Abstract
To ensure genome stability during cell division, all chromosomes must attach to spindles emanating from the opposite spindle pole bodies before segregation. The tension between sister chromatids generated by the poleward pulling force is an integral part of chromosome biorientation. In budding yeast, the residue Gly44 of histone H3 is critical for retaining the conserved Shugoshin protein Sgo1p at the pericentromeres for monitoring the tension status during mitosis. Studies carried out in this work showed that Lys42, Gly44, and Thr45 of H3 form the core of a tension sensing motif (TSM). Similar to the previously reported G44S mutant, K42A, G44A, and T45A alleles all rendered cells unable to respond to erroneous spindle attachment, a phenotype suppressed by Sgo1p overexpression. TSM functions by physically recruiting or retaining Sgo1p at pericentromeres as evidenced by chromatin immunoprecipitation and by in vitro pulldown experiments. Intriguingly, the function of TSM is likely regulated by multiple histone modifying enzymes, including the histone acetyltransferase Gcn5p, and deacetylases Rpd3p and Hos2p. Defects caused by TSM mutations can be suppressed by the expression of a catalytically inactive mutant of Gcn5p. Conversely, G44S mutant cells exhibit prominent chromatin instability phenotype in the absence of RPD3. Importantly, the gcn5− suppressor restores the tension sensing function in tsm− background in a fashion that bypasses the need of stably associating Sgo1p with chromatin. These results demonstrate that the TSM of histone H3 is a key component of a mechanism that ensures faithful segregation, and that interaction with chromatin modifying enzymes may be an important part of the mitotic quality control process.
Keywords: histone H3, chromatin, mitosis, Shugoshin, Saccharomyces cerevisiae
FAITHFUL partitioning of the genome duplicates in mitosis requires that the sister chromatids be engaged in bipolar attachment to mitotic spindle. Once all chromosomes are appropriately captured by the spindles, anaphase starts with the action of separase that cleaves the cohesin complex, thus separating the two sister chromosomes (Nasmyth 2002). Premature anaphase onset causes aneuploidy, a common trait associated with spontaneous abortion, birth defects, and cancer. Chromosome biorientation is a result of sister chromatid cohesion by the cohesin complex, stable attachment of spindles to kinetochores, and that the two sister kinetochores each attach to spindles emanating from different spindle pole bodies (Kschonsak and Haering 2015). Erroneous attachment of spindles to kinetochore activates the spindle assembly checkpoint (SAC), which prevents metaphase-to-anaphase transition so that errors can be corrected (Akera and Watanabe 2016). The two essential elements of biorientation are the spindle–kinetochore interaction and the tension between sister chromatids (Goshima and Yanagida 2000; Pinsky and Biggins 2005; Wang et al. 2014). The latter results from the physical cohesion of the sister chromatids that resists the poleward pulling force from opposing mitotic spindles, a scenario also called “amphitelic attachment.” If one of the two sister kinetochores is not attached (monotelic), or if both kinetochores are attached to spindles from the same spindle pole body (syntelic), tension will not be produced, and both copies of sisters will cosegregate, leading to aneuploidy (Pinsky and Biggins 2005). The physical form of tension detectable by cells remains a subject of investigation (Li and Nicklas 1995). Tension-dependent conformational changes of chromatin and cohesin near kinetochores are likely candidates (Chambers et al. 2012; Haase et al. 2012; Verdaasdonk et al. 2012). Besides the biorientation-induced separation of sister kinetochores within the confinement of cohesion (He et al. 2000), intrachromosomal extension of the distance between adjacent nucleosomes in the pericentric regions has also been suggested to be an outcome of bipolar attachment (Yeh et al. 2008). On the other hand, how cells interpret such structural changes induced by tension is unclear. One key player in tension sensing is the Shugoshin protein (Indjeian et al. 2005; Kitajima et al. 2006; Yamagishi et al. 2008; Yin et al. 2008). Homologs of Shugoshin are found in eukaryotes ranging from yeast to humans and are important for both meiotic and mitotic chromosome segregation (Kitajima et al. 2004; Watanabe 2005). Deleting SGO1, which encodes the sole copy of Shugoshin in the budding yeast, renders cells unable to detect or to respond to tensionless crises (Indjeian et al. 2005; Fernius and Hardwick 2007). During mitosis, Shugoshin is enriched at the centromeres and pericentromeres (Salic et al. 2004; Kiburz et al. 2005; Riedel et al. 2006), from which tension originates (Bloom et al. 2006). Centromeric recruitment of Shugoshin depends critically on the phosphorylation of Ser121 of H2A by the Bub1p kinase, as well as several heterochromatic marks at the pericentromeres (Kiburz et al. 2005; Fernius and Hardwick 2007; Yamagishi et al. 2008; Kawashima et al. 2010). Genetic and biochemical experiments revealed that Shugoshin interacts with Ipl1p, the kinase subunit of the chromosomal passenger complex (Campbell and Desai 2013; Ng et al. 2013), protein phosphatase 2A (PP2A) (Tang et al. 2006; Xu et al. 2009; Tanno et al. 2010; Liu et al. 2013a,b; Eshleman and Morgan 2014), and cohesion (Liu et al. 2013b). It is possible that Shugoshin proteins participate in the detection and/or correction of attachment error. Consistently, evidence has been presented for the biorientation-dependent removal of Shugoshin from pericentromeres (Eshleman and Morgan 2014; Nerusheva et al. 2014), suggesting that retaining this protein at centromeres and pericentromeres may be a crucial element that keeps the spindle assembly checkpoint at an “on” state before the establishment of biorientation. However, how Shugoshin interacts with SAC remains an open question.
Previously we reported that histone H3 plays a critical role in mitotic tension surveillance in budding yeast (Luo et al. 2010). Yeast cells harboring the Gly44-to-Ser (G44S) mutant allele of H3 exhibit phenotypes typical of those resulting from tension sensing defects, including chromosome instability, missegregation, and inability to activate the SAC when tension buildup is perturbed (Indjeian et al. 2005). This mutation apparently impairs the recruitment and retention of Sgo1p at pericentromeres, whereas the centromeric Sgo1p localization remains in large part normal (Luo et al. 2010). Moreover, scanning mutagenesis of H3 helped uncover multiple residues, including Gly44, required for faithful segregation of chromosomes (Kawashima et al. 2011; Ng et al. 2013). Together, these reports attest to the indispensable, yet frequently overlooked function of nucleosomes in the regulation of mitosis.
Nucleosomes are the basal components specifying both the structures and functions of chromatin. Dynamic changes in nucleosomes, including their post-translational modifications, critically affect nuclear activities, including transcription, replication, and recombination. Comparatively, how mitosis might be regulated by chromatin is only beginning to be understood. Here we present evidence that Gly44 of histone H3 is part of the TSM 42KPGT that bridges the N′ tail domain and the central histonefold domain of H3. Genetic assays also revealed that the function of H3 TSM is regulated by two opposing chromatin modifying activities, the histone acetyltransferase Gcn5p and deacetylases Rpd3p and Hos2p. Together, these results further demonstrate that chromatin, besides itself being the cargo of genome partitioning, plays an active role in ensuring faithful segregation.
Materials and Methods
Yeast strains and plasmid constructs
The yeast strains, plasmids, and primers used in this work are listed in Table 1, Table 2, and Table 3.
Table 1.
Yeast strains used in this study
| Strain | Relevant genotype | Source or reference |
|---|---|---|
| 227a | lys1 | Gift of E. Grayhack |
| 70α | thr3 met− | Gift of E. Grayhack |
| yJL171 | MATa ade2-1 bar1∆ can1-100 his3-11, 15::pGAL-MCD1::HIS3 leu2-3, 112 trp1-1::PDS1-Myc13::TRP1 ura3-1 hht1-hhf1::KAN hht2-hhf2::KAN hta1-htb1::Nat hta2-htb2::HPH pQQ18 [ARS CEN LEU2 HTA1-HTB1 HHT2-HHF2] | Luo et al. (2010) |
