Abstract
The organization and stability of higher order structures that form in the extracellular matrix (ECM) to mediate the attachment of muscles are poorly understood. We have made the surprising discovery that a subset of clotting factor proteins are also essential for muscle attachment in the model organism Drosophila melanogaster. One such coagulation protein, Fondue (Fon), was identified as a novel muscle mutant in a pupal lethal genetic screen. Fon accumulates at muscle attachment sites and removal of this protein results in decreased locomotor behavior and detached larval muscles. A sensitized genetic background assay reveals that fon functions with the known muscle attachment genes Thrombospondin (Tsp) and Tiggrin (Tig). Interestingly, Tig is also a component of the hemolymph clot. We further demonstrate that an additional clotting protein, Larval serum protein 1γ (Lsp1γ), is also required for muscle attachment stability and accumulates where muscles attach to tendons. While the local biomechanical and organizational properties of the ECM vary greatly depending on the tissue microenvironment, we propose that shared extracellular protein–protein interactions influence the strength and elasticity of ECM proteins in both coagulation and muscle attachment.
Keywords: Drosophila, muscle attachment sites, Fondue, Tiggrin
REGULATION of protein stability and remodeling in the extracellular environment is essential for the organization of higher order structures that comprise the extracellular matrix (ECM). The biochemical composition of the ECM can differ from one tissue to another. This heterogeneity has a dramatic effect on the strength and elasticity of cell–ECM interactions in development and tissue repair (Frantz et al. 2010). Despite the importance of the ECM in the development and physiology of multicellular organisms, a broad understanding of the shared physical properties among ECM substrates in diverse biological processes is unclear. To uncover mechanisms that underlie ECM biology, several groups including our own study muscle attachment in the Drosophila model.
Larval body wall muscles in Drosophila form in embryogenesis after repeated rounds of myoblast fusion, myofiber migration, and the subsequent attachment of muscles to their target tendon cells (Schnorrer and Dickson 2004; Schejter and Baylies 2010; Schweitzer et al. 2010). Detailed transmission electron micrographs (TEM) revealed two categories of muscle attachment sites (MASs), direct and indirect (Prokop et al. 1998; Alves-Silva et al. 2008). Single muscles, such as the lateral transverse muscles, directly adhere to epidermally derived tendon cells at direct attachment sites (or muscle–tendon junctions) in closely associated conditions (30–40 μm) (Prokop et al. 1998). Indirect muscle attachments (or muscle–muscle junctions) occur at the hemisegmental borders where multiple muscles form attachments to adjoining muscles by connecting to an extended belt of ECM anchored to a cuticle-associated tendon cell. This nomenclature is analogous to vertebrate literature where direct attachments refer to tightly associated muscle–bone interactions or an indirect attachment site, which utilizes a rope-like extension of connective tissues to join muscle to bone. At both direct and indirect muscle attachments, muscle and tendon cells form extensions and invaginations between the opposing plasma membranes of each cell type and connect to the ECM at a myotendinous junction (MTJ). This membrane interdigitation increases the muscle–tendon interface area to allow for increased resistance against forces generated during muscle contraction.
The direct or indirect attachment of muscles to other muscles or to tendon cells relies largely on the function of transmembrane integrin proteins. Individual integrin subunits form obligate heterodimer complexes on the surface of both muscle and tendon cells and link the internal actin cytoskeleton to proteins in the extracellular environment (Maartens and Brown 2015). An αPS1βPS complex accumulates on tendon cell membranes, while αPS2βPS subunits are found on the surface of muscle cells. Mutations in myospheroid (mys), which encodes for the βPS subunit, causes embryonic muscles to detach from tendon cells following muscle contraction (Wright 1960; Leptin et al. 1989). Absence of the muscle-specific αPS2 (inflated, if) subunit leads to similar muscle detachment (Brown 1994), while lack of αPS1 (multiple edematous wings, mew) on tendon cells shows no evidence of detachments (Brower et al. 1995). This attachment role for integrins in muscle and tendon cell adhesion is conserved, as loss of the α (pat-2)- and β (pat-3)-integrin subunits alter muscle attachment in C. elegans (Qadota and Benian 2010). Furthermore, mutations in mouse integrin α7 lead to progressive muscular dystrophy resulting from impairment of MTJ function (Mayer et al. 1997).
