Abstract
The development of gametophytes relies on the establishment of a haploid gametophytic generation that initiates with the specification of gametophytic precursors. The majority of flowering plants differentiate a single gametophytic precursor in the ovule: the megaspore mother cell. Here we show that, in addition to argonaute9 (ago9), mutations in other ARGONAUTE (AGO) genes such as ago4, ago6, and ago8, also show abnormal configurations containing supernumerary gametophytic precursors in Arabidopsis thaliana. Double homozygous ago4 ago9 individuals showed a suppressive effect on the frequency of ovules with multiple gametophytic precursors across three consecutive generations, indicating that genetic interactions result in compensatory mechanisms. Whereas overexpression of AGO6 in ago9 and ago4 ago9 confirms strong regulatory interactions among genes involved in RNA-directed DNA methylation, AGO8 is overexpressed in premeiotic ovules of ago4 ago9 individuals, suggesting that the regulation of this previously presumed pseudogene responds to the compensatory mechanism. The frequency of abnormal meiotic configurations found in ago4 ago9 individuals is dependent on their parental genotype, revealing a transgenerational effect. Our results indicate that members of the AGO4 clade cooperatively participate in preventing the abnormal specification of multiple premeiotic gametophytic precursors during early ovule development in A. thaliana.
Keywords: ARGONAUTE, Arabidopsis, RdDM, gametogenesis, ovule
THE alternation between the diploid-sporophytic and the haploid-gametophytic generation is a crucial distinction between the life cycle of plants and animals. Whereas in mammals and insects the cell lineage giving rise to gametes differentiates during embryogenesis (Bendel-Stenzel et al. 1998; Donoughe et al. 2014), in plants the gametogenic lineage initiates during floral development in the adult organism (Yadegari and Drews 2004). The onset of the female reproductive phase in most sexual flowering plants is defined by the specification of a single diploid megaspore mother cell (MMC) within the sporophytic nucellus, in the developing ovule (Bajon et al. 1999). Before gametogenesis, the MMC divides meiotically to produce four haploid megaspores. As in the majority of sexual species, in Arabidopsis thaliana (Arabidopsis) a single meiotically derived cell [the functional megaspore (FM)] differentiates prior to dividing mitotically and giving rise to the female gametophyte. The nucleus of the FM performs three mitotic divisions generating eight nuclei that subsequently undergo cellularization and differentiation to form accessory cells (the synergids and the antipodals), and two types of gametes: the egg cell and the central cell, which after fertilization will produce the embryo and the endosperm, respectively (Robinson-Beers et al. 1992; Reiser and Fischer 1993; Schneitz et al. 1995; Sheridan et al. 1999; Drews and Yadegari 2002).
Despite its prime importance for plant reproduction, the genetic basis and molecular mechanisms that control the somatic-to-reproductive transition are poorly understood. Only few mutants affecting specification of gamete precursors in the ovule have been found and characterized in different species. Mutants affecting the plant-specific MADS-box domain transcription factor NOZZLE/SPOROCYTELESS (NZZ/SPL) are defective in differentiating both microspore mother cells and MMCs (Yang et al. 1999). Similar to nzz/spl, the Arabidopsis windhouse1 (wih1) and windhouse2 (wih2) mutants are not able to properly differentiate an MMC; instead, nucellar cells acquire a parenchyma-like identity (Lieber et al. 2011). Other mutants promote the differentiation of several MMCs. In maize, for example, the loss of function of MULTIPLE ARCHEOSPORES 1 (MAC1), a gene encoding a leucine-rich repeat receptor-like kinase protein (LRR-RLK), promotes the development of numerous MMCs that can undergo meiosis (Sheridan et al. 1999). A similar phenotype was found in the multiple sporocyte 1 (msp1) mutant of rice, a gene encoding a LRR-RLK protein (Nonomura et al. 2003). In Arabidopsis, the differentiation of more than one female gamete precursor was first reported in mutants affecting small RNA (sRNA) pathways. Dominant argonaute9 (ago9), suppressor of gene silencing 3 (sgs3), rna-dependent rna polymerase 2 (rdr2), rna-dependent rna polymerase 6 (rdr6), dicer-like 3 (dcl3) mutants, and a double mutant defective in both RNA POLYMERASE IV and POLYMERASE V genes (nrpd1a nrpd1b), show multiple gametophytic precursors in the premeiotic ovule. Furthermore, some of these ectopic cells are able to develop female gametophytes that bypass meiosis, a phenotype resembling aposporous mechanisms that prevail in some plant species reproducing by apomixis (Olmedo-Monfil et al. 2010). More recently, a similar phenotype was reported for a dominant mutant affected in MNEME (MEM), a gene encoding an RNA helicase of the DEAD-box family (Schmidt et al. 2011).
Epigenetic mechanisms involved in the development of the animal germline have also been reported (Hajkova et al. 2002; Loriot et al. 2003; Brennecke et al. 2007; Brennecke et al. 2008; Kelly 2014). For example, it is well known that differentiation of primordial germ cells encompasses extensive DNA demethylation and histone replacement (Seki et al. 2005; Leitch et al. 2013). Similarly, recent findings demonstrated that highly active chromatin changes occur during MMC specification in Arabidopsis, suggesting that a reorganization of the epigenetic landscape takes place during the somatic-to-reproductive transition (She et al. 2013). In plants, sRNAs are involved in epigenetic modifications through the RNA-dependent DNA methylation (RdDM) regulation pathway (Matzke and Mosher 2014). RdDM initiates with the transcription of chromatin-enriched loci by the plant-specific DNA-directed RNA Polymerase IV (Pol IV). Pol IV transcripts are converted into long double-stranded RNA molecules by the action of RDR2, and subsequently sliced by DCL3 into 24 nt sRNAs that are loaded by AGO proteins such as AGO4, AGO6, or AGO9. These proteins interact with nascent transcripts produced by Polymerase V (Pol V), promoting the recruitment of de novo DNA methyltransferases such as DOMAINS REARRANGED METHYLTRANSFERASE 2 (DRM2) as well as histone methyltransferases and chromatin remodelers, to ultimately cause the reinforcement of heterochromatin (Law and Jacobsen 2010; Simon and Meyers 2010; Rowley et al. 2011; Zhang and Zhu 2012; Zhong et al. 2014). AGOs are a class of PAZ/PIWI domain-containing proteins that have undergone a high degree of gene duplication in plants (Vaucheret 2008; Zhai et al. 2014). In Arabidopsis, a phylogenetic analysis defined three distinct clades: the AGO1/5/10, the AGO2/3/7, and the so-called AGO4 clade composed of AGO4, AGO6, AGO8, and AGO9 (Supplemental Material, Figure S1; Vaucheret 2008). To date, only AGO4, AGO6, and AGO9 are shown to bind heterochromatic small interfering RNAs (siRNAs) that mostly target repetitive genomic regions and transposable elements (TE) (Zilberman et al. 2004; Zheng et al. 2007, 2013; Duran-Figueroa and Vielle-Calzada 2010; Havecker et al. 2010; Olmedo-Monfil et al. 2010; Eun et al. 2011). By contrast, AGO8 is generally considered to be a pseudogene, mainly because a computational analysis predicted that its presumed coding sequence contains splicing-inducing frame shifts, suggesting the formation of a nonfunctional protein (Takeda et al. 2008).
Here we report that mutations in AGO4, AGO6, or AGO8 lead to abnormal premeiotic ovules harboring more than one female gametophytic precursor, a phenotype reminiscent of defects found in ago9 mutants. We also show that individuals defective in both AGO4 and AGO9 show a suppressive effect on the frequency of ovules harboring this phenotype, revealing a genetic interaction between these two genes that leads to compensatory mechanisms in the control of cell specification. A detailed genetic and cytological analysis indicates that the frequency of premeiotic ovules showing ectopic cells is influenced by parental genotypes involving the function of AGO proteins other than AGO4 and AGO9. Gene expression analysis and in situ protein immunolocalization indicate that AGO6 is overexpressed in ago9 but not in ago4 or ago4 ago9 ovules, suggesting the existence of an interaction between AGO6 and AGO9 that partially depends on the activity of AGO4. By contrast, AGO8 is only overexpressed in ago4 ago9 individuals, suggesting its possible role in the compensatory effect that contributes to restrict gametophytic cell fate. Our results reveal a multigenic network of interactions involving members of the AGO4 clade to control early megaspore formation, opening new possibilities for elucidating the canalized mechanisms that ensure the initial stages of sexual reproduction in Arabidopsis.