| yJL340 | MATa/α his3∆1 leu2∆0 met15∆0 ura3∆0 hht1-hhf1::KAN hht2-hhf2::Nat hta1-htb1::HPH hta2-htb2::Nat pMK439G44S [ARS CEN LEU2 HTA1-HTB1 hht2-G44S-HHF2] | This study |
| yJL343 | MATa ade2-1 can1-100 his3-11, 15 leu2-3, 112 trp1-1::SGO1-6HA::TRP1 ura3-1 hht1-hhf1::KAN hht2-hhf2::KAN hta1-htb1::Nat hta2-htb2::HPH pQQ18 [ARS CEN LEU2 HTA1-HTB1 HHT2-HHF2] | Luo et al. (2010) |
| yJL475 | MATa/α his3∆1 leu2∆0 met15∆0 ura3∆0 hht1-hhf1::KAN hht2-hhf2::Nat hta1-htb1::HPH hta2-htb2::Nat pMK439K42A [ARS CEN LEU2 HTA1-HTB1 hht2-K42A-HHF2] | This study |
| yJL479 | MATa/α his3∆1 leu2∆0 met15∆0 ura3∆0 hht1-hhf1::KAN hht2-hhf2::Nat hta1-htb1::HPH hta2-htb2::Nat pMK439T45A [ARS CEN LEU2 HTA1-HTB1 hht2-T45A-HHF2] | This study |
| yJL486 | MATa ade2-1 can1-100 his3-11, 15 leu2-3, 112 trp1-1 ura3-1::gcn5 hht1-hhf1::KAN hht2-hhf2::KAN hta1-htb1::Nat hta2-htb2::HPH pJH33 [ARS CEN URA3 HTA1-HTB1 HHT2-HHF2] | This study |
| yJL487 | MATa ade2-1 bar1∆ can1-100 his3-11, 15::pGAL-MCD1::HIS3 leu2-3, 112 trp1-1::PDS1-Myc13::TRP1 ura3-1 hht1-hhf1::KAN hht2-hhf2::KAN hta1-htb1::Nat hta2-htb2::HPH pMK439K42A [ARS CEN LEU2 HTA1-HTB1 hht2-K42A-HHF2] | This study |
| yJL492 | MATa ade2-1 bar1∆ can1-100 his3-11, 15::pGAL-MCD1::HIS3 leu2-3, 112 trp1-1::PDS1-Myc13::TRP1 ura3-1 hht1-hhf1::KAN hht2-hhf2::KAN hta1-htb1::Nat hta2-htb2::HPH pMK439T45A [ARS CEN LEU2 HTA1-HTB1 hht2-T45A-HHF2] | This study |
| yJL506 | MATa ade2-1 can1-100 his3-11, 15 leu2-3, 112 trp1-1 ura3-1::gcn5 hht1-hhf1::KAN hht2-hhf2::KAN hta1-htb1::Nat hta2-htb2::HPH pQQ18 [ARS CEN LEU2 HTA1-HTB1 HHT2-HHF2] | This study |
| yJL507 | MATa ade2-1 can1-100 his3-11, 15 leu2-3, 112 trp1-1 ura3-1::gcn5 hht1-hhf1::KAN hht2-hhf2::KAN hta1-htb1::Nat hta2-htb2::HPH pMK439G44S [ARS CEN LEU2 HTA1-HTB1 hht2-G44S-HHF2] | This study |
| yJL508 | MATa ade2-1 can1-100 his3-11, 15 leu2-3, 112 trp1-1 ura3-1::gcn5 hht1-hhf1::KAN hht2-hhf2::KAN hta1-htb1::Nat hta2-htb2::HPH pMK439G44A [ARS CEN LEU2 HTA1-HTB1 hht2-G44A-HHF2] | This study |
| yJL509 | MATa ade2-1 can1-100 his3-11, 15 leu2-3, 112 trp1-1 ura3-1::gcn5 hht1-hhf1::KAN hht2-hhf2::KAN hta1-htb1::Nat hta2-htb2::HPH pMK439K42A [ARS CEN LEU2 HTA1-HTB1 hht2-K42A-HHF2] | This study |
| yJL510 | MATa ade2-1 can1-100 his3-11, 15 leu2-3, 112 trp1-1 ura3-1::gcn5 hht1-hhf1::KAN hht2-hhf2::KAN hta1-htb1::Nat hta2-htb2::HPH pMK439T45A [ARS CEN LEU2 HTA1-HTB1 hht2-T45A-HHF2] | This study |
| yJL540 | MATa ade2-1 can1-100 his3-11, 15 leu2-3, 112 trp1-1::SGO1-6HA::TRP1 ura3-1 hht1-hhf1::KAN hht2-hhf2::KAN hta1-htb1::Nat hta2-htb2::HPH pMK439K42A [ARS CEN LEU2 HTA1-HTB1 hht2-K42A-HHF2] | This study |
| yJL543 | MATa ade2-1 can1-100 his3-11, 15 leu2-3, 112 trp1-1::SGO1-6HA::TRP1 ura3-1 hht1-hhf1::KAN hht2-hhf2::KAN hta1-htb1::Nat hta2-htb2::HPH pMK439T45A [ARS CEN LEU2 HTA1-HTB1 hht2-T45A-HHF2] | This study |
| yMK1141 | MATa ade2-1 can1-100 his3-11, 15 leu2-3, 112 trp1-1 ura3-1 hht1-hhf1::KAN hht2-hhf2::KAN hta1-htb1::Nat hta2-htb2::HPH pMK440 [ARS CEN URA3 HTA1-HTB1 HHT2-HHF2] | Luo et al. (2010) |
| yMK1174 | MATa/α his3∆1 leu2∆0 met15∆0 ura3∆0 hht1-hhf1::KAN hht2-hhf2::Nat hta1-htb1::HPH hta2-htb2::Nat pJH33 [ARS CEN URA3 HTA1-HTB1 HHT2-HHF2] | This study |
| yMK1243 | MATa ade2-1 can1-100 his3-11, 15 leu2-3, 112 trp1-1 ura3-1 hht1-hhf1::KAN hht2-hhf2::KAN hta1-htb1::Nat hta2-htb2::HPH pQQ18 [ARS CEN LEU2 HTA1-HTB1 HHT2-HHF2] | Luo et al. (2010) |
| yXD24 | MATa ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 hht1-hhf1::KAN hht2-hhf2::KAN hta1-htb1::Nat hta2-htb2::HPH hda1∆::TRP1 /pMK440[ARS CEN URA3 HTA1-HTB1 HHT2-HHF2] | This study |
| yXD26 | MATa ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 hht1-hhf1::KAN hht2-hhf2::KAN hta1-htb1::Nat hta2-htb2::HPH sin3∆::TRP1/pMK440[ARS CEN URA3 HTA1-HTB1 HHT2-HHF2] | This study |
| yXD27 | MATa ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 hht1-hhf1::KAN hht2-hhf2::KAN hta1-htb1::Nat hta2-htb2::HPH rpd3∆::TRP1/pMK440[ARS CEN URA3 HTA1-HTB1 HHT2-HHF2] | This study |
| yXD29 | MATa ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 hht1-hhf1::KAN hht2-hhf2::KAN hta1-htb1::Nat hta2-htb2::HPH RPD3-3HA::TRP1/pMK440[ARS CEN URA3 HTA1-HTB1 HHT2-HHF2] | This study |
| yXD87 | MATa ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 hht1-hhf1::KAN hht2-hhf2::KAN hta1-htb1::Nat hta2-htb2::HPH hos1∆::TRP1/pMK440[ARS CEN URA3 HTA1-HTB1 HHT2-HHF2] | This study |
| yXD88 | MATa ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 hht1-hhf1::KAN hht2-hhf2::KAN hta1-htb1::Nat hta2-htb2::HPH hos2∆::TRP1/pMK440[ARS CEN URA3 HTA1-HTB1 HHT2-HHF2] | This study |
| yXD100 | MATa ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 hht1-hhf1::KAN hht2-hhf2::KAN hta1-htb1::Nat hta2-htb2::HPH GCN5-13MYC::TRP1/pMK440[ARS CEN URA3 HTA1-HTB1 HHT2-HHF2] | This study |
Table 2.
Plasmid constructs used in this study
| Plasmid | Main features | Source or reference |
|---|---|---|
| pJH33/pMK440 | pRS316-HTA1-HTB1 HHT2-HHF2 | Luo et al. (2010) |
| pJL51 | 2 μm URA3 pADH1-3xHA-SGO1-tADH1 | This study |
| pJL52 | ARS1 CEN4 URA3 pADH1-3xHA-tADH1 | This study |
| pJL53 | ARS1 CEN4 URA3 pADH1-3xHA-SGO1-tADH1 | This study |
| pJL55 | pGEX-4T-1 3xHA-SGO1 | This study |
| pJL74 | pRS315-HTA1-HTB1 | This study |
| pMK120 | 2 μm URA3 vector with CUP1 promoter | Kuo et al. (1998) |
| pMK144 | 2 μm URA3 pCUP1-GCN5 | Kuo et al. (1998) |
| pMK144E173H | 2 μm URA3 pCUP1-gcn5E173H | Kuo et al. (1998) |
| pMK144F221A | 2 μm URA3 pCUP1-gcn5F221A | Kuo et al. (1998) |
| pMK572 | 2 μm URA3 vector with ADH1 promoter and terminator | Luo et al., (2010) |
| pMK573 | 2 μm URA3 SGO1 | Luo et al., (2010) |
| pQQ18/pMK439 | pRS315-HTA1-HTB1 HHT2-HHF2 | Luo et al., (2010) |
| pXD32 | 2 μm URA3 RPD3 with ADH1 promoter and terminator | This study |
| pXD33 | 2 μm URA3 rpd3H150A with ADH1 promoter and terminator | This study |
Table 3. Oligos used in this study.
| Oligo | Sequence |
|---|---|
| CEN16 S | ATGCAAAGGTTGAAGCCGTTA |
| CEN16 AS | TTTGCCGATTTCGCTTTAGAAC |
| CEN16–0.3 kb S | GAAGCACTCCGACCTTTC |
| CEN16–0.3 kb AS | CTTGCCTTTTCTGGATCAG |
| CEN16–1.7 | GATGAGCACATATGCATG |
| CEN16–1.7as | CTTAATCCATCAATTCTGG |
| CEN16+4.0 | GCCCTGATAAAGTCGACC |
| CEN16+4.0as | GAACTCTTGCAAGTTGAAG |
| CEN16+6.5 | CCGATGATGGTTGTTATG |
| CEN16+6.5as | CTCTAATAGTGGCAATGTTG |
| CEN16+9.1 | AAACTCAATGATGACCTTG |
| CEN16+9.1as | TATGTTACTCTTACGATGTG |
| mk93 | GGC AAG TGG TAT TCC GTA AG |
| mk93as | CTT GGT TTT CCT CTT AAG TG |
| OJL19 | TGTCATCATGCGTATTAGAG |
| OJL20 | CGTATAGGGAATTTAACGTC |
| OJL100 | GACTAAAGTAGAGCAACA |
| OJL101 | AGTGGAGTAATGCCACAT |
| OJL102 | ACGTCTAGCTGAGCATGT |
| OJL103 | GAACTGTCGAAACTGAGT |
| oXD15 | ATCCATATGACGTTCCAGATTACGCTGCTCAGTGCGTATATGAAGCAACACCTTTTGATC |
| oXD16 | TATCGGGGGGATCCACTAGTTCTAGCTAGAGCGGCCTCAATAGAATTCATTGTCATGCTC |
| oXD25 | AGCATGGATTCTGTAATGGTTAAGAAAGAAGTACTGGAAAATTGAAGCTTGATATCGAAT |
| oXD26 | TTCTTCATCACTCCATTCTTCAAACGAATCCAGTATAAAGTCTACGACTCACTATAGGGC |
| oXD33 | AAAATGTCACAGGTTTGGCATAATTCGAATTCGCAATCAAACTGAAGCTTGATATCGAAT |
| oXD34 | TTGAATCTTAGCCCCCTTGTCTGAAGATTCAGTATTCCCAGTTACGACTCACTATAGGGC |
| oXD37 | CAGATGGTATATGAAGCAACACCTTTTGATCCGATCACGGTCTGAAGCTTGATATCGAAT |
| oXD38 | ATAGAATTCATTGTCATGCTCAACATGTAGGTCCCTCGCATATACGACTCACTATAGGGC |
| oXD59 | TCAACTATGCGGGTGGTTTGGCTCATGCAAAAAAATCGGAGGCTTCTGGGTTTTGTTATT |
| oXD60 | AATAACAAAACCCAGAAGCCTCCGATTTTTTTGCATGAGCCAAACCACCCGCATAGTTGA |
| oXD79 | TAATATGAATTAATAAACACCTGTCCATTTTAGAAAAACGCTTGAAGCTTGATATCGAAT |
| oXD80 | TCGCATTATTAATTTGTATTCAAACGACTAATTAAAACTATCTACGACTCACTATAGGGC |
| oXD83 | AGTACGTTAAAATCAGGTATCAAGTGAATAACAACACGCAACTGAAGCTTGATATCGAAT |
| oXD84 | AAAAAAAAAAACGGGAGATTAACCGAATAGCAAACTCTTAAATACGACTCACTATAGGGC |
To assess the effects of histone H3 mutations from lysine 36 to lysine 56 on the benomyl sensitivity, pJL74 (a LEU2 plasmid bearing histones H2A and H2B) was cotransformed with H3 mutant collection from the Boeke group (TRP1 plasmids harboring H3 and H4 genes) that contains specific H3 mutations (Dai et al. 2008). 5-FOA selection was conducted to select for yeast cells that had lost the plasmid pMK440 (a URA3 plasmid bearing all four core histone genes). All other studies were carried out with histone mutations generated in pMK439 by two-step PCR site-directed mutagenesis (Luo et al. 2010).