In the developing Drosophila musculature, the α-integrin subunits cannot substitute for one another (Martin-Bermudo and Brown 1996), but rather impart extracellular ligand binding specificity. Laminins are trimeric ECM proteins that consist of α-, β-, and γ-chains. The α-chains are encoded by two genes, Laminin A (LanA) and wing blister (wb), which associate with the αPS1βPS or αPS2βPS heterodimer complexes, respectively (Gotwals et al. 1994; Graner et al. 1998). Weak muscle detachment defects are present in wb, but not in LanA mutants (Prokop et al. 1998; Martin et al. 1999), suggesting that the muscle-specific αPS2 subunit is crucial for muscle attachment. However, it is also possible that functional redundancy of LanA precludes the observation of phenotypic consequences in LanA mutants.
Interactions between αPS2 and Laminin are mediated by the tripeptide RGD sequence present in the LanA α-chain (Graner et al. 1998). The Drosophila-specific ECM protein Tiggrin (Tig) also possesses RGD integrin binding activity (Fogerty et al. 1994). Tig is produced in fat body and hemocytes and accumulates at the site of muscle–muscle junctions (Fogerty et al. 1994). Consistent with a role in integrin-mediated cell adhesion, Tig mutants exhibit a weak larval muscle detachment phenotype that appears after the onset of muscle contraction (Bunch et al. 1998). In screens aimed at identifying new muscle patterning genes, two groups identified thrombospondin (Tsp) as an additional αPS2 integrin ligand (Chanana et al. 2007; Subramanian et al. 2007). Tsp contains the alternate KGD tripeptide motif and is secreted from the tendon cell into the extracellular space at the junctions between muscle and tendon contact zones. Additional secreted proteins, including M-spondin (Mspo) and Masquerade (Mas) also accumulate at Drosophila embryonic MASs (Murugasu-Oei et al. 1995; Umemiya et al. 1997). However, only mutations in mas exhibit loss of muscle attachment, once again suggesting that redundancy could account for the lack of somatic muscle defects observed in mspo mutants.
In an effort to identify new muscle mutants, we screened a collection of lethals for abnormal pupal morphology due to inefficient muscle contraction during the larval-to-pupal transition. One such mutant, named fon, was originally identified for its role in Drosophila hemolymph coagulation (Scherfer et al. 2006). The muscle detachment phenotype in fon mutants was remarkably similar to that observed in Tig mutants (Bunch et al. 1998). Moreover, Fon and Tig protein expression overlaps at MASs. Since Fon and Tig are also both components of the hemolymph clot (Karlsson et al. 2004; Scherfer et al. 2004), we reasoned that there may exist other secreted coagulation proteins required for muscle attachment. Indeed, Larval serum protein 1γ (Lsp1γ) is found on the surface of larval tendon cells and Lsp1γ deficiency results in myofiber detachment. These data suggest that a specific subset of hemolymph proteins that participate in the larval clot coordinately function in the MAS matrix to mediate muscle attachment stability.
Materials and Methods
Fly genetics
Drosophila stocks were raised on cornmeal medium under standard laboratory conditions at 25° unless otherwise indicated. The lab control strain yw was used for detachment and gap distance experiments, while w1118 was used as a control in all other experiments. The following stocks were used to drive tissue-specific expression: tubP(αTub84B.PL)-Gal4 (BL-5138), 24B-Gal4 (BL-1767), sr-Gal4 and sr-Gal4, UAS-CD8-GFP (gifts from T. Volk), ppl-Gal4 (a gift from L. Dobens), and da-Gal4 (originally BL-37291 outcrossed 10 times to w1118 to remove background lethals). The following fondue mutations were used: the null alleles fonΔ24 and fonΔ17 are deletions that remove only fon coding sequence (Bajzek et al. 2012); hypomorphic allele w1118; Mi{ET1}fonMB11923/SM6a (fonMB; BL-29262) (Bajzek et al. 2012), fon RNAi [originally from R. Ueda; described in Scherfer et al. (2006)], and w1118; P{UAS-fon.GFP}28e [(fon-GFP; BL-43646) (Lindgren et al. 2008)]. Additional alleles and/or stocks analyzed are as follows: TigA1 and TigX (Bunch et al. 1998), fonΔ24A; da-Gal4 (Bajzek et al. 2012), UAS-Tig RNAi (BL-31570), UAS-Tsp RNAi (VDRC; v10072s), UAS-Lsp1γ RNAi (BL-55389), UAS-Gelsolin RNAi (BL-31205; BL-41704), and P{PTT-un1}vkgG00454 (Morin et al. 2001). Deficiency (Df) stocks Df(2L)Exel6043 and Df(2L)BSC185 were used to remove fon and Tig, respectively. All fon and Tig mutant alleles and Dfs were maintained over a Cyo-Act-GFP balancer. Non-GFP individuals were manually selected for mutant analysis. RNA interference (RNAi) experiments were performed at 29° except for crosses involving Lsp1γ RNAi (BL-55389), which were performed at 27° to minimize larval lethality.