Materials and Methods
Plant material and growth conditions
The ago4-6 (SALK_071772; Strickler et al. 2013), ago5-4 (SALK_050483; Tucker et al. 2012), ago6-2 (SALK_031553; Zheng et al. 2007), ago8-1 (Havecker et al. 2012), ago8-2 (SALK_010058), and ago9-3 (SAIL_34_G10; Olmedo-Monfil et al. 2010) mutant alleles are in the Columbia (Col) background; whereas ago1-37 (Yang et al. 2006) and ago4-1 (Zilberman et al. 2003) are in the Landsberg erecta (Ler) background. Both Col and Ler wild-type plants were used as controls in the quantitative analysis of single mutant genotypes, whereas Col × Ler F1 individuals were used as controls in the analysis of double mutant genotypes. Seeds were surface sterilized with chlorine gas and germinated under long-day conditions (16 hr light/8 hr dark) in MS medium at 22°. Seedlings were grown under greenhouse or growth chamber conditions (24°). Primer pairs used for genotyping are listed in Table S2.
Cytological analysis of ovule development
For cytological examination of premeiotic ovules, gynoecia of 0.4–0.6 mm in length from wild-type and mutant plants were harvested and fixed in formalin-acetic acid-alcohol solution (40% formaldehyde, glacial acetic acid, 50% ethanol; in a 5:5:90 volume ratio) for 24 hr at room temperature. After fixation, samples were washed five times with absolute ethanol and stored in 70% ethanol at room temperature for 24 hr. Fixed gynoecia were dissected with hypodermic needles (1 mm insulin syringes), cleared in Herr’s solution (phenol:chloral hydrate:85% lactic acid:xylene:clove oil in a 1:1:1:0.5:1 proportion), and observed by differential interference contrast microscopy using a Leica DMR microscope.
Quantitative real-time PCR
Total RNA from gynoecia bearing premeiotic ovules (0.5–0.6 mm in length) was isolated using Trizol (Invitrogen, Carlsbad, CA). Complementary DNA (cDNA) was synthesized from 1 µg of total RNA, using oligo dT and SuperScript reverse transcriptase II (Invitrogen). Primers for PCR were designed using the online program Primer 3 (v.0.4.0) and verified with OligoEvaluator (Sigma Chemical, St. Louis, MO) to discard dimer structure formation. PCR efficiencies of the target and reference genes were determined by generating standard curves, based on serial dilutions prepared from cDNA templates. PCR efficiency was calculated according to the slope of the standard curve (primers with 100% efficiency, the fold equals to 2). Each quantitative real-time PCR (qPCR) reaction was performed in a 10-µl volume consisting of 5 µl of 2× SYBR Green PCR Reaction Mix (Applied Biosystems, Foster City, CA), 3.5 µl of DNA template (10 ng/µl), 0.5 µl of forward primer (5 µM), 0.5 µl of reverse primer (5 µM), and 0.5 µl of ultrapure water. The qPCR reactions were performed using the StepOne Applied Biosystems and the data were analyzed using the StepOne software v2.2.2. The thermal profile consisted of 10 min at 95°, 40 cycles of 15 sec at 95°, and 1 min at 60°. Amplification results were collected at the end of the extension step. Primer sequences used for qPCR amplifications are listed in Table S2 and Table S4. A comparative 2−ΔΔCt method was used for determining a relative target quantity of gene expression, and ACTIN2 was used as a control (Czechowski et al. 2004). Reproducibility of the results was evaluated for each sample by running three technical and three biological replicates of each of the reactions and each genotype.
Whole-mount immunolocalization
Whole-mount immunolocalization was performed as described in Escobar-Guzmán et al. (2015), with minor modifications. Gynoecia of 0.6 mm were harvested and fixed in paraformaldehyde (1× PBS, 4% paraformaldehyde, 2% triton), for 2 hr under continuous agitation on ice. After fixation, the samples were washed three times in 1× PBS and embedded in a matrix of 15% acrylamide:bysacrilamide (29:1) over positively charged slides (ProbeOn Plus; Fisher Scientific, Pittsburgh, PA) previously treated with Poly-L-lysine. After embedding, the samples were digested in an enzymatic cocktail (1% driselase, 0.5% cellulose, 1% pectolyase) in 1× PBS for 1 hr at 37°. Then, the samples were permeabilized for 2 hr in 1× PBS:2% triton and blocked by incubating them with 1% BSA (Hoffman La Roche, Nutley, NJ) for 1 hr at 37°. Incubation with AGO6 (Havecker et al. 2010) primary antibody was carried out overnight at 4° at a dilution of 1:100. Samples were washed for 8 hr in 1× PBS:0.2% triton, refreshing the solution each 2 hr. Subsequently, samples were incubated overnight with the secondary antibody Alexa Fluor 488 (Molecular Probes, Eugene, OR) at a dilution of 1:300. After a washing step of 8 hr, the samples were incubated with propidium iodide (500 µg/ml) in 1× PBS for 20 min and washed in 1× PBS for 40 min. Finally, samples were mounted in PROLONG (Molecular Probes). Sections of premeiotic ovules were captured on a laser scanning confocal microscope (LSM 510 META; Carl Zeiss, Thornwood, NY) with multitrack configuration for detecting propidium iodide [excitation with diode pump solid state (DPSS) laser at 568 nm, emission collected using band pass filter (BP) 575–615 nm] and Alexa 488 (excitation with Argon laser at 488 nm, emission collected using band pass filter (BP) 500–550 nm). Laser intensity and gain were equivalently set for all samples.
Data availability
Sequence data from this article can be found in the European Molecular Biology Laboratory /GenBank data libraries under accession numbers At2g27040, At2g32940, At5g21030, and At5g21150. Additional mutant strains are available upon request. Table S2 contains names of primers used for genotyping and qPCR assays.
Results
All members of the AGO4 clade are involved in the specification of female gametophytic precursors
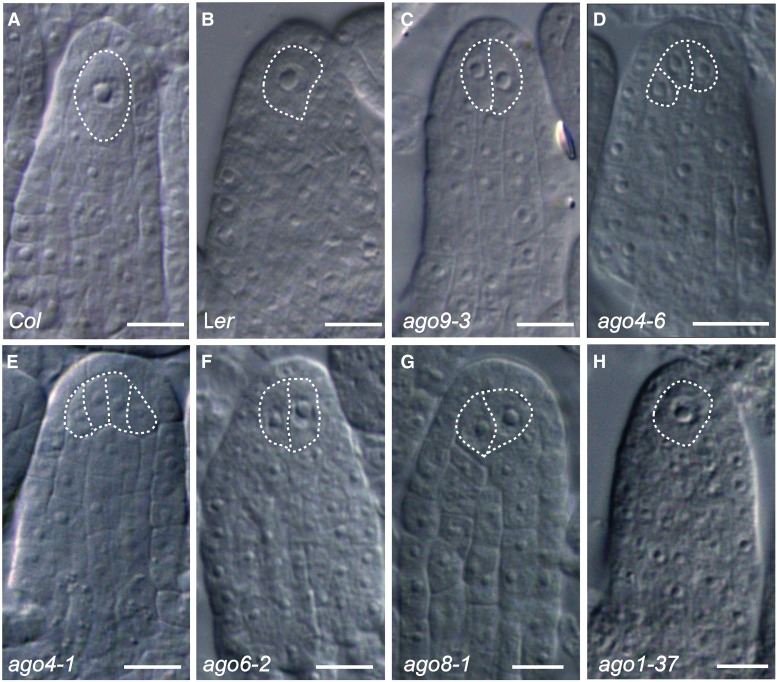
It was previously reported that mutations in AGO9 promote the development of more than one premeiotic gametophytic precursor during early ovule development (Olmedo-Monfil et al. 2010). The incomplete penetrance of this defect led us to suspect that additional factors could act redundantly to restrict cell specification in the ovule. To elucidate if close relatives of AGO9 could have a role in the somatic-to-reproductive transition, we cytologically characterized early ovule development in mutant plants defective in AGO4, AGO6, and AGO8. We started by conducting a quantitative characterization of stage 1 ovules following the classification reported by Rodríguez-Leal et al. (Rodríguez-Leal et al. 2015). Stage 1 corresponds to premeiotic ovule primordia having a well-defined proximal-distal axis and absence of integument initiation. For this type of ovule, we defined three phenotypic classes based on the number of enlarged subepidermal cells reminiscent of gametophytic precursors corresponding to the MMC: class I includes ovules with a single gametophytic precursor, which by its subepidermal position corresponds to the MMC (Figure 1, A and B); class II corresponds to premeiotic ovules with two gametophytic precursors resembling twin MMCs (Figure 1C); and class III corresponds to premeiotic ovules containing more than two cells resembling the MMC (Figure 1, D and E). Under greenhouse conditions, 9.8% (n = 934) and 17.2% (n = 429) of Col and Ler ovules showed two MMCs, respectively (Table 1), confirming that Arabidopsis ecotypes exhibit naturally occurring variation in the number of ovules with ectopic gametophytic precursors (Rodríguez-Leal et al. 2015). In contrast to wild type, ago9-3 behaved as a dominant mutation showing 20.68% ± 2.18 (n = 385) and 28.93% ± 3.36 (n = 508) class II and class III ovules in heterozygous and homozygous mutant individuals, respectively.