To study the functions of GCN5 on the H3-Sgo1p tension sensing mechanism, H3 mutations (on pMK439 LEU2+) were introduced into yJL486 (gcn5∆, pMK440 URA3+) via plasmid shuffling and 5-FOA selection, resulting in strains yJL506 to yJL510 (see Table 1). yJL486 was constructed by transforming a 4.6-kb gcn5::URA3 fragment, which is released from pMK147 by XhoI and XbaI, into yMK1141 (pMK440 URA3+). Dominant negative HAT inactive mutants of gcn5, gcn5E173H, or gcn5F221A were carried on pMK144 and were directly transformed into cells bearing H3 mutations.
To delete RPD3, primers oXD37 and oXD38 were used to amplify the Kluyveromyces lactis TRP1 selective marker from plasmid pBS1479. The PCR product was transformed into yMK1141 for tryptophan prototroph selection. The same strategy was used for knockout of SIN3 (oXD33 and oXD34), HDA1 (oXD25 and oXD26), HOS1 (oXD79 and oXD80), and HOS2 (oXD83 and oXD84). The single knockout strains were then subjected to plasmid shuffling and 5-FOA selection to obtain either WT or G44A H3.
To create pXD32, a 2-µm URA3 RPD3 plasmid, primers oXD15 and oXD16, bearing 42 bp of homology to the vector pMK595 at the 5′ end, were used to amplify RPD3 ORF from the yeast genome. The PCR product was cotransformed with NotI-digested pMK595 into yMK839. Ura+ transformants were subjected to DNA extraction for bacterial transformation. Miniprep DNA was then verified by sequencing. To introduce the H150A catalytically dead mutation to Rpd3p, primers oXD59 and oXD60 were used for site-directed mutagenesis PCR with pXD32 as template, generating pXD33. The mutagenesis was confirmed by sequencing as well.
Myc-tagged Gcn5p and HA-tagged Rpd3p strains were created as previously described (Liu et al. 2010).
Yeast methods
Yeast growth media, conditions, and transformation were based on standard procedures (Sherman 1991). When appropriate, 5% casamino acids (CAAs) were used to substitute for synthetic amino acid mixtures as selective medium for uracil, tryptophan, or adenine prototroph. Yeast transformation was done with the lithium acetate method (Gietz et al. 1992).
Chromosome stability assays were conducted by measuring the mating behavior of diploid strains bearing WT or selective tension sensing mutants. Homozygous diploid cells created by the transformation of YCp50-HO (Herskowitz and Jensen 1991) were grown overnight in YPD, and then patched onto YPD plates and incubated at 30° for 2–3 days until saturation. Cell plates were replica plated to other fresh YPD plates covered with ∼5 × 107 MATa or MATα tester cells and allowed to mate at 30° for 10 hr, followed by further replica plating to minimum medium plates. Mating between the tester and the subject strains resulted in complete complementation of nutrient requirement and were able to survive in the minimal medium. Genomic PCR that examined the status of the MAT loci on chromosome III was conducted by using the primers OJL100, OJL101, OJL102, and OJL103 targeting the MAT locus but not either of the two silent loci. The tension sensing test using the PGAL1-MCD1 strains was precisely according to Indjeian et al. (2005) using strains yJL171, yJL487, and yJL492. Western analyses of yeast proteins and benomyl washout assays were conducted as mentioned in Luo et al. (2010).
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) was conducted as previously described (Kuo and Allis 1999; Luo et al. 2010) using primers listed in Table 3. To quantify the ChIP results, PCR products were purified and resolved by 9% polyacrylamide gel electrophoresis and stained by ethidium bromide. The captured gel images were then quantified by National Institutes of Health ImageJ. Intensities of each CEN/pericentric fragment were compared to a common internal control (DED1, PGK1, or TEL). The ratio was further normalized to 0.1% input DNA for PCR amplification carried out in parallel of all reactions. The ChIP data were obtained from at least three independent yeast cultures.
Sgo1p–H3 interaction
Histones were prepared according to Edmondson et al. (1996). Recombinant Sgo1p was prepared as previously described (Luo et al. 2010). For pulldown assays, ∼5 µg of soluble recombinant Sgo1p was incubated with ∼3 µg of yeast histones in 150 µl of HEMGT buffer at 4° for 1 hr. A total of 6 µl of glutathione beads along with 150 µl of the HEMGT buffer were added to the reactions and rocked gently at 4° for another hour. Beads were washed with 500 µl of the HEMGT buffer three times, 5 min each, followed by boiling in 2× SDS-PAGE loading buffer for 5 min. Eluate was resolved by 15% SDS-PAGE and blotted for anti-H3 Western analyses (Luo et al. 2010).
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results
Mutations of a cluster of amino acid residues of H3 cause mitotic chromosome instability
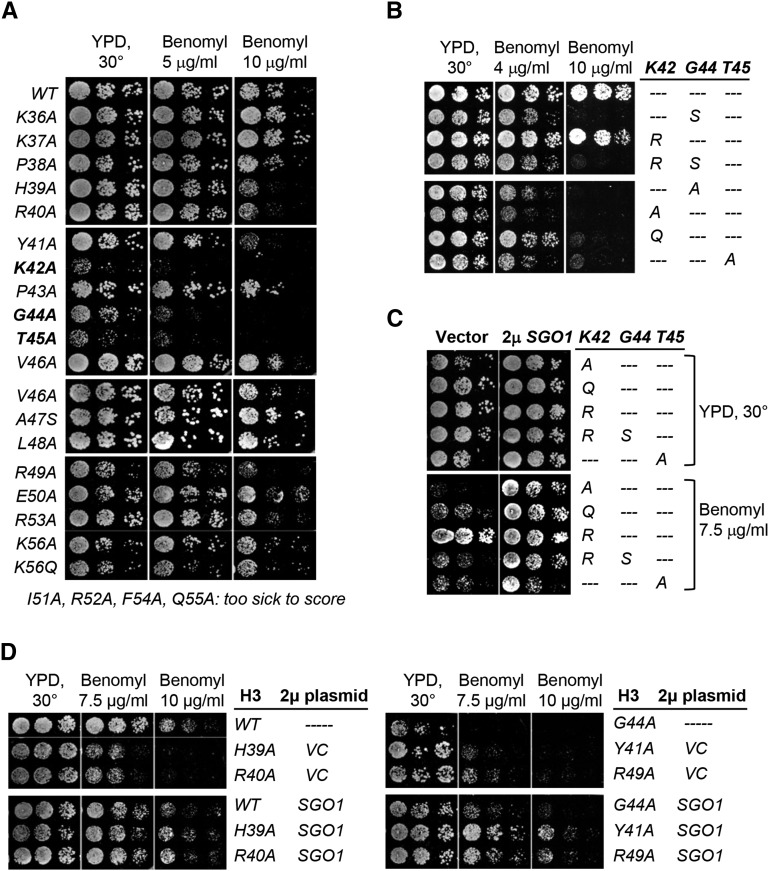
Our initial discovery that Gly44 of H3 is important for Sgo1p interaction and tension sensing (Luo et al. 2010) suggests that this residue is part of a motif directly responsible for Sgo1p recruitment. Several scanning mutagenesis studies of histone H3 indeed identified residues, some are close to Gly44, to be important for maintaining mitotic integrity and chromatin stability (Dai et al. 2008; Kawashima et al. 2011; Ng et al. 2013). To more specifically map the functional domain integrating Gly44 for tension sensing, we first compared benomyl hypersensitivity of strains bearing histone H3 mutations from K36 (the end of the tail domain) through K56 (the end of the αN helix of the histone core). Benomyl depolymerizes mitotic spindles. Cellular hypersensitivity to benomyl is a trait shared by many mutants with defects in mitotic regulation. Figure 1A shows that K42A, G44A, and T45A mutations caused strong hypersensitivity to benomyl, and that a milder phenotype was caused by the adjacent H39A, R40A, Y41A, and R49A mutations. Intriguingly, P43A, flanked by K42 and G44, apparently is phenotypically neutral.
Figure 1.
Identification of the tension sensing motif of histone H3. (A) Scanning mutagenesis of histone H3 residues from K36 to K56. Yeast cells bearing a sole copy of H3 containing the indicated mutations were tested for their sensitivity to benomyl. (B and C) More detailed analyses of 42KPGT TSM. (D) Mutations in and near the 42KPGT TSM are suppressed by a multicopy plasmid bearing the SGO1 gene.
Crystal structures of nucleosomal particles show that the 42KPGT region adapts a unique turn structure transitioning from the flexible tail domain to the well-structured histonefold domain (White et al. 2001) (Supplemental Material, Figure S1). Curiously, both K42 and T45 have been shown to be modified post-translationally (Baker et al. 2010; Hyland et al. 2011) for the control of gene expression and replication, respectively. Their role in mitosis, if any, is unclear. To examine the potential involvement of K42 and T45 modifications in mitosis, we introduced additional mimetic mutations and tested the cellular response to benomyl treatment. Figure 1B shows that the K42R nonacetylatable mutant behaved similarly to the WT but K42Q cells were hypersensitive to benomyl. The T45E phosphomimetic mutation caused cell death, due possibly to dysregulation of replication (Baker et al. 2010). The differential effects of alanine, glutamine, and arginine substitutions at K42 suggest that maintaining a positive charge at this position is critical but the actual side chain, that is, lysine or arginine, is not as critical. On the other hand, the K42R mutation alone could not rescue the benomyl hypersensitivity phenotype of G44S (which shared comparable traits with G44A) (fourth row, Figure 1B), indicating that glycine at this position is essential and cannot be rescued by a constitutive, positive charge at position 42.
The original G44S mutation that impairs tension sensing can be suppressed by overexpressing Sgo1p, the key factor for this checkpoint function ensuring error-free segregation (Indjeian et al. 2005; Luo et al. 2010). If the newly identified mutant alleles also perturbed the function of tension sensing, we predicted that they would be suppressed by Sgo1p overexpression as well. Results confirm this hypothesis (Figure 1C). In the presence of a multicopy plasmid bearing a constitutively expressed SGO1 gene, all mutant alleles tested showed apparent restoration of robust growth in the presence of benomyl. Neighboring residues replaced by alanine (i.e., His39, Arg40, Tyr41, and Arg49) were all rescued by 2µ SGO1 (Figure 1D). These data suggest that K42, G44, and T45 form the core of a TSM that also requires several nearby residues for full function.
