.6004/jnccn.2015.0102. < http://nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 3.Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–1704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 4.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357(17):1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 5.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 6.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 7.Haddad RI, Tishler RB, Norris C, et al. Phase I study of C-TPF in patients with locally advanced squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27(27):4448–4453. doi: 10.1200/JCO.2009.22.1333. [DOI] [PubMed] [Google Scholar]
- 8.Kies MS, Holsinger FC, Lee JJ, et al. Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. J Clin Oncol. 2010;28(1):8–14. doi: 10.1200/JCO.2009.23.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Argiris A, Heron DE, Smith RP, et al. Induction docetaxel, cisplatin and cetuximab followed by concurrent radiotherapy, cisplatin and cetuximab and maintenance cetuximab in patients with locally advanced head and neck cancer. J Clin Oncol. 2010;28(36):5294–5300. doi: 10.1200/JCO.2010.30.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adkins D, Ley J, Trinkaus K, et al. A phase 2 trial of induction nab-paclitaxel and cetuximab given with cisplatin and 5-fluorouracil followed by concurrent cisplatin and radiation for locally advanced squamous cell carcinoma of the head and neck. Cancer. 2013;119(4):766–773. doi: 10.1002/cncr.27741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai N, Trieu V, Damascelli B, et al. SPARC expression correlates with tumor response to albumin-bound paclitaxel in head and neck cancer patients. Transl Oncol. 2009;2(2):59–64. doi: 10.1593/tlo.09109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Commisso C, Davidson SM, Soydaner-Azeloglu RD, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–638. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lui VW, Hedberg ML, Li H, et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discovery. 2013;3:761–769. doi: 10.1158/2159-8290.CD-13-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rampias T, Giagini A, Siolos S, et al. RAS/PI3K crosstalk and cetuximab resistance in head and neck squamous carcinoma. Clin Cancer Res. 2014;20(11):2933–2946. doi: 10.1158/1078-0432.CCR-13-2721. [DOI] [PubMed] [Google Scholar]
- 15.Pickering CR, Zhang J, Yoo SY, et al. Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discovery. 2013;3:770–781. doi: 10.1158/2159-8290.CD-12-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agrawal N, Frederick MJ, Pickering CR, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris LG, Taylor BS, Bivona TG, et al. Genomic dissection of the epidermal growth factor receptor (EGFR)/PI3K pathway reveals frequent deletion of the EGFR phosphatase PTPRS in head and neck cancers. PNAS. 2011;108(47):19024–19029. doi: 10.1073/pnas.1111963108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schell A, Ley J, Wu N, et al. Nab-paclitaxel-based compared to docetaxel-based induction chemotherapy regimens for locally advanced squamous cell carcinoma of the head and neck. Cancer Med. 2015;4(4):481–489. doi: 10.1002/cam4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edge SB, Byrd DR, Compton CC, editors. American Joint Committee on Cancer Staging Manual. New York: Springer; 2009. [Google Scholar]
- 21.Ensley JF, Jacobs JR, Weaver A, et al. Correlation between response to cisplatinum-combination chemotherapy and subsequent radiotherapy in previously untreated patients with advanced squamous cell cancers of the head and neck. Cancer. 1984;54(5):811–814. doi: 10.1002/1097-0142(19840901)54:5<811::aid-cncr2820540508>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Piccirillo JF, Lacy PD, Basu A, et al. Development of a new head and neck cancer-specific comorbidity index. Otolaryngol Head Neck Surg. 2002;128:1172–1179. doi: 10.1001/archotol.128.10.1172. [DOI] [PubMed] [Google Scholar]
- 24.Scantlebury JB, Luo J, Thorstad WL, et al. Cyclin D1—a prognostic marker in oropharyngeal squamous cell carcinoma that is tightly associated with high-risk human papillomavirus status. Human Pathol. 2013;44(8):1672–1680. doi: 10.1016/j.humpath.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assn. 1958;53:457–481. [Google Scholar]
- 26.Fakhry C, Zhang Q, Nguyen-Tan PF, et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol. 2014;32(30):3365–3378. doi: 10.1200/JCO.2014.55.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen EEW, Karrison TG, Kocherginsky M, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014;32(15):2735–2747. doi: 10.1200/JCO.2013.54.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haddad R, O’Neill A, Rabinowits G, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomized phase 3 trial. Lancet Oncol. 2013;14:257–264. doi: 10.1016/S1470-2045(13)70011-1. [DOI] [PubMed] [Google Scholar]
- 30.Spaulding MB, Fischer SG, Wolf GT. Tumor response, toxicity, and survival after neoadjuvant organ-preserving chemotherapy for advanced laryngeal carcinoma. The department of Veterans Affairs Cooperative Laryngeal Cancer Study Group. J Clin Oncol. 1994;12:1592–1599. doi: 10.1200/JCO.1994.12.8.1592. [DOI] [PubMed] [Google Scholar]
- 31.ECOG-1308: A phase II trial of induction chemotherapy followed by cetuximab (Erbitux) with low dose vs. standard dose IMRT in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx. NCT01084083. 2010 doi: 10.1200/JCO.2016.68.3300. ClinicalTrials.gov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adkins D, Ley J, Michel L, et al. Looking beyond the CRT paradigm: why induction chemotherapy is worthy of pursuit. Oral Oncol. 2015;51(2):103–104. doi: 10.1016/j.oraloncology.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Haigentz M, Cohen EEW, Wolf GT, Strojan P, Eisbruch A, Ferlito A. The future of induction chemotherapy for head and neck squamous cell carcinoma. Oral Oncol. 2012;48:1065–1067. doi: 10.1016/j.oraloncology.2012.08.009. [DOI] [PubMed] [Google Scholar]