Figure 1.
Phenotypic characterization of stage 1 ovules in wild type and ago mutants. (A) Wild-type ovule of Col showing a single MMC (class I). (B) Wild-type ovule of Ler showing a single MMC (class I). (C) ago9-3 ovule showing two enlarged cells (class II), (D) ago4-6 ovule showing three abnormal enlarged cells (class III), (E) ago4-1 ovule showing four enlarged cells (F) ago6-2 ovule showing two enlarged cells (class III), (G) ago8-1 ovule showing two enlarged cells (class II), and (H) ago1-37 ovule showing a single MMC (class I). Bar, 20 µm.
Table 1. Phenotypic analysis of wild-type and mutant ovules at stage 1.
| Genotype | na | Class Ib | Class IIc | Class IIId | Class II and III | AIe |
|---|---|---|---|---|---|---|
| Col | 934 | 89.5 ± 1.23 | 10.49 ± 1.23 | 0 | 10.49 ± 1.23 | 3 |
| Ler | 429 | 82.9 ± 1.61 | 16.52 ± 1.39 | 0.56 ± 0.33 | 17.24 ± 1.62 | 3 |
| ago1-37 (Ler) | 369 | 86.90 ± 0.47 | 11.81 ± 0.83 | 0.97 ± 1.02 | 12.8 ± 0.54 | 3 |
| ago5-1 (Col) | 375 | 92.3 ± 0.55 | 7.59 ± 0.41 | 0 | 7.59 ± 0.41 | 3 |
| ago9-3/+ (Col) | 385 | 78.48 ± 2.63 | 20.68 ± 2.18 | 0.82 ± 0.47 | 21.50 ± 2.63 | 4 |
| ago9-3 (Col) | 508 | 71.05 ± 3.36 | 28.69 ± 3.36 | 0.32 ± 0.22 | 28.93 ± 3.36 | 4 |
| ago4-1/+ (Ler) | 270 | 79.77 ± 1.47 | 18.20 ± 0.94 | 2.00 ± 0.62 | 20.21 ± 1.47 | 3 |
| ago4-1 (Ler) | 276 | 61.58 ± 0.56 | 37.32 ± 0.90 | 1.07 ± 0.34 | 38.40 ± 0.56 | 4 |
| ago4-6/+ (Col) | 649 | 83.16 ± 0.61 | 14.43 ± 0.43 | 2.39 ± 0.31 | 16.82 ± 0.61 | 3 |
| ago4-6 (Col) | 356 | 66.54 ± 2.57 | 27.74 ± 0.25 | 5.70 ± 0.24 | 33.44 ± 1.48 | 3 |
| ago6-2/+ (Col) | 426 | 87.69 ± 1.94 | 11.53 ± 1.99 | 0.75 ± 0.05 | 13.54 ± 1.85 | 3 |
| ago6-2 (Col) | 220 | 74.31 ± 3.58 | 18.10 ± 2.18 | 7.56 ± 2.00 | 25.66 ± 3.58 | 4 |
| ago8-1/+ (Col) | 334 | 87.50 ± 0.52 | 12.24 ± 0.41 | 0.24 ± 0.41 | 12.48 ± 0.52 | 3 |
| ago8-1 (Col) | 1023 | 71.97 ± 3.28 | 26.11 ± 3.10 | 1.73 ± 0.71 | 27.84 ± 3.23 | 6 |
| ago8-2 (Col) | 456 | 70.29 ± 1.62 | 24.77 ± 1.67 | 4.91 ± 1.67 | 29.69 ± 1.62 | 5 |
Values are given as a percentage of the total number of ovules analyzed.
Total of ovules analyzed.
Ovules with a single MMC.
Ovules with two enlarged MMC-like cells.
Ovules with more than two enlarged MMC-like cells.
Number of individuals included in the analysis.
Heterozygous ago4-1/+ and ago4-6/+ mutant individuals also showed abnormal numbers of ovules with gametophytic precursors, suggesting that these mutants are also dominant for the phenotype analyzed. The frequency of class II and class III ovules in heterozygous ago4-1/+ (Ler background) and ago4-6/+ (Col background) individuals was 20% (n = 270) and 16.79% (n = 649), respectively (Table 1). As expected, homozygous ago4-1 and ago4-6 plants exhibited larger frequencies of class II and class III ovules compared to heterozygous plants (38.4 ± 0.56 and 33.44 ± 1.48, respectively; Table 1). Because past studies have shown that mutations in AGO4 act as recessive loss-of-function alleles that can be rescued by hemizygous complementation (Zilberman et al. 2003), we conducted an additional cytological analysis in a randomly selected group of F2 and F3 individuals segregating for ago4-6, scoring a total of eight individuals per genotype at each generation. Whereas wild-type F2 plants that inherited two wild-type copies of AGO4 showed a frequency of abnormal class II and class III ovules equivalent to wild-type Col (average of 10.65% ± 0.96; Table S1), F2 heterozygous ago4-6/+ individuals showed a frequency of class II and class III ovules ranging between 28.84 and 37.71% (average of 31.82% ± 1.2; Table S1), which is similar to the frequency obtained for F2 homozygous ago4/6 individuals (average of 33.23 ± 0,98; Table S1). Similar frequencies were obtained for segregating individuals of the F3 population resulting from self-pollination of a F2 heterozygous individual (Table S2), confirming that mutations in AGO4 consistently cause dominant defects during megasporogenesis (Table S3).
In addition, heterozygous ago6-2/+ individuals showed 13.54% (n = 426) of ovules harboring more than one gametophytic precursor (Table 1). Homozygous ago6-2 plants exhibited the same phenotype at 25.66% (n = 220; Figure 1F, Table 1), indicating that AGO6 also has a role in restricting the number of gametophytic precursors specified in the ovule primordium. Similar to homozygous ago4-6, homozygous ago6-2 individuals showed a higher frequency of class III ovules than homozygous ago9-3 individuals (Table 1). Finally, heterozygous ago8-1/+ plants showed 12.48% (n = 334) of stage1 ovules harboring ectopic gametophytic precursors, whereas homozygous ago8-1 and ago8-2 individuals showed 27.84% (n = 1023) and 29.69% (n = 456) respectively (Figure 1G); indicating that AGO8 is also involved in the restriction of cell specification, despite being previously reported as a pseudogene (Takeda et al. 2008). To determine if mutations in AGO genes not belonging to the AGO4 clade could also show defects in female gametophytic precursor specification, we analyzed ago1-37, a weak allele of AGO1, the main protein involved in microRNA-dependent regulatory pathways (Yang et al. 2006); and ago5-1, which had been previously shown to be involved in the promotion of mitosis during female gametogenesis in Arabidopsis (Tucker et al. 2012). Despite strong vegetative defects exhibited by ago1-37 at almost all developmental stages (Yang et al. 2006), the frequency of ovules with ectopic gametophytic precursors in homozygous ago1-37 and ago5-1 individuals was no different from wild type (Figure 1H, Table 1). We therefore conclude that it is specifically the AGO4 clade that plays an important role in restricting the number of female gametophytic precursors in the developing ovule.