Immunostaining and microscopy
L3 larvae were filleted and fixed with 4% formaldehyde prior to staining as previously described (LaBeau-DiMenna et al. 2012). The following primary antibodies were used: mouse anti-βPS-integrin [1:50, Developmental Studies Hybridoma Bank (DSHB)], mouse anti-Tig [(1:1000) (Fogerty et al. 1994)], mouse anti-αPS2-integrin [1:10, (DSHB)], anti-Talin [1:10, (DSHB)], anti-Perlecan [(1:1000; (Friedrich et al. 2000)], anti-DLG [1:300, (DSHB)], and rabbit anti-GFP (1:1000, Medical and Biological Laboratories). The following secondary antibodies were used at 1:400 for fluorescent detection: Alexa Fluor anti-mouse 488, Alexa Fluor anti-rabbit 488, and Alexa Fluor anti-mouse 647 (Molecular Probes). Phalloidin 488, 594, and 647 were used for F-actin labeling (1:400; Molecular Probes, Eugene, OR). Fluorescent images were taken with a Zeiss 700 confocal microscope. Images were processed, analyzed, and compiled into figures using Zen Black (Zeiss), ImageJ (National Institutes of Health) software, and/or Adobe Photoshop Elements.
Brefeldin A treatment
Brefeldin A (BFA) treatment was modified from a published protocol developed for embryo analysis (Dong et al. 2014). Briefly, larvae were dissected live in Schneider’s Insect Medium buffer (Sigma Chemical, St. Louis, MO) and incubated in either a DMSO control solution or a BFA solution [20 μg/ml in DMSO (Cell Signaling Technology, Danvers, MA)] for 1.5 hr at 29°. Fillets were washed three times quickly with PBS and fixed with 4% formaldehyde. Fillets were then stained for F-actin and GFP and imaged as described above. To quantitatively assess Fon–GFP retention in the fat body, GFP fluorescence intensity was calculated using the measurement function in ImageJ. Briefly, the interior of individual fat body cells was selected for analysis in 20× images, with three cells measured across each image. A background measurement was collected outside of the frame of the fat body lobe. Corrected total fluorescence (CTF) was calculated using the following equation: CTF = integrated density − (area of selected cell × mean fluorescence of background) (McCloy et al. 2014). Raw data and statistical analysis for both BFA-treated and DMSO-treated fat bodies were compiled and analyzed using GraphPad Prism 6.0.
Transmission electron microscopy
Drosophila L3 larvae were filleted and fixed overnight in 1× Trump’s fixative (4% formaldehyde/1% glutaraldehyde in phosphate buffer). Fillets were processed with osmium tetroxide and put through a graded alcohol dehydration series before embedding in Spurr resin. Ultrathin sections of the dissected fillets were taken in a parasagittal orientation starting at the dorsal edges of muscle hemisegments using uranyl acetate and lead citrate for contrast. Samples were observed and imaged with a FEI Tecnai 12 Bio-Spirit Transmission electron microscope. Images were prepared using the Gatan Microscopy Suite software.
Quantitative PCR analysis
To assess the effectiveness of RNAi knockdown, RNA transcripts were collected from three wandering L3 larvae using the RNeasy Mini Kit (QIAGEN, Valencia, CA) for each line of interest. The driver line, da-Gal4, was used as a control genotype. Complementary DNA (cDNA) synthesis of 125 ng RNA was performed using the SuperScript II First-Strand Synthesis System Kit (Invitrogen, Carlsbad, CA). Dilutions of cDNA were optimized for primer pairs (Supplemental Material, Table S2) and combined with the SYBR Select Master Mix (Applied Biosystems, Foster City, CA) for quantitative measurement of transcripts on the CFX96 Touch Real-Time PCR Detection System with CFX Manager software (Bio-Rad, Hercules, CA). Both the housekeeping gene rp49 and the gene of interest were measured for control and RNAi knockdown larvae. Results from three biological replicates and a minimum of three technical replicates were averaged to obtain Ct values. Fold expression change was calculated using the 2−ΔΔCt method, graphed using GraphPad Prism 6.0, and analyzed using the Kruskal–Wallis statistical test.