Chromosome instability conferred by K42, G44, and T45 mutants
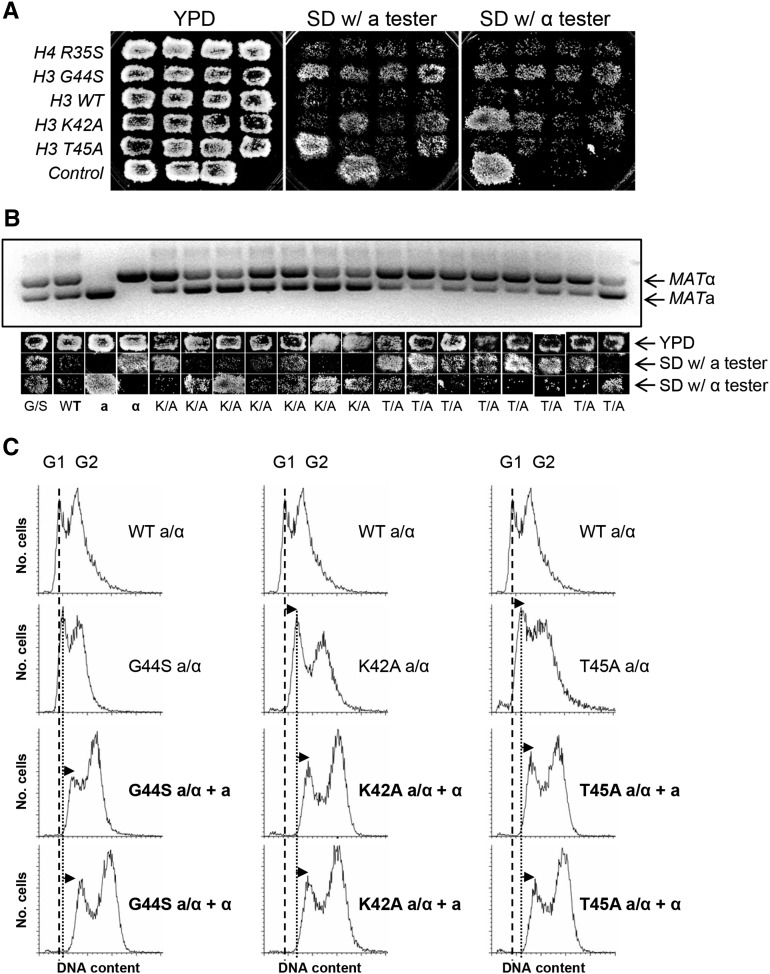
Mitotic defects frequently trigger chromosome instability and aneuploidy, a phenotype that can be realized by the acquisition of the ability of diploid cells to mate. In budding yeast, only haploids can mate. Normal diploid cells inherit the transcriptionally active MATa and MATα mating loci on chromosome III from the two haploid parents. Concomitant expression of these two loci in diploid cells represses genes essential for mating. The a/α diploid cells thus are nonmater. If there is an increase in chromosome instability, sporadic loss of one of the two chromosome III homologs from a population will enable the underlying strain to mate as both MATa and MATα mating types, regardless of the ploidy for the rest of the genome. The emergence of this bimater phenotype thus manifests chromosome instability and aneuploidy (Yuen et al. 2007). To take advantage of this method to examine the effects of TSM mutations on chromosome stability, we first generated diploid strains bearing homozygous K42A, G44S, or T45A mutation. As an additional control, we included another novel histone mutant allele, H4 R35S, which was also hypersensitive to benomyl but was not suppressed by Sgo1p overexpression (J. Luo and M.-H. Kuo, unpublished data). Diploid cells from single colonies were grown on solid medium as patches before replica plating to haploid mating tester strains. Successful mating to either tester strain complemented the nutrient marker gene defects, hence allowing cells to grow on the synthetic defined (SD) minimal plate. Figure 2A shows that a substantial portion of diploid cells bearing any of these H3 mutant alleles were able to mate with both tester strains, while WT or H4 R35S mutant cells maintained their diploid nonmater character. Genomic PCR using primers differentiating MATa and MATα loci demonstrated that the nonmater isolates maintained both copies of chromosome III, that is, the two PCR fragments were of roughly equal intensities. On the other hand, those capable of mating, WT haploid or mutant diploid, showed substantial bias toward one of the two copies. The mating type correlated well with the intensities of the two bands (Figure 2B), strongly suggesting that the bimater phenotype was due to imbalance of the two chromosome III homologs. To further rule out the possibility that these maters were a result of meiosis before or during experiments that would have generated mating-capable haploids, we did fluorescence activated cell sorting (FACS) analysis to examine the overall DNA content of cells from the patches on YPD (before mating) and SD minimal plates (after mating). Figure 2C shows that prior to mating with the tester, all starting diploid cells contained 2N or slightly higher DNA content. After mating with the haploid tester cells, the DNA content further increased (marked by the arrows of the G1 peaks), confirming the fusion of two sets of genome that resulted in triploidy.
Figure 2.
Mutations introduced to the TSM cause chromosome instability. (A) Diploid cells bearing homozygous mutant alleles of histone H3 or H4 were tested for their mating behavior. Cells mate with a tester strain (227a or 70α, see Table 1) acquire the ability to grow in SD minimal medium. Cells containing the R35S mutation in histone H4 are hypersensitive to benomyl and do not affect the tension sensing function. The three WT control strains on the bottom roll are, from left to right, MATα, MATa, and MATa/α. (B) Genomic PCR reveals the stochastic loss of one of the two copies of chromosome III. Cells scraped from the YPD (before mating) patches were subjected to DNA isolation and PCR, using primers amplifying both silent mating loci concomitantly. The corresponding mating behaviors of each patch on YPD and SD plate are shown below each lane of the DNA gel. The relative intensity of the two mating loci PCR products correlates with the mating ability. (C) FACS analysis of the DNA content of asynchronous diploid strains before and after mating. Successful mating increases the DNA content. The G1 peak of the WT and each mutant diploid is marked by a broken and a solid vertical line, respectively. The right shift of the G1 peak after mating is indicated by arrows.
In addition to high frequencies of aneuploidy (i.e., bimater phenotype), the K42A, G44A, G44S, and T45A homozygous diploid cells had either a very low sporulation efficiency, or, when tetrads were formed, low germination rate (data not shown). Finally, in contrast to the histone H3 tension sensing mutants, the H4 R35S mutant behaved like the WT cells in mating tests (Figure 2A), arguing against the possibility that all benomyl hypersensitive mutants suffer from chromosome instability. We surmise that K42, G44, and T45 are important for cells to maintain mitotic chromosome stability.
K42A and T45A mutations compromise tension sensing function and Sgo1p recruitment
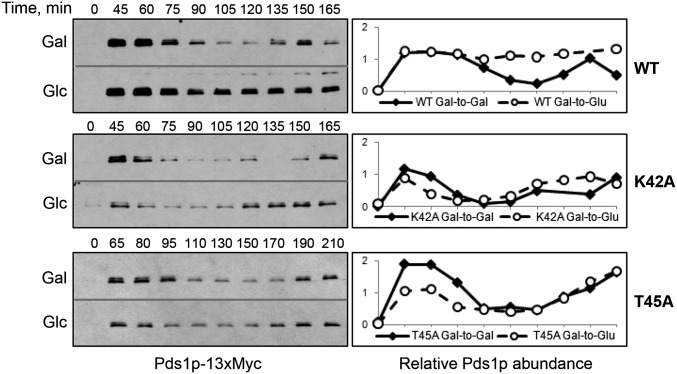
To more firmly link 42KPGT to the tension sensing function, we tested the spindle assembly checkpoint activation in response to a tensionless crisis. Benomyl treatment depolymerizes microtubules, eliminating both attachment and tension, and consequently activates the SAC. Both K42A and T45A mutant cells were able to stabilize the securing protein Pds1p in response to benomyl insult (Figure S2). We then tested the spindle checkpoint activation induced specifically by the lack of tension. To this end, we placed the MCD1 gene under the control of the galactose-repressible GAL1–10 promoter (Indjeian et al. 2005). MCD1 (also known as SCC1) encodes an integral component of the cohesin complex that holds sister chromatids together. WT and mutant strains were synchronized at, and released from G1 phase in a galactose-containing medium. Normal mitotic progression is manifested by the fluctuation of the securin protein Pds1p (Figure 3, solid lines). If cells were released from G1 arrest into a glucose-containing medium that repressed MCD1 transcription, Mcd1p deficiency disrupted cohesion and prevented tension buildup. Such a tensionless crisis activated the SAC by stabilizing Pds1p (broken line, top row, Figure 3). On the other hand, K42A and T45A mutant cells showed Pds1p fluctuation in both galactose- and glucose-containing media (middle and bottom rows, Figure 3). The inability of cells to respond to the lack of tension is in excellent agreement with the documented tension sensing defects caused by the G44S mutation (Luo et al. 2010). We therefore conclude that K42, G44, and T45 together form a TSM.
Figure 3.
K42A and T45A mutations diminish the ability to activate the SAC when tension is absent. A cohesin complex component Mcd1p is under the control of the GAL promoter. Yeast cells were released from G1 arrest into galactose (Gal) or glucose (Glc) medium. The abundance of Pds1p securin was examined by immunoblotting and quantified by comparing with an internal control glucose-6-phosphate dehydrogenase (G-6-PDH) (see Figure S3).
Yeast and other eukaryotic cells monitor the tension status via the Shugoshin family proteins (Marston 2015). Recruitment of the yeast Sgo1p to the pericentromeres is essential for the tension sensing function (Fernius and Hardwick 2007; Luo et al. 2010). Given that overexpressing SGO1 suppresses the benomyl hypersensitivity phenotype of both K42A and T45A mutants (Figure 1C), we suspected that these two alleles also crippled the ability of H3 to retain Sgo1p at the pericentric region, hence compromising the tension sensing activity. To test this hypothesis, we conducted ChIP experiments to check the localization of Sgo1p in K42A or T45A mutant strains. Indeed, Sgo1p was recruited efficiently to CEN16 in all strains tested (Figure 4, A and B). However, the pericentric Sgo1p was significantly reduced in both mutants, despite the fact that the total levels of Sgo1p were comparable in all three strains tested (Figure 4C). The abrupt descent of the Sgo1p signal at as close as 0.3 kb to CEN16 in these two mutants strongly suggested that the recruitment of Sgo1p to the centromere, in which the canonical H3 is replaced by a centromere-specific H3 variant Cse4p, is mediated by a mechanism different from that for pericentric Sgo1p enrichment. This notion was further confirmed by in vitro pulldown assays that examined the physical interaction between H3 and Sgo1p. Figure 4D shows that the wild-type H3 purified from yeast was able to interact with a GST-tagged Sgo1p. K42A and T45A mutations weakened, but did not eliminate, the affinity for Sgo1p (Figure S4). Consistent with the observation that the P43A mutation did not cause benomyl hypersensitivity (Figure 1A), histone H3 bearing the P43A mutation bound Sgo1p similarly to WT. We thus conclude that both K42A and T45A mutations compromise severely the ability of H3 to interact with Sgo1p in vitro and in vivo, and that the TSM of H3 functions through physically recruiting or retaining Sgo1p at the pericentromeres.