Genetic interactions between AGO4 and AGO9 affect the specification of female gametophytic precursors
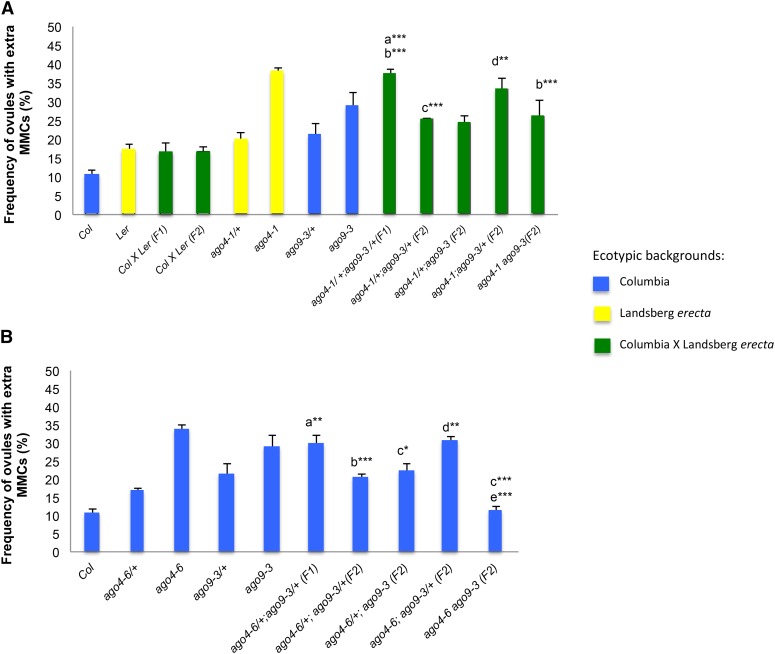
To determine possible genetic interactions between AGO4 and AGO9, we conducted a phenotypic analysis in different genotypes involving the double mutant ago4 ago9. Homozygous ago4-1 individuals show reduced DNA methylation primarily at CHG and CHH methylation sites (where H = A, T, or C) and less frequently at CG sites (Zilberman et al. 2003; Stroud et al. 2013). We crossed ago4-1 to ago9-3 individuals and produced plants segregating for both mutations. F1 double heterozygous ago4-1/+;ago9-3/+ individuals showed a frequency of ovules with ectopic gametophytic precursors reflecting an additive effect between these two mutants (Figure 2A). To analyze additional genotypic combinations, F2 individuals segregating for both mutations were also cytologically quantified. Interestingly, the frequency of ovules showing ectopic gametophytic precursors in F2 ago4-1/+;ago9-3/+ was significantly lower than those expected under additive effects, and similar to frequencies observed in heterozygous plants for each single mutant (Figure 2A). Furthermore, F2 ago4-1/+;ago9-3/ago9-3 plants exhibited a slightly lower frequency of abnormal ovules compared to single homozygous ago9-3 individuals (Figure 2A), indicating that heterozygosity of ago4-1 in the homozygous ago9-3 background promotes a suppression of the mutant phenotype. On the other hand, F2 ago4-1/ago4-1;ago9-3/+ individuals showed a frequency of ectopic gametophytic precursors lower than single homozygous ago4-1 but higher than F2 ago4-1/+;ago9-3/ago9-3 plants (Figure 2A). In addition, F2 double homozygous ago4-1 ago9-3 individuals showed a significantly lower frequency of ovules with ectopic gametophytic precursors than homozygous ago4-1 or ago9-3 single mutant individuals (Figure 2A). These results indicate that the simultaneous absence of AGO4 and AGO9 tends to repress the differentiation of ectopic gametophytic precursors in the ovule. This repressive effect is less severe in F2 ago4-1/ago4-1;ago9-3/+ plants, suggesting that a functional allele of AGO9 in a homozygous ago4 background tends to exacerbate the mutant phenotype. Strikingly, the frequency of ovules harboring multiple gamete precursors in F2 ago4-1/+;ago9-3/+, ago4-1/+;ago9-3/ago9-3, and ago4-1/ago4-1;ago9-3/ago9-3 was similar, suggesting that additional genetic factors must contribute to restrict the specification of gametophytic precursors in the premeiotic ovule.
Figure 2.
Genetic interactions between ago4 and ago9 during female gametophytic cell specification. (A) Quantitative analysis of stage 1 ovules showing more than one gametophytic precursor in genotypes of single and double ago9-3 (Col) ago4-1 (Ler) mutant individuals. Letters indicate pairwise results of two-tailed Fisher’s exact tests used to estimate statistical significance of possible differences between genotypes: a, comparison to ago9-3/+; b, comparison to ago4-1/+; c, comparison to ago4-1/+;ago9-3/+ (F1); d, comparison to ago9-3;ago4-1/+ (F2). * P < 0.05, ** P < 0.01, *** P < 0.001. (B) Quantitative analysis of stage 1 ovules showing more than one gametophytic precursor in genotypes of single and double ago9-3 (Col) ago4-6 (Col) mutant individuals. Letters indicate pairwise results of two-tailed Fisher’s exact tests used to estimate statistical significance of possible differences between genotypes: a, comparison to ago4-6/+; b, comparison to ago4-6/+;ago9-3/+ (F1); c, comparison to ago9-3; d, comparison to ago4-6/+;ago9-3 (F2); e, comparison to homozygous ago4-6. * P < 0.05, ** P < 0.01, *** P < 0.001. SDs were calculated on the basis of biological replicates for each genotype.
To determine if the ecotypic background could influence interactions between AGO4 and AGO9, we conducted the same phenotypic analysis in ago4-6, a mutation generated in the Col and not the Ler ecotype. As in the case of interactions between ago4-1 and ago9-3, the frequency of abnormal stage 1 ovules was higher in F1 ago4-6/+;ago9-3/+ individuals than in single heterozygous ago4-6/+ or ago9-3/+ mutants (Figure 2B). Also, F2 ago4-6/+; ago9-3/+ individuals exhibited a lower frequency of abnormal ovules than F1 ago4-6/+;ago9-3/+ plants (Figure 2B), confirming the results obtained with ago4-1. In addition, the suppressive effect observed in double homozygous individuals was stronger in ago4-6 ago9-3 than in ago4-1 ago9-3 individuals (Figure 2B), suggesting that the Ler background exerts a stronger ecotypic effect than Col over the restriction of female gametophytic precursors. Overall, these results suggest that AGO9 and AGO4 genetically interact to restrict the differentiation of additional premeiotic precursors in the ovule, but also that additional genetic factors participate in this developmental process.
Genetic interactions between AGO4 and AGO9 are influenced by parental genotypes
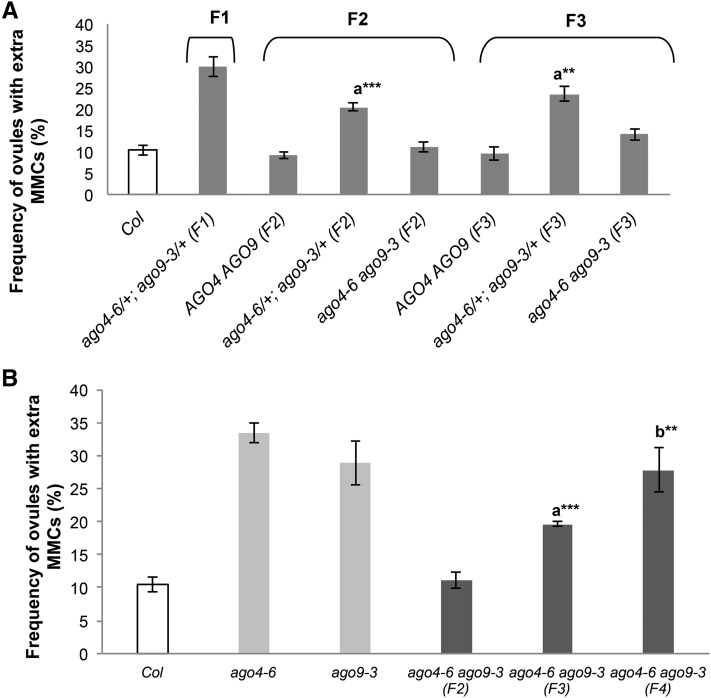
Emerging evidence suggests that mutations affecting epigenetic pathways can originate transgenerational, stable, epigenetic modifications (Reinders and Paszkowski 2009; Johannes and Colome-Tatche 2011; Mari-Ordonez et al. 2013). To test if this type of epigenetic effect could influence the function of AGO4 or AGO9, we quantified the frequency of premeiotic stage 1 gametophytic precursors in the progeny of double heterozygous individuals segregating for mutations in these two genes. Equivalent frequencies were found in F2 and F3 wild-type segregant individuals (Figure 3A), discarding the possibility of strict transgenerational epigenetic effects and indicating that any abnormal frequency is caused by loss-of-function alleles for any of the two genes. We also analyzed stage 1 ovules in homozygous and heterozygous progeny of double heterozygous individuals. F3 double heterozygous plants exhibited a similar frequency of ectopic gametophytic precursors than F2 double heterozygous individuals, and double homozygous F3 individuals exhibited a frequency equivalent to double homozygous F2 individuals (Figure 3A). By contrast, the frequency of stage 1 ovules harboring ectopic gametophytic precursors increased progressively in F3 and F4 progeny originating from double homozygous plants (Figure 3B), indicating that the suppressive effect previously described is mitigated if not suppressed in progeny from double homozygous plants. These results indicate that the role of AGO4 and AGO9 in premeiotic gametophytic specification is influenced by the parental genotype, suggesting that loss of function of both genes triggers a compensatory effect that prevents the differentiation of ectopic cells in the developing ovule.