Phenotypic quantification and statistical analysis
Pupal axial ratio:
White pupae of the appropriate genotype were removed from vials, oriented dorsal side up, and attached to slides using a small drop of nail polish. Images were taken with a Leica M165 FC stereomicroscope. Length and width measurements of each pupae were performed in ImageJ using the line and measure functions. Values were deposited into an Excel spreadsheet and the axial ratio (length/width) was calculated for each individual. The raw data were imported into Graphpad Prism 6.0 and graphed as a box and whiskers plot.
Gap distance:
Average gap distance quantifies the space between dorsal oblique 1 and 2 (external muscles 9 or 10) across the hemisegmental border using the “distance between two polylines” plug-in for ImageJ. Images were taken at 40× magnification and gap distances were calculated as an average of distances along the length of the muscle attachment surface for each genotype. Average distances were compiled in Excel and graphed as a dot plot using GraphPad Prism 6.0.
Locomotion:
Larval locomotion studies were performed on apple juice agar plates with a minimum of 15 individuals per genotype. Larvae crawling patterns were filmed for 1 min and analyzed using the “grid” plugin in ImageJ. Velocity was calculated from the distance the organism crawled (conversion of no. of squares crawled through to distance in centimeters) per second in Excel, graphed as average ± SD detachment. For all experiments, muscles were characterized as detached if: (1) muscles had rounded up following detachment or had clearly separated at an attachment site; (2) muscles were in the process of stripping away but were attached through muscle–muscle connections in another hemisegment; or (3) muscles were missing from the fillet. A detailed, individual muscle phenotyping was performed for fon alleles, tissue-specific fon knockdown, and to analyze fon and Tig genetic interactions. The following indirect and direct subsets were scored within muscle (m.) hemisegments: dorsal acute [DA1-3 (m. 1–3)]; longitudinal lateral [LL1 (m. 4)]; lateral oblique [LO1 (m. 5)]; ventral longitudinal [VL1-4 (m. 6–7, 12–13)]; segment border muscle [SBM (m. 8)]; dorsal oblique [DO1-2 (m. 9–10)]; DO3-4 (m. 11,19); ventral oblique [VO4-6 (m. 15–17)]; lateral transverse [LT1-4 (m. 21–24)]. Direct muscle subsets were quantified as LT1-4 (m. 21–24), the medial attachments of VO4-6 (m. 15–17), and the dorsal attachment site of DO3-4 (m. 11, 19). All other muscles quantified were considered indirect attachments. Percent detachment was calculated as the number of individual detached muscles divided by the total number of muscles quantified per fillet. Individual detachment percentages were plotted and represented as a bar graph (average ± SD). For the fon sensitized background experiments, detachment was quantified as the percentage of hemisegments containing one or more detached muscles. Individual percentages were plotted per genotype and represented as a bar plot.
Statistical analysis:
All data points in each set of experiments/graphs were first analyzed for Gaussian distribution sampling. None of the data sets conformed to these parameters and were subjected to the Kruskal–Wallis test, a nonparametric test that compares three or more unmatched groups that do not conform to a Gaussian distribution. The Bliss independence test was used to determine the expected contribution of additive phenotypes (Fitzgerald et al. 2006). Significance values are indicated in each figure legend.
Generating Lsp1γ transgenics
Total RNA was isolated from L3 larvae and reverse transcribed. The open reading frame for Lsp1γ was PCR amplified from this cDNA pool using the forward primer 5′-CACCATGAAGTTGACCCTTGTTATATT-3′ and the reverse primer 5′-GTATTCAATGGAGTAGTCGAAGGTGC-3′, inserted into the Gateway pENTR/D-TOPO vector (Invitrogen), and recombined into the pTWV destination plasmid [(Drosophila Genomics Resource Center (DGRC)] using standard procedures to generate UAS–Lsp1γ–YFP (hereafter referred to as UAS–Lsp1γ–GFP). This construct was injected for the generation of transgenic flies by Rainbow Transgenic Flies.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results
Novel genetic screen to identify muscle mutants
We and others have identified mutants, including thin(tn)/abba, sallamus (sls), Mlp84B, and Tiggrin (Tig), that are defective in various aspects of larval muscle structure and/or function, including myofiber stability, sarcomere maintenance, and/or muscle attachment (Bunch et al. 1998; Clark et al. 2007; LaBeau-DiMenna et al. 2012; Domsch et al. 2013). A shared feature among these mutants is pupal lethality and an abnormally elongated, or curved, pupal morphology. We reasoned that this extended pupal case, caused by the inability of muscles to contract during the larval-to-pupal transition, could serve as the basis for a genetic screen to identify novel genes essential for larval muscle contraction.