Figure 4.
Defective TSM fails to retain Sgo1p in pericentromeres. (A and B) Chromatin immunoprecipitation of Sgo1p epitope-tagged with HA. Cells were arrested at G2/M by benomyl before ChIP. Each PCR reaction contained a pair of locus-specific primers and another common internal control corresponding to the PGK1 gene. Quantification results of PCR reactions are shown in B and C. The steady-state abundance of Sgo1p is not affected by mutations of the TSM. Yeast whole cell lysates were probed with anti-HA antibodies to detect the HA-tagged Sgo1p. (D) Sgo1p interaction with H3 is weakened by K42A, G44A, and T45A, but not by P43A mutation. Bacterially expressed GST–Sgo1p was incubated with core histones purified from yeast. H3 retained by GST–Sgo1p on the glutathione beads was eluted and resolved by SDS-PAGE. Results of Western blotting with and anti-H3 antibodies are shown. Error bars were calculated from at least three biological replica.
The acetyltransferase Gcn5p is a negative regulator of the TSM
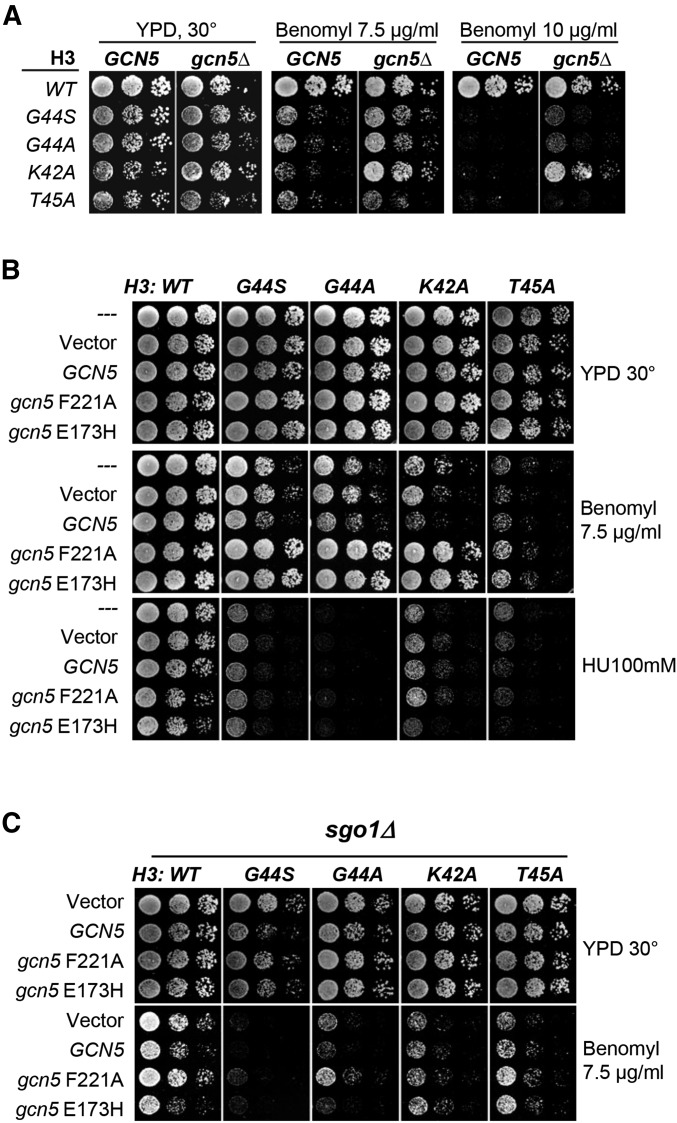
While it is highly likely that Sgo1p was first recruited to the centromeres and then spread to pericentromeres (Figure 4) (Fernius and Hardwick 2007; Kawashima et al. 2010; Luo et al. 2010), it is enigmatic as to how Sgo1p is confined at such loci when H3 is nearly ubiquitous in chromosomes. One possibility is that the H3–Sgo1p interaction is subjected to direct or indirect regulation at or surrounding centromeres such that only the pericentric H3 is amenable to Sgo1p association. Because bacterially expressed Sgo1p and H3 interact well (Luo et al. 2010), it is possible that the H3–Sgo1p interaction might be negatively regulated in vivo by a reversible modification. Of the many chromatin modifying activities, we were particularly interested in the potential involvement of Gcn5p, a prototypical histone acetyltransferase well known for its role in transcriptional regulation. Additionally, Gcn5p controls the chromatin structure at the centromeric region, an activity shown to be important for ensuring timely mitotic progression (Zhang et al. 1998; Vernarecci et al. 2008). We suspected that Gcn5p might also be involved in the tension sensing function of H3 and therefore examined the effect of deleting GCN5 from strains bearing mutations within the TSM. Figure 5A shows that deleting GCN5 effectively suppressed the benomyl hypersensitivity of G44S, G44A, and K42A strains. Intriguingly, the T45A mutant was much less susceptible to this suppression, arguing against the idea that deleting GCN5 resulted in a global change in benomyl flux or metabolism. To further delineate whether the suppression was related to the HAT (or lysine acetyltransferase) (KAT) activity of Gcn5p, we overexpressed two dominant negative alleles of GCN5, gcn5 F221A and gcn5 E173H (Kuo et al. 1998; Liu et al. 2005), that were devoid of the catalytic activity. Essentially identical results as those from GCN5 ORF deletion were obtained (Figure 5B). The HAT activity of Gcn5p was thus concluded to be elemental to the mitotic function of Gcn5p. In addition to benomyl hypersensitivity, mutations at K42, G44, and T45 also caused cellular hypersensitivity to a nucleoside analog, hydroxyurea (HU) (Figure 5B). However, overexpression of the GCN5 HAT inactive mutants had no effect on HU hypersensitivity, indicating that the genetic interaction between Gcn5p and H3 is specific to mitotic functions. The notion that Gcn5p acted as a negative regulator for the H3 mitotic function was further supported by the enhanced benomyl sensitivity when the WT Gcn5p was overexpressed in the K42A, G44A, and G44S mutants (Figure 5B). Moreover, the suppression brought about by gcn5 F221A and gcn5 E173H alleles was reverted upon SGO1 deletion (Figure 5C), suggesting that Gcn5p regulates H3 function in the same pathway as Sgo1p.
Figure 5.
Gcn5p histone acetyltransferase is a negative regulator of the histone H3 TSM. (A) Deleting GCN5 rescued the benomyl hypersensitivity phenotype of mutations at K42 and G44, but to a lesser extent for T45A allele. (B) The HAT activity of Gcn5p is linked specifically to the mitotic defect of TSM mutations. Dominant negative, catalytically inactive mutant Gcn5p bearing the E173H or F221A mutation (Kuo et al. 1998) was overexpressed in different TSM mutant strains. The cellular sensitivity to benomyl and HU was tested. Only benomyl hypersensitivity was rescued. (C) The E173H and F221A Gcn5p suppressors for TSM mutations require Sgo1p. SGO1 was deleted in WT and different TSM mutants expressing different GCN5 alleles. The cellular tolerance to benomyl was assessed.
The E173H gcn5− suppressor for mitotic defects caused by G44S was further examined by two additional methods (Figure 6). First, the cellular viability decline caused by progressively longer incubation with benomyl was assessed. Benomyl treatment depolymerizes microtubules and arrests cells at the G2/M phase. After returning to a benomyl-free medium, cells reestablish spindle–kinetochore attachment for metaphase-to-anaphase transition. Cell cycle progression with uncorrected attachment mistakes leads to aneuploidy and cell death. Defects in tension sensing thus cause elevated death rates (Luo et al. 2010) (Figure 6A). Overexpressing the dominant negative mutant Gcn5p restored viability of the G44S mutant to nearly the WT level (black broken line, Figure 6A). Second, the chromosome missegregation rate of the G44S mutant with or without the E173H allele of Gcn5p was further quantified by examining haploid cells bearing a GFP-marked TRP1 locus 12 kb from CEN4 (Straight et al. 1996). Haploid G1 phase cells with two GFP dots indicated missegregation from the previous round of mitosis. Figure 6B shows that the percentage of two-dotted G44S cells was significantly reduced by the gcn5− E173H allele. From Figure 5 and Figure 6, we concluded that the histone acetyltransferase Gcn5p functions as a negative regulator for the mitotic regulatory function of the histone H3 TSM.
Figure 6.
Gcn5p and Rpd3p affect chromosome stability via genetic interaction with the TSM. (A) WT and G44S strains bearing a gcn5 E173H overexpressing plasmid were treated with benomyl for the indicated time before plating to a benomyl-free medium to assess cellular viability. G44S cells lost viability fast but could be rescued by the dominant negative allele of Gcn5p. (B) Segregation of a GFP-marked chromosome was assessed by fluorescent microscopy. G1 phase cells bearing two GFP dots, indicating cosegregation of the indicator chromosome, were scored and expressed as percent of missegregation. (C) Deleting RPD3 augments chromosome instability caused by a tension sensing mutation. The indicated yeast strains bearing an artificial chromosome that rescues the ade2− red colony phenotype were plated to YPD medium after overnight growth. Retention of the artificial chromosome gives rise to white colonies, the loss of which renders colonies exhibiting red color. The relative size of the red sector is dictated by the time of the chromosome loss during colonization. Total red colonies result from chromosome loss before inoculation to the plate. G44S rpd3Δ double mutant cells produce practically only all-red colonies. Error bars from A and B were calculated from at least three biological replica.