Figure 3.
The number of female gametophytic precursors is influenced by the parental genotype. (A) Quantitative analysis of stage 1 ovules showing more than one gametophytic precursor in F2 and F3 segregant populations derived from ago4-6/+ (Col); ago9-3/+ (Col) individuals. The letter indicates pairwise results of two-tailed Fisher’s exact tests used to estimate statistical significance of differences between genotypes: a, comparison to ago4-6/+ ; ago9-3/+ (F1). ** P < 0.01, *** P < 0.001. (B) The compensatory effect resulting from simultaneous loss of function of AGO4 and AGO9 is progressively diminished through consecutive generations. Letters indicate pairwise results of two-tailed Fisher’s exact tests used to estimate statistical significance of differences between genotypes: a, comparison to ago4-6 ago9-3 (F2); b, comparison to ago4-6 ago9-3 (F3). ** P < 0.01, *** P < 0.001. SDs were calculated on the basis of biological replicates for each genotype.
AGO6 and AGO8 influence gametophytic specification in ago4 and ago9 mutant backgrounds
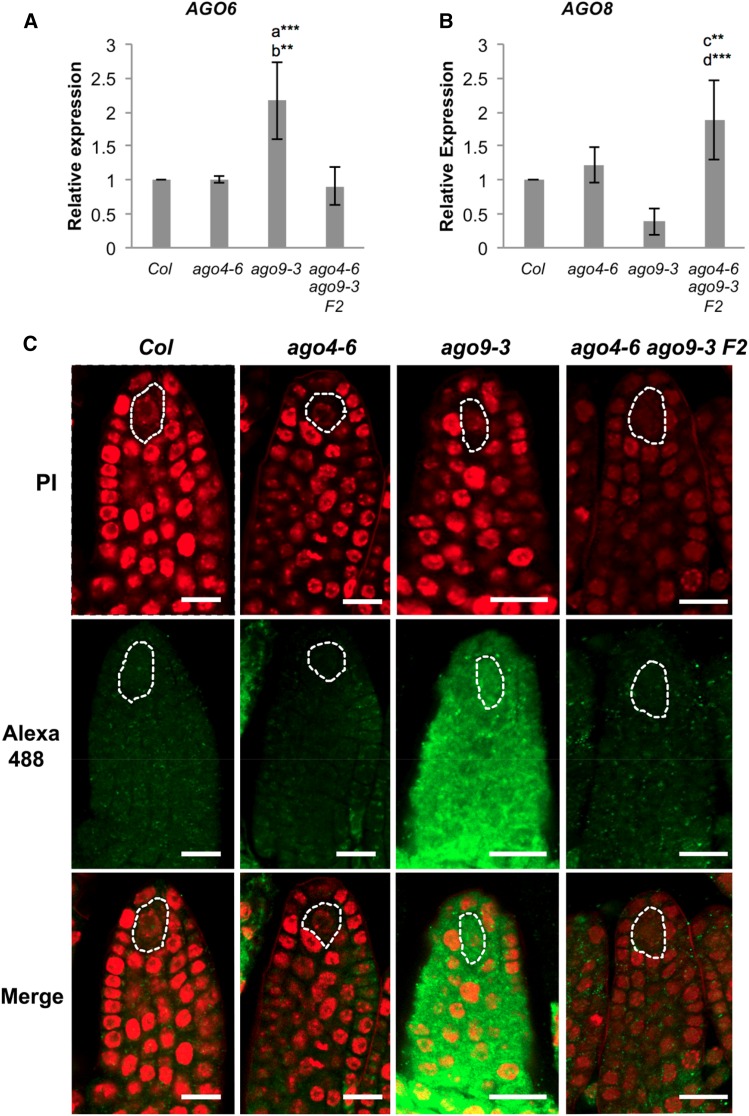
To explore if the compensatory mechanism triggered by the simultaneous loss of function of AGO4 and AGO9 could be related to the activity of other members of the AGO4 clade, we conducted qPCR to determine the expression of AGO6 and AGO8 in developing gynoecia harboring premeiotic ovules of single ago4-6, ago9-3, and double homozygous ago4-6 ago9-3 individuals (Figure 4, A and B). In ago4-6, the expression of AGO6 was equivalent to wild type (Col); however, the expression of AGO6 was significantly increased in ago9-3 (Figure 4A), indicating a differential response in the expression of AGO6 to the absence of AGO4 or AGO9 functional activity. Despite being overexpressed in ago9-3, premeiotic gynoecia of the double mutant ago4-6 ago9-3 exhibited normal AGO6 expression, indicating that loss of function of AGO4 in the ago9-3 background negatively regulates AGO6 activity. In contrast to AGO6, the expression of AGO8 was not affected in ago4-6 and ago9-3 single mutants (Figure 4B), but was significantly increased in premeiotic gynoecia of ago4-6 ago9-3 individuals (Figure 4B). These results indicate that interactions between AGO4-clade members are reflected at the level of transcriptional gene activity, suggesting that robust redundant mechanisms among members of the AGO4 clade contribute to restrict gametophytic cell fate in the premeiotic ovule. Increased expression of AGO8 in ovules lacking AGO4 and AGO9 activity could contribute to explain the compensatory phenotypic effect exhibited by premeiotic ovules.
Figure 4.
The expression of AGO6 and AGO8 is affected by loss-of-function mutations in ago4 or ago9. (A) Expression of AGO6 in developing gynoecia containing premeiotic ovules. (B) Expression of AGO8 in developing gynoecia containing premeiotic ovules. The comparative 2−ΔΔCt method was used for determining the relative level of gene expression as compared to wild type, using ACTIN2 as internal control (Czechowski et al. 2004). Each histogram represents the mean of three biological replicates and shows the corresponding SD. Letters indicate pairwise results of two-tailed Fisher’s exact tests used to estimate statistical significance of possible differences between genotypes: a, comparison to Col; b, comparison to ago4-6 ago9-3 F2; c, comparison to Col; d, comparison to ago9-3. ** P < 0.01, *** P < 0.001. (C) Whole-mount immunolocalizations showing the expression of AGO6 in wild-type and mutant premeiotic ovules. Alexa 488 fluorescence (green) denotes the localization of the antibody raised against AGO6; samples were counterstained with propidium iodide (red). Bar, 15 µm.
To determine if the qPCR experiments could reflect the levels of protein expression in the premeiotic ovule, we performed whole-mount immunolocalization in wild-type Col, ago4-6, ago9-3, and double mutant ago9-3 ago4-6 ovules; using an antibody raised against AGO6 (Figure 4, C–F; Havecker et al. 2010). In wild-type ovules, AGO6 was localized in the cytoplasm and sometimes the nucleus of most sporophytic cells of the premeiotic primordium, including the premeiotic MMC (Figure 4D). The localization and level of AGO6 expression was similar to wild type in premeiotic ovules of ago4-6 and ago4-6 ago9-3 individuals (Figure 4E), a result in agreement with our previous qPCR assay. At the onset of integumentary initiation, a region of preferential AGO6 expression was identified in the dorsal region of the primordium in both genetic backgrounds (Figure 4D); suggesting that the absence of AGO4 activity might cause subtle differences in the pattern of AGO6 localization, but no differences in the level of AGO6 protein expression. By contrast, AGO6 was abundantly localized throughout the ovule primordium of ago9-3 plants, including L1 cells of the apical region and the MMC (Figure 4F). These results show that overexpression of AGO6 in ago9-3 ovules is reflected at the protein level, suggesting that the genetic interactions that control premeiotic gametophytic specification imply dosage effects at the protein level among AGO4-clade members.