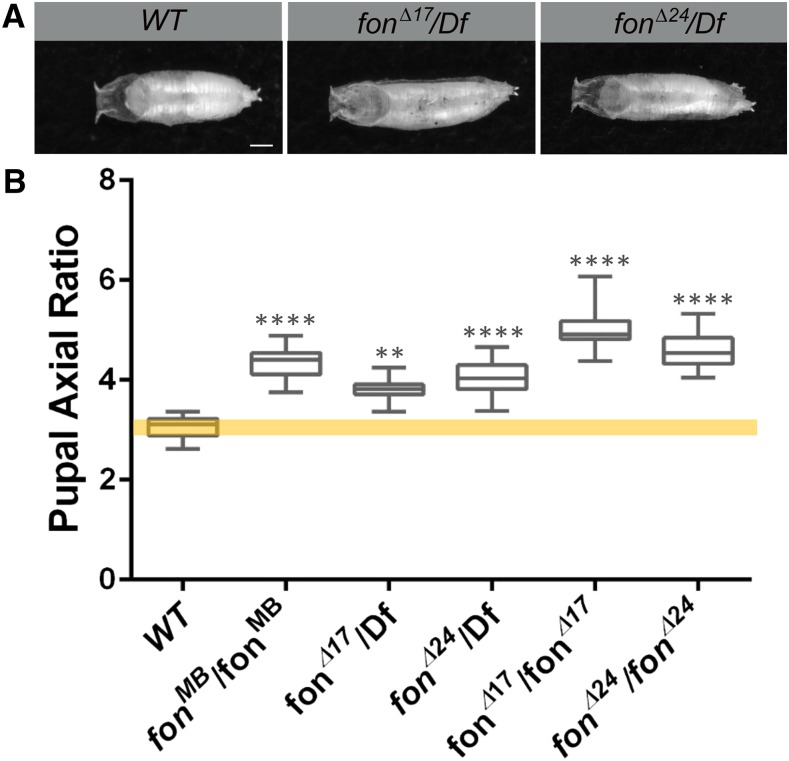
We visually inspected 9323 lethal stocks at the Bloomington Drosophila Stock Center (BDSC) and identified 184 possible stocks (∼1.9%) that exhibited an elongated and/or curved pupal phenotype. We screened 15 of these candidates for muscle morphology defects in third instar (L3) larvae (Table S1). For one candidate, this abnormal pupal phenotype was caused by a Minos insertion into the fondue (fon) locus (Mi{ET1}fonMB11923 referred to hereafter as fonMB). Interestingly, the Dushay group had previously reported that homozygous fon mutants exhibit longer or curved pupae that failed to eclose (Figure 1A) (Scherfer et al. 2006; Bajzek et al. 2012). To further characterize the pupal morphology phenotype, we measured the axial ratio (length/width) in fon alleles compared to wild-type (WT) control pupae. In fonMB/fonMB or deletion mutants (fonΔ17 or fonΔ24) that remove portions of fon coding region (Bajzek et al. 2012), pupae exhibited a greater axial ratio than WT individuals (Figure 1B). This lethality and associated pupal morphological changes seemed unlikely to result solely from the role of Fon in hemolymph coagulation, as this phenomenon occurred in unwounded individuals, and other more severe clotting mutants (e.g., hemolectin) did not exhibit this same phenotype (Lesch et al. 2007).
Figure 1.
Mutations in fon result in elongated pupal phenotypes. (A) Pupal cases of control (WT) or fon mutations (fonΔ17 and fonΔ24) analyzed over a deficiency chromosome (Df) that removes the fon locus. (B) Measurement and quantitation of axial ratios (length/width) of the indicated pupal genotypes demonstrate that fon mutants are defective in the ability to shorten their pupal case (17 ≤ n ≤ 35 for each genotype). Mean ± SD; P-values: ** P < 0.01, **** P < 0.001. Bars, 0.75μm.