Histone deacetylases Rpd3p and Hos2 interact genetically with the TSM of H3
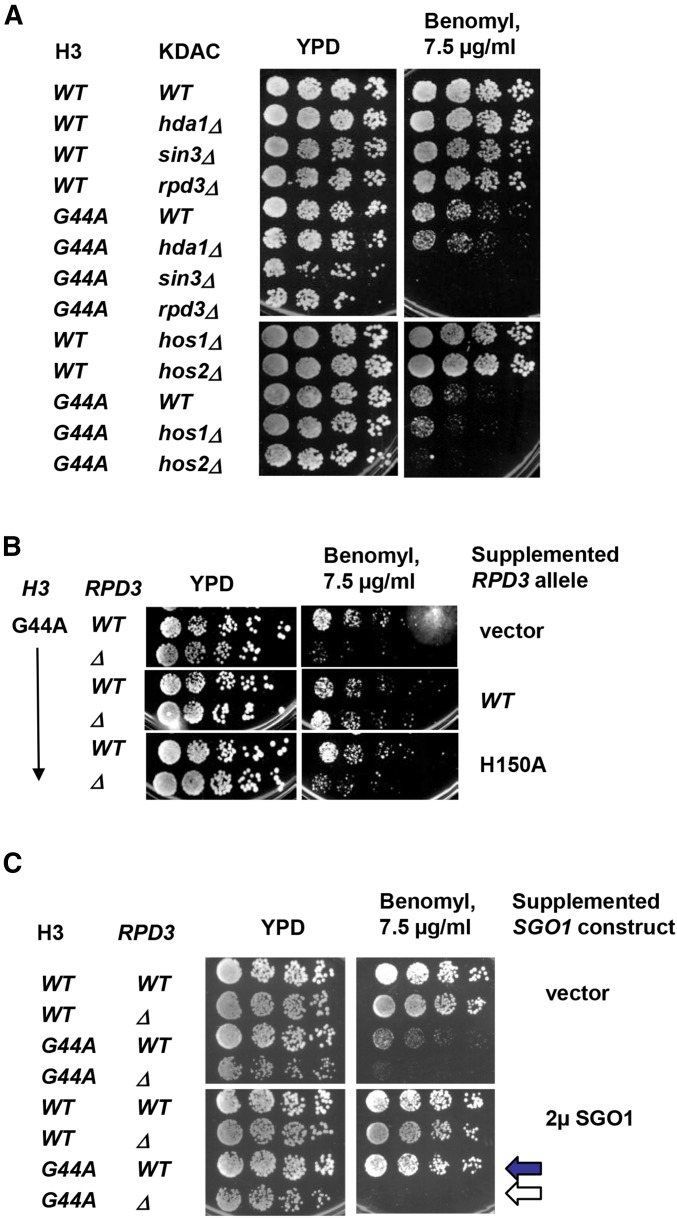
If the HAT activity of Gcn5p plays a negative role in tension sensing, a lysine deacetylase(s) (KDAC) would likely be involved as well. If true, deleting such a deacetylase gene (equivalent to upregulating the HAT activity of Gcn5p) was predicted to cause a phenotype opposite of deleting GCN5, e.g., elevated benomyl intolerance. Indeed, when RPD3 or HOS2 was deleted, the G44A cells exhibited benomyl hypersensitivity more severe than G44A, rpd3Δ, and hos2Δ single mutants (Figure 7A). Knocking out SIN3, which encodes a partner of Rpd3p for protein deacetylation (Kasten et al. 1997), caused the same phenotype as that of G44A rpd3Δ (Figure 7A), strongly suggesting that the Rpd3p/Sin3p deacetylase complex was involved. In contrast, the null alleles of two other KDAC genes HDA1 and HOS1 failed to cause a similar synthetic phenotype, indicating functional differentiation among these enzymes in mitosis. Besides benomyl hypersensitivity, the combination of rpd3Δ and the H3 G44S allele caused significant frequency of chromosome loss, as revealed by the sectoring assays using a nonessential artificial chromosome (Figure 6C) (Spencer et al. 1990). Deleting RPD3 alone did not cause a discernible effect on chromosome stability. Moreover, similar to the observation that a single catalytic dead mutation of Gcn5p was sufficient to elicit the suppression phenotype (see Figure 5 above), an H150A point mutation introduced to the active center of Rpd3p KDAC (Kadosh and Struhl 1998) was sufficient to cause synthetic benomyl hypersensitivity in the H3 G44A background (Figure 7B), indicating that the KDAC activity of Rpd3p was responsible for the observed mitotic defects. Western blotting of whole cell lysates demonstrated comparable abundance of the WT and the H150A species of Rpd3p (Figure S5). Moreover, all TSM mutants are suppressed by Sgo1p overexpression (Luo et al. 2010; Ng et al. 2013) (Figure 1D above). This suppression was ablated by deleting RPD3 (black vs. white arrows, Figure 7C), indicating that Rpd3p is essential for Sgo1p to function in the surveillance of biorientation when the TSM is crippled (Figure S6).
Figure 7.
Histone deacetylases Rpd3p and Hos2p functionally interact with the H3 TSM. (A) Different HDAC genes were deleted in the G44A background to assess the effect on the benomyl hypersensitivity of the G44A cells. (B) The HDAC activity of Rpd3 underlies the genetic interaction between RPD3 and H3 G44A allele. WT and G44A cells with an rpd3Δ null allele were transformed with an empty vector, WT RPD3, or rpd3 bearing an inactivating H150A mutation. The cellular sensitivity to benomyl was examined. (C) Sgo1p multicopy suppressor for the G44A mutant allele of the TSM requires Rpd3p.
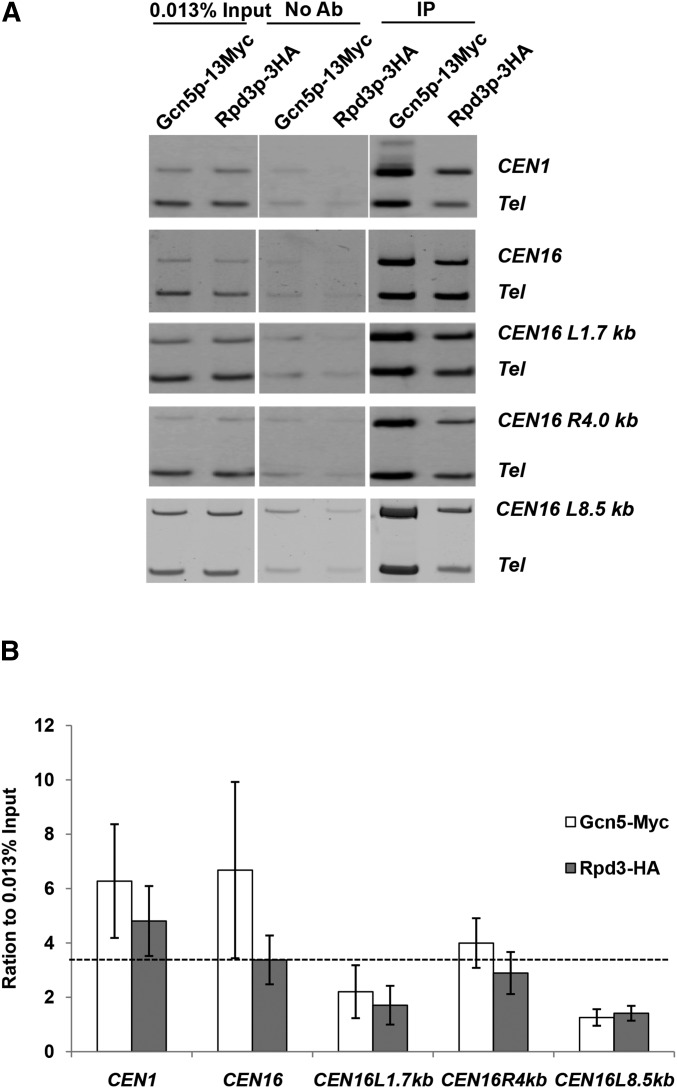
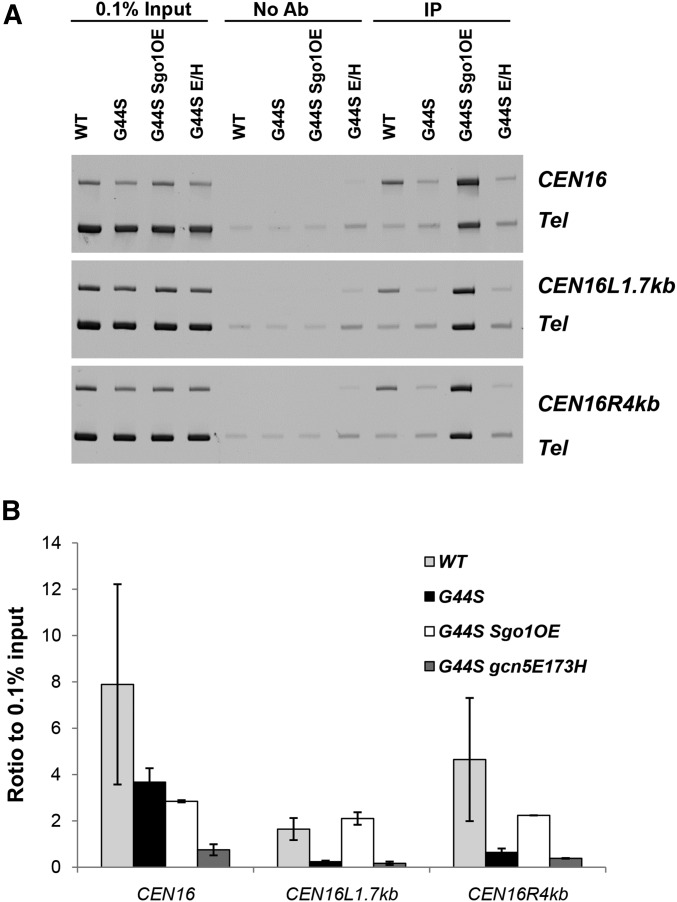
A role of Rpd3p and Gcn5p in tension sensing was further supported by ChIP that showed enrichment of both enzymes at centromeres and pericentromeres (Figure 8). When these two proteins were epitope tagged (13×Myc for Gcn5p and 3×HA for Rpd3p) and ChIP’ed from benomyl-arrested G2/M phase cells, both CEN and pericentromeres harbored significant levels of Gcn5p and Rpd3p when compared with a transcriptionally silenced subtelomeric region. The enrichment of Gcn5p and Rpd3p at the centromeres and nearby regions is consistent with the notion that the mitotic function of Sgo1p and TSM are subjected to the local regulation by these two enzymes. To further delineate the molecular underpinning of how Gcn5p may control the Sgo1p–TSM interaction, ChIP was conducted to examine the recruitment of Sgo1p in the gcn5 E173H suppressor background. In contrast to the common notion that Sgo1p monitors the biorientation status only at centromeres and pericentromeres, the gcn5 E173H suppressor apparently rescues the G44S TSM mutant in a manner that does not require persistent presence of Sgo1p at the centromeres or the pericentromeric region (Figure 9). Conversely, overexpressing Sgo1p in the same mutant background successfully reestablished the pericentric Sgo1p recruitment (Figure 9B, white bars). These results suggest that Sgo1p may also have an activity that does not require stable association with centromeres or pericentromeres, and that this activity, liberated in gcn5− cells, complements the defects of the TSM of H3.
Figure 8.
Both Gcn5p and Rpd3p are present in centromeres and pericentromeres. Myc-tagged Gcn5p and HA-tagged Rpd3p were ChIP’ed with the cognate antibodies. Quantitative PCR was conducted with primers corresponding to a selective chromosomal locus and a common telomeric region (Tel) as the internal control. The ratio of selective vs. the telomere control were calculated and normalized to that of input. The dotted line in B represents the experiment-to-input baseline. Error bars are from three biological replica.
Figure 9.
gcn5 E173H suppressor rescues tension sensing motif mutation independently of pericentric Sgo1p recruitment. Association of Sgo1p with CEN16 and the nearby pericentric regions was examined by ChIP. See Figure 8 legends for assay details. OE, overexpression. Errors were calculated from two biological replica.