Discussion
All genes of the AGO4 clade play an important role during the somatic-to-reproductive transition in the ovule
ARGONAUTE genes have been described as fundamental factors controlling specific aspects of germline development in yeast, Drosophila, Caenorhabditis elegans, mammals, and plants (Lin and Spradling 1997; Kennerdell et al. 2002; Yigit et al. 2006; Nonomura et al. 2007; Olmedo-Monfil et al. 2010). Here we provide genetic evidence indicating that all members of the AGO4-clade contribute to restrict the number of gametophytic precursors in premeiotic ovules of Arabidopsis. Distinct mutations in members of the AGO4 clade result in variable phenotypic frequencies, suggesting that these genes hierarchically contribute to this restriction. Whereas AGO4 and AGO9 appear to play a prevalent role in the mechanism that impedes the differentiation of multiple MMCs, mutations in AGO6 and AGO8 show equivalent phenotypic effects, albeit at lower frequencies; suggesting a less determinant redundant function. The role of AGO6 in gametophytic precursor specification is unexpected as the presence of its messenger RNA is not detected in nucellus or MMCs according to previously published data (Schmidt et al. 2011). Although AGO8 was previously reported as a pseudogene (Takeda et al. 2008), the ago8-1 insertional allele exhibits a frequency of ectopic configurations significantly higher than wild type. This same phenotype has been also found in ago8-2, suggesting a functional activity necessary for gametophytic precursor specification. By contrast, and despite harboring severe vegetative defects that could precede pleiotropic abnormalities during female reproductive development, homozygous ago1-37 and ago5-1 individuals did not show defects in meiosis or gametophytic precursor differentiation, indicating that the canonical microRNA-dependent pathway is not essential for megasporogenesis, and that premeiotic ovules of individuals defective in AGO5 are also indistinguishable from wild type (Tucker et al. 2012); confirming that MMC specification is not dependent on microRNA function but strictly controlled by members of the AGO4 clade.
Since AGO4, AGO6, and AGO9 bind heterochromatic sRNAs through the RdDM pathway, our results reinforce the important participation of epigenetic processes in regulating gametophytic precursor specification in the ovule (Zilberman et al. 2003; Zheng et al. 2007; Duran-Figueroa and Vielle-Calzada 2010; Olmedo-Monfil et al. 2010; Eun et al. 2011; Duan et al. 2014). Our results also show that mutations in any of the AGO4-clade genes, including AGO4, are dominant over wild-type alleles for defects affecting gametophytic cell specification in the ovule. This type of inheritance had already been reported for ago9-2 and ago9-3 (Olmedo-Monfil et al. 2010), suggesting that a dosage-dependent mechanism acting nonautonomously during megasporogenesis is responsible for the mutant phenotype in members of the AGO4 clade; a model for the mode of action has been proposed elsewhere (Armenta-Medina et al. 2011) . A recent report showed that the MMC is marked by a reduction of heterochromatin content compared to its surrounding nucellar cells (She et al. 2013). A similar depletion of heterochromatic elements is observed in the ectopic configurations of ago9, sgs3, and rdr6 individuals (She et al. 2013). In addition, genome-wide studies have proven that loss of RdDM components such as AGO4 and AGO6 results in changes of chromatin integrity (Huettel et al. 2007; Pikaard et al. 2008; Law and Jacobsen 2010; Zhang and Zhu 2012; Stroud et al. 2013). A direct role of AGO proteins in chromatin modification has been also described in C. elegans, where HRDE-1 directs trimethylation of histone H3 at Lysine 9 (Maine and Kimble 1993; Nishiwaki and Miwa 1998; Buckley et al. 2012). Furthermore, Ago-1 of Drosophila plays a crucial role in heterochromatin formation (Pushpavalli et al. 2012). Taken together, these results support the hypothesis proposing that members of the AGO4 clade are required for maintaining the chromatin configuration of nucellar cells in Arabidopsis in a dosage-dependent manner. The disruption of any of these genes during the somatic-to-reproductive transition would confer a novel chromatin status that might lead to ectopic differentiation of gametophytic precursors.
Complex interactions between AGO4-clade members
Although genetic interactions between AGO members have been addressed by comparing the nature and abundance of their interacting sRNAs as well as the consequence of their functional loss for genomic DNA methylation (Zheng et al. 2007; Havecker et al. 2010; McCue et al. 2015), little emphasis has been given to their possible developmental role, mainly due to a lack of obvious mutant phenotypes during vegetative growth. Here we show that AGO4 and AGO9 genetically interact during the somatic-to-reproductive transition in the ovule. With the exception of double heterozygous individuals, all genotypic combinations of alleles simultaneously affecting AGO4 and AGO9 exhibit nonadditive effects during megasporogenesis. Interestingly, the complete loss of function of these genes can result in the absence of a mutant phenotype, as compared to wild-type plants; however, our results also suggest that suppression of this mutant phenotype is dependent on the ago4 allelic variants tested and their genetic backgrounds, likely contributing to the compensatory phenomenon revealed by genetic interactions among members of the AGO4 clade. Recent reports showed that different ecotypes of Arabidopsis exhibit equivalent phenotypes at variable frequencies, and that interecotypic hybridization exacerbates the frequency of supernumerary gametophytic precursors, suggesting that multiple loci control cell specification at the onset of female meiosis (Rodríguez-Leal et al. 2015).
Our qPCR experiments show that AGO8 is overexpressed in homozygous F2 ago4 ago9 ovules, but not in ovules of ago4 or ago9 single mutants, suggesting that the mitigation of the abnormal phenotype in the double homozygous mutant background could be influenced by the activity AGO8. Contrary to AGO8, the expression of AGO6 is increased in ago9-3 but not in in ago4-6, nor in double homozygous ago4 ago9 individuals. These contrasting effects in AGO6 and AGO8 activity suggest that, despite their common contribution to restricting gametophytic precursor specification at the onset of meiosis, each gene is differentially regulated in response to loss of function of AGO4 or AGO9. The recovery of normal expression levels for AGO6 in the double homozygous ago4 ago9 but not in single ago9-3 individuals indicates that AGO4 influences AGO6 expression only in the absence of AGO9 activity. Our overall results suggest that AGO4-clade gene members coordinately act to restrict the ectopic formation of gametophytic precursors in the ovule. Because AGO4 and AGO9 are importantly required for silencing repetitive elements such as TEs in the germline (Zilberman et al. 2003; Duran-Figueroa and Vielle-Calzada 2010), and AGO9 has been implicated in male meiosis and somatic DNA repair (Oliver et al. 2014), the compensatory effect revealed by interactions among AGO4-clade members is likely to be extended to a broader developmental context. In addition, genome-wide DNA methylation analysis has revealed nonredundant interactions between AGO4 and AGO6 in most of their target loci, as both are required to confer a wild-type DNA methylation status (Duan et al. 2014). Interestingly, methylation is not completely suppressed at target loci in ago4 ago6 mutants, suggesting that additional AGOs are also required to maintain the global methylation pattern (Duan et al. 2014).
The pattern of protein localization of AGO4, AGO6, and AGO9 is in agreement with the evidence indicating they play redundant functions during early ovule formation. Whereas AGO4 is ubiquitously expressed throughout development and localized in both nucleus and cytoplasm (Li et al. 2006; Ye et al. 2012), AGO6 is reported to localize in the cytoplasm of foliar parenchyma cells (Zheng et al. 2007; McCue et al. 2015). AGO9 is preferentially expressed in the L1 layer of the premeiotic ovule primordium, and in the cytoplasm of nucellar cells (Olmedo-Monfil et al. 2010; Escobar-Guzmán et al. 2015). Recent evidence indicates that AGO4 and AGO6 differ in their subnuclear colocalization as compared to the RNA polymerases required for RdDM (McCue et al. 2015); whereas Pol V and AGO4 are colocalized in perinuclear foci, Pol II and AGO6 are absent (McCue et al. 2014).
Parental genotypes influence the somatic-to-reproductive transition during consecutive generations
A significantly different frequency of ectopic configurations was obtained in double heterozygous ago4/+ ago9/+ F1 and F2 individuals, indicating that epigenetic factors are influencing the number of ectopic configurations that differentiate at each generation. Epigenetic variability between parental lines can cause additive effects for developmental traits scored in the progeny (Groszmann et al. 2013; Groszmann et al. 2014; Escobar-Guzmán et al. 2015). Despite the large proportion of potential RdDM target loci shared by both genes, there is also evidence suggesting specific epigenetic regulation by one or the other (Havecker et al. 2010), suggesting potential epi-allelism for each mutant. Strikingly, segregant ago4/+ ago9/+ F3 individuals showed frequencies equivalent to ago4/+ ago9/+ F2 plants, suggesting that double heterozygous progeny from outcrossed plants behave differently than heterozygous progeny of self-fertilized individuals.
Double homozygous ago4 ago9 individuals derived from self-pollination of double heterozygous plants showed frequencies of ectopic configurations equivalent to wild type. In contrast, ago4 ago9 individuals derived from self-pollination of a double homozygous plant showed an abnormal frequency of ectopic configurations, revealing an influence of the parental genotype on the capacity for restricting ectopic differentiation of female gamete precursors. The frequency of ectopic configurations in double homozygous ago4 ago9 individuals increased progressively throughout consecutive generations, indicating that the compensatory effect is progressively diminished. These results suggest that AGO4 and AGO9 are necessary for maintaining the epigenetic marks that ensure the restriction of gametogenic commitment to a single cell, over consecutive generations. There is growing evidence that epigenetic modifications occurring within the plant germline in one generation can be stably inherited at subsequent generations (Iwasaki and Paszkowski 2014). For example, components of the RdDM pathway have been implicated in preventing transgenerational accumulation of some Ty1/Copia-like retrotransposons in plants affected by abiotic stress in Arabidopsis (Ito et al. 2011); and in C. elegans, proteins such as HRDE-1 are required for transmitting the RNA-interference silencing signal to subsequent generations (Buckley et al. 2012). Our results indicate that AGO4 and AGO9 are required to establish the transgenerational epigenetic information that is necessary to restrict gametophytic fate in the ovule, confirming that members of the AGO4 clade cooperatively participate in preventing the abnormal specification of multiple premeiotic gametophytic precursors during early ovule development.