The extracellular Fon protein is essential for stable muscle attachment
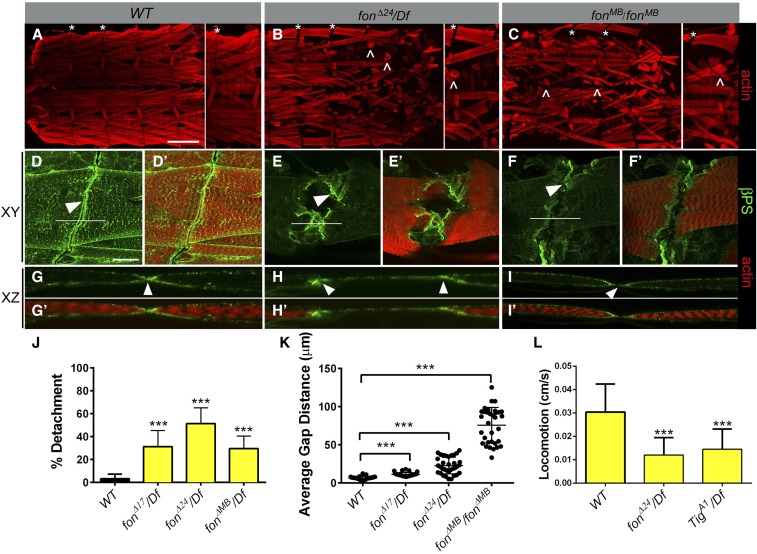
To examine whether defects in muscle structure and/or function could be responsible for the elongated fon pupal phenotype, we dissected and immunostained muscles in late wandering L3 larvae, just prior to puparium formation. Fillets of WT L3 individuals revealed a precise pattern of segmentally repeated myofibers that were rectangular in shape and firmly attached to other muscles or directly to the larval exoskeleton (Figure 2A). As expected in WT animals, the dorsal oblique muscles 9 and 10 (Figure 2A, DO1 and DO2, respectively) were in close proximity at the segment borders (Figure 2A, asterisks), with no obvious gaps between adjacent muscles in higher magnification confocal images viewed in an XY (Figure 2, D and D′) or XZ planes (Figure 2, G and G′).
Figure 2.
Fon is required for muscle attachment stability and larval locomotion. (A–I′) Internal views of larval fillets at low (A–C) or high magnification (D–I′) in WT or fon mutant larvae in XY (A–F′) or XZ (G–I′) focal planes. (A) WT larval muscles are rectangular when firmly attached to other muscles or tendon cells. (B and C) Deletion of the fon locus (fonΔ24/Df; B) or an insertion that disrupts fon (fonMB/fonMB; C) results in many detached muscles (carets). (D–I′) Phalloidin (red) and βPS integrin (green) staining in the indicated genotypes. (D and G) WT dorsal oblique muscle 10 (DO2) shows accumulation of βPS integrin at attachment sites between adjacent muscles (white arrowhead). (E, F, H, and I) fon mutant DO2 muscles reveal large gaps between adjacent hemisegments, yet retain βPS integrin accumulation at muscle edges (white arrowheads). (J) Analysis of different fon mutant alleles show an increased percentage of detached myofibers. (K) The dorsal oblique muscles 9 and 10 (DO1 and 2) exhibit a variable, but significant increase in gap distance between adjacent hemisegments in fon mutants. (L) L3 wandering larvae (29 ≤ n ≤ 46 for each genotype) with mutations in the fon locus traverse across agar plates at a velocity lower than their WT counterparts, but similar to Tig mutants. Mean ± SD; P-values: *** P < 0.005. Bars, 200 µm for A–C; 50 µm for D–I′.