Discussion
This work characterizes a TSM in histone H3 that is important for mitotic checkpoint control. The core residues of this motif, Lys42, Gly44, and Thr45, are important for interactions with Sgo1p both in vitro and in vivo. Alanine substitutions of these three residues share similar mitotic phenotypes, and are all susceptible to the overexpression of SGO1, suggesting that they function in the same pathway for monitoring tension between sister chromatids. These results are consistent with the scanning mutagenesis experiments reported by others (Kawashima et al. 2011; Ng et al. 2013). ChIP assays showed that mutating residues of the TSM selectively diminished the pericentric, but not the centromeric recruitment of Sgo1p. We suggest that Sgo1p is first recruited to the centromeres by factors such as the kinase Bub1p and phosphorylated histone H2A (Fernius and Hardwick 2007; Kawashima et al. 2010; Williams et al. 2016), and then relocates to pericentromeres where histone H3 is part of the canonical nucleosomes (Luo et al. 2010). Retention of pericentric Sgo1p is mediated through the TSM of H3. The loss of pericentric domains of Sgo1p results in malfunction in tension sensing and chromosome missegregation. Intriguingly, the mitotic function of TSM appears to be regulated by the histone acetyltransferase Gcn5p, and at least two deacetylases, Rpd3p and Hos2p. That changing GCN5, RPD3, or HOS2 status alone does not cause a significant mitotic phenotype in the WT H3 background suggests that these enzymes function within the TSM–Sgo1p tension sensing pathway, and likely play auxiliary roles that become indispensable when TSM is crippled.
The benomyl hypersensitivity phenotype caused by K42A, G44A, and G44S alleles is suppressed by deleting GCN5 or inactivating its HAT activity and is augmented by the loss of RPD3, SIN3, or HOS2 deacetylase genes. Chromosome instability is also rescued by the E173H catalytically inactive mutant of Gcn5p (Figure 6, A and B), but drastically augmented by the deletion of RPD3 (Figure 6C). Importantly, the suppressor activity of gcn5− alleles depends critically on Sgo1p (Figure 5C). The SGO1 high-copy suppressor for G44A is practically eliminated in the absence of RPD3 (Figure 5C), whereas the chromosome loss rate of G44S cells rises greatly (Figure 6C). Together, these results strongly suggest that protein acetylation is a critical component of tension sensing executed by H3 and Sgo1p. Indeed, both Gcn5p and Rpd3p are found to be enriched at centromeres and pericentromeres during mitosis (Figure 8). It is tempting to speculate that one or more centromeric and pericentric proteins are acetylated by Gcn5p in its antagonizing the H3–Sgo1p mitotic function. While the TSM includes a potentially acetylatable lysine residue, it is unlikely that Lys42 is a functional acetylation target herein because the K42R mutation, which mimics a constitutively unacetylated state, does not suppress the concomitant G44S mutant (Figure 1B). Instead, one can envision that acetylation of Sgo1p by Gcn5p diminishes the affinity for the TSM. The biochemical test of this hypothesis is currently being pursued. In addition to Sgo1p, cohesin may also be targeted by Gcn5p and Rpd3p. Cohesin plays an important role in localizing Sgo1p to the pericentromeres (Hou et al. 2007; Gutierrez-Caballero et al. 2012; Liu et al. 2013a). The recruitment of mammalian cohesion complex to centromeres and pericentromeres requires the acetyltransferase activity of San (Hou et al. 2007) and Esco1 (Whelan et al. 2012). However, our genetic data indicate that the acetylation is a negative regulator of tension sensing. If one of the cohesin subunits is acetylated by Gcn5p, this action may inhibit either the pericentric loading of cohesin or its interaction with Sgo1p.
It is also intriguing that the suppression brought about by eliminating the activity of Gcn5p does not require stable association of Sgo1p with centromeres and pericentromeres (Figure 9), which is in stark contrast to the observation that overexpressing Sgo1p restores the pericentric Sgo1p enrichment. One explanation is that the association between Sgo1p and chromatin, though restored, is more dynamic in the gcn5− background due to, e.g., changes in centromeric and pericentric chromatin structure, so that the standard ChIP procedures fail to efficiently detect pericentric Sgo1p. Alternatively, it is possible that Sgo1p’s tension sensing function includes a phase that is separable from chromatin binding. This function is normally kept dormant by Gcn5p and is awakened upon deleting GCN5. For example, Jin and Wang (2013) suggested that Sgo1p functions in the SAC silencing network. However, the physical form or location of Sgo1p in this network remains to be delineated. The pericentromere and centromere binding-independent function of Sgo1p as revealed by the gcn5− suppressor may provide a direction for more work.
Compared with other mutations in the TSM, the T45A allele responds poorly to the suppression by gcn5 and less well to multicopy SGO1 overexpression (Figure 1C and Figure 5B, respectively). Because K42A, G44A, G44S, and T45A alleles all show weakened interactions with Sgo1p (Figure 4D) (Luo et al. 2010), it seems likely that Thr45 plays a qualitatively distinct role in mediating H3–Sgo1p interaction. One possibility is that the H3–Sgo1p interaction requires the side chain of Thr45, whereas Lys42 and Gly44 together provide an environment (e.g., the sharp-turn structure, Figure 1E) that facilitates the Thr45–Sgo1p contact. If true, this hypothesis suggests that a post-translational modification at this residue would impose a significant impact on Sgo1p interaction. Indeed, Thr45 phosphorylation has been reported in both yeast and humans. The yeast Dbf4p–Cdc7p complex catalyzes Thr45 phosphorylation for DNA replication (Baker et al. 2010), whereas the mammalian Thr45 phosphorylation event is linked to apoptosis (Hurd et al. 2009) and to DNA damage (Lee et al. 2015). The phosphomimetic T45E mutation causes cell death (Baker et al. 2010 and data not shown), preventing detailed genetic experiments for the possible involvement of Thr45 phosphorylation in mitosis. However, our preliminary biochemical experiments showed that phosphorylation at Thr45 blocked Sgo1p interaction in vitro (data not shown), suggesting that Dbf4p–Cdc7p or another kinase may control the H3–Sgo1p interaction via Thr45 phosphorylation. Additionally, given the potential contribution of acetylation in the regulation of the TSM, it is worth noting that serine and threonine can also be acetylated (Mukherjee et al. 2006, 2007; Tweedie-Cullen et al. 2012). Whether Gcn5p could use Thr45 as an acetylation target for mitotic control awaits to be examined.
Sgo1p undergoes biorientation-dependent removal from chromatin (Nerusheva et al. 2014), suggesting that Sgo1p and, in particular, its interaction with chromatin is tightly linked to the status of tension. Pericentric chromatin structural changes seem to be an obligatory outcome of bipolar attachment (Haase et al. 2012; Verdaasdonk et al. 2012; Stephens et al. 2013). In tension defects, sgo1Δ cells either cannot activate the spindle checkpoint (Indjeian et al. 2005) or cannot prevent the silencing of SAC (Jin and Wang 2013). The latter model was supported by a recent report that Sgo1p, along with Ipl1p, Dam1p, and protein phosphatase 1 (PP1), is required to keep SAC on in the absence of tension (Jin and Wang 2013). The data presented above demonstrate a causal role of the loss of pericentric Sgo1p in crippling the tension sensing function. We suggest that the TSM of histone H3 in the pericentromeres functions initially as a docking site for Sgo1p, which is recruited to the centromeres by Bub1p and phosphorylated H2A, while cells establish bipolar attachment. Biorientation generates tension, which in turn alters chromatin structure in the pericentromeres (Haase et al. 2012; Verdaasdonk et al. 2012; Stephens et al. 2013), resulting in conformational changes of the TSM that disrupt Sgo1p–H3 interaction. The eviction of Sgo1p molecules from the pericentromere of the last pair of chromosomes under tension silences the checkpoint, hence allowing cells to progress from metaphase to anaphase. Whether the mere absence of Sgo1p or the exiting Sgo1p proactively shuts off SAC awaits to be delineated.
Acknowledgments
This work was supported by a grant (MCB1050132) from the National Science Foundation to M.H.-Kuo. X. Deng and C. Buehl were partly supported by fellowships from the College of Natural Science of Michigan State University.
Footnotes
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.192443/-/DC1.