Conclusions
We show a surprising degree of cooperative interaction among gene members of the AGO4 clade during meiosis and megaspore formation in Arabidopsis. Our study reveals unforeseen levels of epigenetic control acting to canalize a developmental process that is essential for sexual plant reproduction in flowering plants.
Acknowledgments
We thank Arnaud Ronceret and Isaac Rodríguez for constructive comments on the manuscript, and June Simpson for support with real-time PCR. We also thank the Arabidopsis Biological Resource Center for their long-standing support sending Arabidopsis seeds to Mexico. E.H-.L and D.R-.L. were recipients of a graduate scholarship from Consejo Nacional de Ciencia y Tecnología. This research was supported by grants from Consejo Nacional de Ciencia y Tecnología (CON47436), the International Scholar Program of the Howard Hughes Medical Institute, and the DuPont Pioneer regional initiatives to benefit local subsistence farmers.
Author contributions: E.H-.L. and J-.P.V-.C. designed the research. E.H-.L., J-.P.V-.C., and J.L. conducted the experiments. J-.P.V-.C, D.R-.L., and E.H-.L. analyzed the results and wrote the paper.
Footnotes
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.188151/-/DC1.
Communicating editor: J. A. Birchler
Literature Cited
- Bajon C., Horlow C., Motamayor J. C., Sauvanet A., Robert D., 1999. Megasporogenesis in Arabidopsos thaliana L.: an ultrastructural study. Sex. Plant Reprod. 12: 99–109. [Google Scholar]
- Bendel-Stenzel M., Anderson R., Heasman J., Wylie C., 1998. The origin and migration of primordial germ cells in the mouse. Semin. Cell Dev. Biol. 9: 393–400. [DOI] [PubMed] [Google Scholar]
- Brennecke J., Aravin A. A., Stark A., Dus M., Kellis M., et al. , 2007. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 128: 1089–1103. [DOI] [PubMed] [Google Scholar]
- Brennecke J., Malone C. D., Aravin A. A., Sachidanandam R., Stark A., et al. , 2008. An epigenetic role for maternally inherited piRNAs in transposon silencing. Science 322: 1387–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley B. A., Burkhart K. B., Gu S. G., Spracklin G., Kershner A., et al. , 2012. A nuclear argonaute promotes multigenerational epigenetic inheritance and germline immortality. Nature 489: 447–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowski T., Bari R. P., Stitt M., Scheible W. R., Udvardi M. K., 2004. Real-time RT-PCR profiling of over 1400 Arabidopsis transcription factors: unprecedented sensitivity reveals novel root- and shoot-specific genes. Plant J. 38: 366–379. [DOI] [PubMed] [Google Scholar]
- Donoughe S., Nakamura T., Ewen-Campen B., Green D. A., II, Henderson L., et al. , 2014. BMP signaling is required for the generation of primordial germ cells in an insect. Proc. Natl. Acad. Sci. USA 111: 4133–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews G. N., Yadegari R., 2002. Development and function of the angiosperm female gametophyte. Annu. Rev. Genet. 36: 99–124. [DOI] [PubMed] [Google Scholar]
- Duan C. G., Zhang H., Tang K., Zhu X., Qian W., et al. , 2014. Specific but interdependent functions for Arabidopsis AGO4 and AGO6 in RNA-directed DNA methylation. EMBO J. 34: 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Figueroa N., Vielle-Calzada J. P., 2010. ARGONAUTE9-dependent silencing of transposable elements in pericentromeric regions of Arabidopsis. Plant Signal. Behav. 5: 1476–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Guzmán R., Rodríguez-Leal D., Vielle-Calzada J.-P., Ronceret A., 2015. Whole-mount immunolocalization to study female meiosis in Arabidopsis. Nat. Protoc. 10: 1535–1542. [DOI] [PubMed] [Google Scholar]
- Eun C., Lorkovic Z. J., Naumann U., Long Q., Havecker E. R., et al. , 2011. AGO6 functions in RNA-mediated transcriptional gene silencing in shoot and root meristems in Arabidopsis thaliana. PLoS One 6: e25730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groszmann M., Greaves I. K., Fujimoto R., Peacock W. J., Dennis E. S., 2013. The role of epigenetics in hybrid vigour. Trends Genet. 29: 684–690. [DOI] [PubMed] [Google Scholar]
- Groszmann M., Gonzalez-Bayon R., Greaves I. K., Wang L., Huen A. K., et al. , 2014. Intraspecific Arabidopsis hybrids show different patterns of heterosis despite the close relatedness of the parental genomes. Plant Physiol. 166: 265–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajkova P., Erhardt S., Lane N., Haaf T., El-Maarri O., et al. , 2002. Epigenetic reprogramming in mouse primordial germ cells. Mech. Dev. 117: 15–23. [DOI] [PubMed] [Google Scholar]
- Havecker E. R., Wallbridge L. M., Hardcastle T. J., Bush M. S., Kelly K. A., et al. , 2010. The Arabidopsis RNA-directed DNA methylation argonautes functionally diverge based on their expression and interaction with target loci. Plant Cell 22: 321–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havecker E. R., Wallbridge L. M., Fedito P., Hardcastle T. J., Baulcombe D. C., 2012. Metastable differentially methylated regions within Arabidopsis inbred populations are associated with modified expression of non-coding transcripts. PLoS One 7: e45242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel B., Kanno T., Daxinger L., Bucher E., van der Winden J., et al. , 2007. RNA-directed DNA methylation mediated by DRD1 and Pol IVb: a versatile pathway for transcriptional gene silencing in plants. Biochim. Biophys. Acta 1769: 358–374. [DOI] [PubMed] [Google Scholar]
- Ito H., Gaubert H., Bucher E., Mirouze M., Vaillant I., et al. , 2011. An siRNA pathway prevents transgenerational retrotransposition in plants subjected to stress. Nature 472: 115–119. [DOI] [PubMed] [Google Scholar]
- Iwasaki M., Paszkowski J., 2014. Epigenetic memory in plants. EMBO J. 33: 1987–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes F., Colome-Tatche M., 2011. Quantitative epigenetics through epigenomic perturbation of isogenic lines. Genetics 188: 215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly W. G., 2014. Transgenerational epigenetics in the germline cycle of Caenorhabditis elegans. Epigenetics Chromatin 7: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerdell J. R., Yamaguchi S., Carthew R. W., 2002. RNAi is activated during Drosophila oocyte maturation in a manner dependent on aubergine and spindle-E. Genes Dev. 16: 1884–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law J. A., Jacobsen S. E., 2010. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 11: 204–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch H. G., McEwen K. R., Turp A., Encheva V., Carroll T., et al. , 2013. Naive pluripotency is associated with global DNA hypomethylation. Nat. Struct. Mol. Biol. 20: 311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. F., Pontes O., El-Shami M., Henderson I. R., Bernatavichute Y. V., et al. , 2006. An ARGONAUTE4-containing nuclear processing center colocalized with Cajal bodies in Arabidopsis thaliana. Cell 126: 93–106. [DOI] [PubMed] [Google Scholar]
- Lieber D., Lora J., Schrempp S., Lenhard M., Laux T., 2011. Arabidopsis WIH1 and WIH2 genes act in the transition from somatic to reproductive cell fate. Curr. Biol. 21: 1009–1017. [DOI] [PubMed] [Google Scholar]
- Lin H., Spradling A. C., 1997. A novel group of pumilio mutations affects the asymmetric division of germline stem cells in the Drosophila ovary. Development 124: 2463–2476. [DOI] [PubMed] [Google Scholar]
- Loriot A., Boon T., De Smet C., 2003. Five new human cancer-germline genes identified among 12 genes expressed in spermatogonia. Int. J. Cancer 105: 371–376. [DOI] [PubMed] [Google Scholar]
- Maine E. M., Kimble J., 1993. Suppressors of glp-1, a gene required for cell communication during development in Caenorhabditis elegans, define a set of interacting genes. Genetics 135: 1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari-Ordonez A., Marchais A., Etcheverry M., Martin A., Colot V., et al. , 2013. Reconstructing de novo silencing of an active plant retrotransposon. Nat. Genet. 45: 1029–1039. [DOI] [PubMed] [Google Scholar]
- Matzke M. A., Mosher R. A., 2014. RNA-directed DNA methylation: an epigenetic pathway of increasing complexity. Nat. Rev. Genet. 15: 394–408. [DOI] [PubMed] [Google Scholar]
- McCue A. D., Panda K., Nuthikattu S., Choudury S. G., Thomas E. N., et al. , 2015. ARGONAUTE 6 bridges transposable element mRNA-derived siRNAs to the establishment of DNA methylation. EMBO J. 34: 20–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiwaki K., Miwa J., 1998. Mutations in genes encoding extracellular matrix proteins suppress the emb-5 gastrulation defect in Caenorhabditis elegans. Mol. Gen. Genet. 259: 2–12. [DOI] [PubMed] [Google Scholar]
- Nonomura K., Miyoshi K., Eiguchi M., Suzuki T., Miyao A., et al. , 2003. The MSP1 gene is necessary to restrict the number of cells entering into male and female sporogenesis and to initiate anther wall formation in rice. Plant Cell 15: 1728–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonomura K., Morohoshi A., Nakano M., Eiguchi M., Miyao A., et al. , 2007. A germ cell specific gene of the argonaute family is essential for the progression of premeiotic mitosis and meiosis during sporogenesis in rice. Plant Cell 19: 2583–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmedo-Monfil V., Duran-Figueroa N., Arteaga-Vazquez M., Demesa-Arevalo E., Autran D., et al. , 2010. Control of female gamete formation by a small RNA pathway in Arabidopsis. Nature 464: 628–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikaard C. S., Haag J. R., Ream T., Wierzbicki A. T., 2008. Roles of RNA polymerase IV in gene silencing. Trends Plant Sci. 13: 390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushpavalli S. N., Bag I., Pal-Bhadra M., Bhadra U., 2012. Drosophila Argonaute-1 is critical for transcriptional cosuppression and heterochromatin formation. Chromosome Res. 20: 333–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinders J., Paszkowski J., 2009. Unlocking the Arabidopsis epigenome. Epigenetics 4: 557–563. [DOI] [PubMed] [Google Scholar]
- Reiser L., Fischer R. L., 1993. The ovule and the embryo sac. Plant Cell 5: 1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Beers K., Pruitt R. E., Gasser C. S., 1992. Ovule development in wild-type Arabidopsis and two female-sterile mutants. Plant Cell 4: 1237–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Leal D., León-Martínez G., Abad-Vivero U., Vielle-Calzada J. P., 2015. Natural variation in epigenetic pathways affects the specification of female gamete precursors in Arabidopsis. Plant Cell 4: 1034–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley M. J., Avrutsky M. I., Sifuentes C. J., Pereira L., Wierzbicki A. T., 2011. Independent chromatin binding of ARGONAUTE4 and SPT5L/KTF1 mediates transcriptional gene silencing. PLoS Genet. 7: e1002120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Wuest S. E., Vijverberg K., Baroux C., Kleen D., et al. , 2011. Transcriptome analysis of the Arabidopsis megaspore mother cell uncovers the importance of RNA helicases for plant germline development. PLoS Biol. 9: e1001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneitz K., Hülskamp M., Pruitt R. E., 1995. Wild-type ovule development in Arabidopsis thaliana: a light microscope study of cleared whole-mount tissue. Plant J. 7: 731–749. [Google Scholar]
- Seki Y., Hayashi K., Itoh K., Mizugaki M., Saitou M., et al. , 2005. Extensive and orderly reprogramming of genome-wide chromatin modifications associated with specification and early development of germ cells in mice. Dev. Biol. 278: 440–458. [DOI] [PubMed] [Google Scholar]
- She W., Grimanelli D., Rutowicz K., Whitehead M. W., Puzio M., et al. , 2013. Chromatin reprogramming during the somatic-to-reproductive cell fate transition in plants. Development 140: 4008–4019. [DOI] [PubMed] [Google Scholar]
- Sheridan W. F., Golubeva E. A., Abrhamova L. I., Golubovskaya I. N., 1999. The mac1 mutation alters the developmental fate of the hypodermal cells and their cellular progeny in the maize anther. Genetics 153: 933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. A., Meyers B. C., 2010. Small RNA-mediated epigenetic modifications in plants. Curr. Opin. Plant Biol. 14: 148–155. [DOI] [PubMed] [Google Scholar]
- Strickler S. R., Tantikanjana T., Nasrallah J. B., 2013. Regulation of the S-locus receptor kinase and self-incompatibility in Arabidopsis thaliana. G3 (Bethesda) 3: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud H., Greenberg M. V., Feng S., Bernatavichute Y. V., Jacobsen S. E., 2013. Comprehensive analysis of silencing mutants reveals complex regulation of the Arabidopsis methylome. Cell 152: 352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A., Iwasaki S., Watanabe T., Utsumi M., Watanabe Y., 2008. The mechanism selecting the guide strand from small RNA duplexes is different among argonaute proteins. Plant Cell Physiol. 49: 493–500. [DOI] [PubMed] [Google Scholar]
- Tucker M. R., Okada T., Hu Y., Scholefield A., Taylor J. M., et al. , 2012. Somatic small RNA pathways promote the mitotic events of megagametogenesis during female reproductive development in Arabidopsis. Development 139: 1399–1404. [DOI] [PubMed] [Google Scholar]
- Vaucheret H., 2008. Plant argonautes. Trends Plant Sci. 13: 350–358. [DOI] [PubMed] [Google Scholar]
- Yadegari R., Drews G. N., 2004. Female gametophyte development. Plant Cell 16: S133–S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Huang W., Wang H., Cai R., Xu Y., et al. , 2006. Characterizations of a hypomorphic argonaute1 mutant reveal novel AGO1 functions in Arabidopsis lateral organ development. Plant Mol. Biol. 61: 63–78. [DOI] [PubMed] [Google Scholar]
- Yang W. C., Ye D., Xu J., Sundaresan V., 1999. The SPOROCYTELESS gene of Arabidopsis is required for initiation of sporogenesis and encodes a novel nuclear protein. Genes Dev. 13: 2108–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R., Wang W., Iki T., Liu C., Wu Y., et al. , 2012. Cytoplasmic assembly and selective nuclear import of Arabidopsis Argonaute4/siRNA complexes. Mol. Cell 46: 859–870. [DOI] [PubMed] [Google Scholar]
- Yigit E., Batista P. J., Bei Y., Pang K. M., Chen C. C., et al. , 2006. Analysis of the C. elegans Argonaute family reveals that distinct Argonautes act sequentially during RNAi. Cell 127: 747–757. [DOI] [PubMed] [Google Scholar]
- Zhai L., Sun W., Zhang K., Jia H., Liu L., et al. , 2014. Identification and characterization of Argonaute gene family and meiosis-enriched Argonaute during sporogenesis in maize. J. Integr. Plant Biol. 56: 1042–1052. [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhu J. K., 2012. Seeing the forest for the trees: a wide perspective on RNA-directed DNA methylation. Genes Dev. 26: 1769–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q., Rowley M. J., Bohmdorfer G., Sandhu D., Gregory B. D., et al. , 2013. RNA polymerase V targets transcriptional silencing components to promoters of protein-coding genes. Plant J. 73: 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Zhu J., Kapoor A., Zhu J. K., 2007. Role of Arabidopsis AGO6 in siRNA accumulation, DNA methylation and transcriptional gene silencing. EMBO J. 26: 1691–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X., Du J., Hale C. J., Gallego-Bartolome J., Feng S., et al. , 2014. Molecular mechanism of action of plant DRM de novo DNA methyltransferases. Cell 157: 1050–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberman D., Cao X., Jacobsen S. E., 2003. ARGONAUTE4 control of locus-specific siRNA accumulation and DNA and histone methylation. Science 299: 716–719. [DOI] [PubMed] [Google Scholar]
- Zilberman D., Cao X., Johansen L. K., Xie Z., Carrington J. C., et al. , 2004. Role of Arabidopsis ARGONAUTE4 in RNA-directed DNA methylation triggered by inverted repeats. Curr. Biol. 14: 1214–1220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequence data from this article can be found in the European Molecular Biology Laboratory /GenBank data libraries under accession numbers At2g27040, At2g32940, At5g21030, and At5g21150. Additional mutant strains are available upon request. Table S2 contains names of primers used for genotyping and qPCR assays.