Communicating editor: S. Biggins
Literature Cited
- Akera T., Watanabe Y., 2016. The spindle assembly checkpoint promotes chromosome bi-orientation: a novel Mad1 role in chromosome alignment. Cell Cycle 15: 493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S. P., Phillips J., Anderson S., Qiu Q., Shabanowitz J., et al. , 2010. Histone H3 Thr 45 phosphorylation is a replication-associated post-translational modification in S. cerevisiae. Nat. Cell Biol. 12: 294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom K., Sharma S., Dokholyan N. V., 2006. The path of DNA in the kinetochore. Curr. Biol. 16: R276–R278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C. S., Desai A., 2013. Tension sensing by aurora B kinase is independent of survivin-based centromere localization. Nature 497: 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers A. L., Ormerod G., Durley S. C., Sing T. L., Brown G. W., et al. , 2012. The INO80 chromatin remodeling complex prevents polyploidy and maintains normal chromatin structure at centromeres. Genes Dev. 26: 2590–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., Hyland E. M., Yuan D. S., Huang H., Bader J. S., et al. , 2008. Probing nucleosome function: a highly versatile library of synthetic histone H3 and H4 mutants. Cell 134: 1066–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D. G., Smith M. M., Roth S. Y., 1996. Repression domain of the yeast global repressor Tup1 interacts directly with histones H3 and H4. Genes Dev. 10: 1247–1259. [DOI] [PubMed] [Google Scholar]
- Eshleman H. D., Morgan D. O., 2014. Sgo1 recruits PP2A to chromosomes to ensure sister chromatid bi-orientation during mitosis. J. Cell Sci. 127: 4974–4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernius J., Hardwick K. G., 2007. Bub1 kinase targets Sgo1 to ensure efficient chromosome biorientation in budding yeast mitosis. PLoS Genet. 3: e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz D., St Jean A., Woods R. A., Schiestl R. H., 1992. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20: 1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G., Yanagida M., 2000. Establishing biorientation occurs with precocious separation of the sister kinetochores, but not the arms, in the early spindle of budding yeast. Cell 100: 619–633. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Caballero C., Cebollero L. R., Pendas A. M., 2012. Shugoshins: from protectors of cohesion to versatile adaptors at the centromere. Trends Genet. 28: 351–360. [DOI] [PubMed] [Google Scholar]
- Haase J., Stephens A., Verdaasdonk J., Yeh E., Bloom K., 2012. Bub1 kinase and Sgo1 modulate pericentric chromatin in response to altered microtubule dynamics. Curr. Biol. 22: 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Asthana S., Sorger P. K., 2000. Transient sister chromatid separation and elastic deformation of chromosomes during mitosis in budding yeast. Cell 101: 763–775. [DOI] [PubMed] [Google Scholar]
- Herskowitz I., Jensen R. E., 1991. Putting the HO gene to work: practical uses for mating-type switching. Methods Enzymol. 194: 132–146. [DOI] [PubMed] [Google Scholar]
- Hou F., Chu C. W., Kong X., Yokomori K., Zou H., 2007. The acetyltransferase activity of san stabilizes the mitotic cohesin at the centromeres in a shugoshin-independent manner. J. Cell Biol. 177: 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd P. J., Bannister A. J., Halls K., Dawson M. A., Vermeulen M., et al. , 2009. Phosphorylation of histone H3 Thr45 is linked to apoptosis. J. Biol. Chem. 284: 16575–16583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland E. M., Molina H., Poorey K., Jie C., Xie Z., et al. , 2011. An evolutionarily ‘young’ lysine residue in histone H3 attenuates transcriptional output in Saccharomyces cerevisiae. Genes Dev. 25: 1306–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indjeian V. B., Stern B. M., Murray A. W., 2005. The centromeric protein Sgo1 is required to sense lack of tension on mitotic chromosomes. Science 307: 130–133. [DOI] [PubMed] [Google Scholar]
- Jin F., Wang Y., 2013. The signaling network that silences the spindle assembly checkpoint upon the establishment of chromosome bipolar attachment. Proc. Natl. Acad. Sci. USA 110: 21036–21041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadosh D., Struhl K., 1998. Histone deacetylase activity of Rpd3 is important for transcriptional repression in vivo. Genes Dev. 12: 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten M. M., Dorland S., Stillman D. J., 1997. A large protein complex containing the yeast Sin3p and Rpd3p transcriptional regulators. Mol. Cell. Biol. 17: 4852–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima S., Nakabayashi Y., Matsubara K., Sano N., Enomoto T., et al. , 2011. Global analysis of core histones reveals nucleosomal surfaces required for chromosome bi-orientation. EMBO J. 30: 3353–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima S. A., Yamagishi Y., Honda T., Ishiguro K., Watanabe Y., 2010. Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science 327: 172–177. [DOI] [PubMed] [Google Scholar]
- Kiburz B. M., Reynolds D. B., Megee P. C., Marston A. L., Lee B. H., et al. , 2005. The core centromere and Sgo1 establish a 50-kb cohesin-protected domain around centromeres during meiosis I. Genes Dev. 19: 3017–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima T. S., Kawashima S. A., Watanabe Y., 2004. The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature 427: 510–517. [DOI] [PubMed] [Google Scholar]
- Kitajima T. S., Sakuno T., Ishiguro K., Iemura S., Natsume T., et al. , 2006. Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature 441: 46–52. [DOI] [PubMed] [Google Scholar]
- Kschonsak M., Haering C. H., 2015. Shaping mitotic chromosomes: from classical concepts to molecular mechanisms. BioEssays 37: 755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M. H., Allis C. D., 1999. In vivo cross-linking and immunoprecipitation for studying dynamic Protein:DNA associations in a chromatin environment. Methods 19: 425–433. [DOI] [PubMed] [Google Scholar]
- Kuo M. H., Zhou J., Jambeck P., Churchill M. E., Allis C. D., 1998. Histone acetyltransferase activity of yeast Gcn5p is required for the activation of target genes in vivo. Genes Dev. 12: 627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H., Kang B. H., Jang H., Kim T. W., Choi J., et al. , 2015. AKT phosphorylates H3-threonine 45 to facilitate termination of gene transcription in response to DNA damage. Nucleic Acids Res. 43: 4505–4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Nicklas R. B., 1995. Mitotic forces control a cell-cycle checkpoint. Nature 373: 630–632. [DOI] [PubMed] [Google Scholar]
- Liu H., Jia L., Yu H., 2013a Phospho-H2A and cohesin specify distinct tension-regulated Sgo1 pools at kinetochores and inner centromeres. Curr. Biol. 23: 1927–1933. [DOI] [PubMed] [Google Scholar]
- Liu H., Rankin S., Yu H., 2013b Phosphorylation-enabled binding of SGO1–PP2A to cohesin protects sororin and centromeric cohesion during mitosis. Nat. Cell Biol. 15: 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Xu X., Singh-Rodriguez S., Zhao Y., Kuo M. H., 2005. Histone H3 Ser10 phosphorylation-independent function of Snf1 and Reg1 proteins rescues a gcn5- mutant in HIS3 expression. Mol. Cell. Biol. 25: 10566–10579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Xu X., Kuo M. H., 2010. Snf1p regulates Gcn5p transcriptional activity by antagonizing Spt3p. Genetics. 184: 91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Xu X., Hall H., Hyland E. M., Boeke J. D., et al. , 2010. Histone h3 exerts a key function in mitotic checkpoint control. Mol. Cell. Biol. 30: 537–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston A. L., 2015. Shugoshins: tension-sensitive pericentromeric adaptors safeguarding chromosome segregation. Mol. Cell. Biol. 35: 634–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Hao Y. H., Orth K., 2007. A newly discovered post-translational modification–the acetylation of serine and threonine residues. Trends Biochem. Sci. 32: 210–216. [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Keitany G., Li Y., Wang Y., Ball H. L., et al. , 2006. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science 312: 1211–1214. [DOI] [PubMed] [Google Scholar]
- Nasmyth K., 2002. Segregating sister genomes: the molecular biology of chromosome separation. Science 297: 559–565. [DOI] [PubMed] [Google Scholar]
- Nerusheva O. O., Galander S., Fernius J., Kelly D., Marston A. L., 2014. Tension-dependent removal of pericentromeric shugoshin is an indicator of sister chromosome biorientation. Genes Dev. 28: 1291–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T. M., Lenstra T. L., Duggan N., Jiang S., Ceto S., et al. , 2013. Kinetochore function and chromosome segregation rely on critical residues in histones H3 and H4 in budding yeast. Genetics 195: 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky B. A., Biggins S., 2005. The spindle checkpoint: tension vs. attachment. Trends Cell Biol. 15: 486–493. [DOI] [PubMed] [Google Scholar]
- Riedel C. G., Katis V. L., Katou Y., Mori S., Itoh T., et al. , 2006. Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature 441: 53–61. [DOI] [PubMed] [Google Scholar]
- Salic A., Waters J. C., Mitchison T. J., 2004. Vertebrate shugoshin links sister centromere cohesion and kinetochore microtubule stability in mitosis. Cell 118: 567–578. [DOI] [PubMed] [Google Scholar]
- Sherman F., 1991. Getting started with yeast. Methods Enzymol. 194: 3–21. [DOI] [PubMed] [Google Scholar]
- Spencer F., Gerring S. L., Connelly C., Hieter P., 1990. Mitotic chromosome transmission fidelity mutants in Saccharomyces cerevisiae. Genetics 124: 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens A. D., Haggerty R. A., Vasquez P. A., Vicci L., Snider C. E., et al. , 2013. Pericentric chromatin loops function as a nonlinear spring in mitotic force balance. J. Cell Biol. 200: 757–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight A. F., Belmont A. S., Robinett C. C., Murray A. W., 1996. GFP tagging of budding yeast chromosomes reveals that protein-protein interactions can mediate sister chromatid cohesion. Curr. Biol. 6: 1599–1608. [DOI] [PubMed] [Google Scholar]
- Tang Z., Shu H., Qi W., Mahmood N. A., Mumby M. C., et al. , 2006. PP2A is required for centromeric localization of Sgo1 and proper chromosome segregation. Dev. Cell 10: 575–585. [DOI] [PubMed] [Google Scholar]
- Tanno Y., Kitajima T. S., Honda T., Ando Y., Ishiguro K., et al. , 2010. Phosphorylation of mammalian Sgo2 by Aurora B recruits PP2A and MCAK to centromeres. Genes Dev. 24: 2169–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie-Cullen R. Y., Brunner A. M., Grossmann J., Mohanna S., Sichau D., et al. , 2012. Identification of combinatorial patterns of post-translational modifications on individual histones in the mouse brain. PLoS One 7: e36980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdaasdonk J. S., Gardner R., Stephens A. D., Yeh E., Bloom K., 2012. Tension-dependent nucleosome remodeling at the pericentromere in yeast. Mol. Biol. Cell 23: 2560–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernarecci S., Ornaghi P., Bagu A., Cundari E., Ballario P., et al. , 2008. Gcn5p plays an important role in centromere kinetochore function in budding yeast. Mol. Cell. Biol. 28: 988–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Jin F., Higgins R., McKnight K., 2014. The current view for the silencing of the spindle assembly checkpoint. Cell Cycle 13: 1694–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., 2005. Shugoshin: guardian spirit at the centromere. Curr. Opin. Cell Biol. 17: 590–595. [DOI] [PubMed] [Google Scholar]
- Whelan G., Kreidl E., Wutz G., Egner A., Peters J. M., et al. , 2012. Cohesin acetyltransferase Esco2 is a cell viability factor and is required for cohesion in pericentric heterochromatin. EMBO J. 31: 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C. L., Suto R. K., Luger K., 2001. Structure of the yeast nucleosome core particle reveals fundamental changes in internucleosome interactions. EMBO J. 20: 5207–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. J., Abrieu A., Losada A., 2016. Bub1 targeting to centromeres is sufficient for Sgo1 recruitment in the absence of kinetochores. Chromosoma. DOI: 10.1007/s00412-016-0592-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Cetin B., Anger M., Cho U. S., Helmhart W., et al. , 2009. Structure and function of the PP2A-shugoshin interaction. Mol. Cell 35: 426–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi Y., Sakuno T., Shimura M., Watanabe Y., 2008. Heterochromatin links to centromeric protection by recruiting shugoshin. Nature 455: 251–255. [DOI] [PubMed] [Google Scholar]
- Yeh E., Haase J., Paliulis L. V., Joglekar A., Bond L., et al. , 2008. Pericentric chromatin is organized into an intramolecular loop in mitosis. Curr. Biol. 18: 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S., Ai J. S., Shi L. H., Wei L., Yuan J., et al. , 2008. Shugoshin1 may play important roles in separation of homologous chromosomes and sister chromatids during mouse oocyte meiosis. PLoS One 3: e3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen K. W., Warren C. D., Chen O., Kwok T., Hieter P., et al. , 2007. Systematic genome instability screens in yeast and their potential relevance to cancer. Proc. Natl. Acad. Sci. USA 104: 3925–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Bone J. R., Edmondson D. G., Turner B. M., Roth S. Y., 1998. Essential and redundant functions of histone acetylation revealed by mutation of target lysines and loss of the Gcn5p acetyltransferase. EMBO J. 17: 3155–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.