Dissection of L3 fon mutants (fonΔ24/Df or fonMB/fonMB) revealed two obvious muscle phenotypes. First, myofibers were detached and rounded due to their inability to remain attached during muscle contraction (Figure 2, B and C, carets). The penetrance of detached muscles varied among fon mutant genotypes, ranging from 28.8 to 54.2% of all muscles examined (Figure 2J and Table 1). Loss of Fon affected all muscle subsets and both direct and indirect linkages. The second morphological phenotype we observed in fon mutants was large gaps between muscles 9 and 10 (Figure 2, B and C, asterisks E and F, K, and Table 2). Since Fon is a secreted protein (Vierstraete et al. 2003; Scherfer et al. 2006), we hypothesized that the apparent gaps and detached muscles were a consequence of changes in the extracellular environment rather than intracellular defects. Consistent with this, we did not observe detachment of the actin cytoskeleton from the sarcolemma in fon mutants (Figure 2, H and I, arrowheads).
Table 1. Muscle detachment in L3 larvae of the indicated genotypes.
| Detached muscles (% of total muscles) | Number of muscles analyzed (n) | Duplicated muscles (% of hemisegments) | Number of hemisegments (n) | |
|---|---|---|---|---|
| yw | 2.0 | 2667 | 2.3a | 127 |
| fonΔ17/Df(2L)Exel6043 | 29.9 | 3801 | 0.0 | 181 |
| fonΔ24/Df(2L)Exel6043 | 54.2 | 2457 | 0.0 | 117 |
| fonMB/Df(2L)Exel6043 | 28.8 | 3969 | 0.0 | 189 |
| fonΔ24/+ | 6.3 | 2772 | 4.5a | 132 |
| TigA1/+ | 9.9 | 2898 | 0.0 | 140 |
| +; fonΔ24/TigA1, + | 20.9 | 1995 | 21.0a,b,c,d | 95 |
Lateral transverse (muscles 21–24).
Lateral longitudinal (muscle 4).
Dorsal oblique (muscles 9–11 and 19).
Dorsal acute muscles (muscles 1–3).
Table 2. Gap distance between muscles 9 and 10.
| Genotype | Average gap distance (μm) | Gap distance range (μm) | (n) |
|---|---|---|---|
| yw | 6.44 | 3.0–11.2 | 25 |
| fonΔ17/Df(2L)Exel6043 | 12.3 | 7.3–20.0a | 35 |
| fonΔ24/Df(2L)Exel6043 | 24.1 | 12.8–37.4b | 35 |
| fonΔ24/+ | 8.5 | 3.8–16.1 | 35 |
| TigA1/+ | 7.2 | 3.1–15.1 | 35 |
| +; fonΔ24/TigA1, + | 11.8 | 5.5–22.5 | 31 |
Outlier = 64.5 μm.
Outlier = 81.2 μm.
Structurally, both vertebrate and invertebrate MASs are composed of integrin heterodimer complexes located within the plasma membranes of muscle cells that link the ECM to the internal muscle contractile apparatus. Mammals display 18 α- and 8 β-subunits, so far known to comprise 24 distinct integrin heterodimers (Hynes 2002). Drosophila has only 5 α- and 2 β-position specific (PS) integrin chains, (called αPS1-5, βPS, and βν) that assemble into cell-type-specific heterodimer complexes (Bulgakova et al. 2012). The αPS2βPS integrin subunits accumulate at the ends of migrating myofibers, while the αPS1βPS heterodimer is found solely on the surface of target tendon cells (Schejter and Baylies 2010; Schweitzer et al. 2010; Maartens and Brown 2015). Thus, we next examined if the detached muscles in fon mutants could be due to a loss or mislocalization of integrin protein. We found that the βPS subunit accumulated normally at the muscle cell surface in both WT (Figure 2, D and D′, arrowhead) or fon mutant (Figure 2, E and F, arrowhead) larvae. Examination of the localization and relative protein levels of αPS2 and Talin, an indicator of intact integrin signaling, were also unaffected, as was the accumulation of Tig (Figure S1). Consistent with previous results that basement membrane components are absent from the MTJ (Alves-Silva et al. 2008), we found that loss of Fon did not alter the localization of Perlecan or Collagen IV, the latter of which is encoded by the viking (vkg) gene (Figure S1).
The severe muscle detachment observed in fon mutants would be predicted to affect organismal movement. Indeed, fon mutant larvae moved across agar plates at a significantly decreased rate compared to WT individuals (Figure 2L). Loss of Fon also did not affect the bouton number of type I synapses (Figure S2), suggesting that the locomotion defects are not an indirect effect of defective neuronal connections. Taken together, these data demonstrate that Fon is essential for the attachment of muscles and does not affect the linkage of actin filaments or the relative membrane localization of integrins and known ECM proteins.
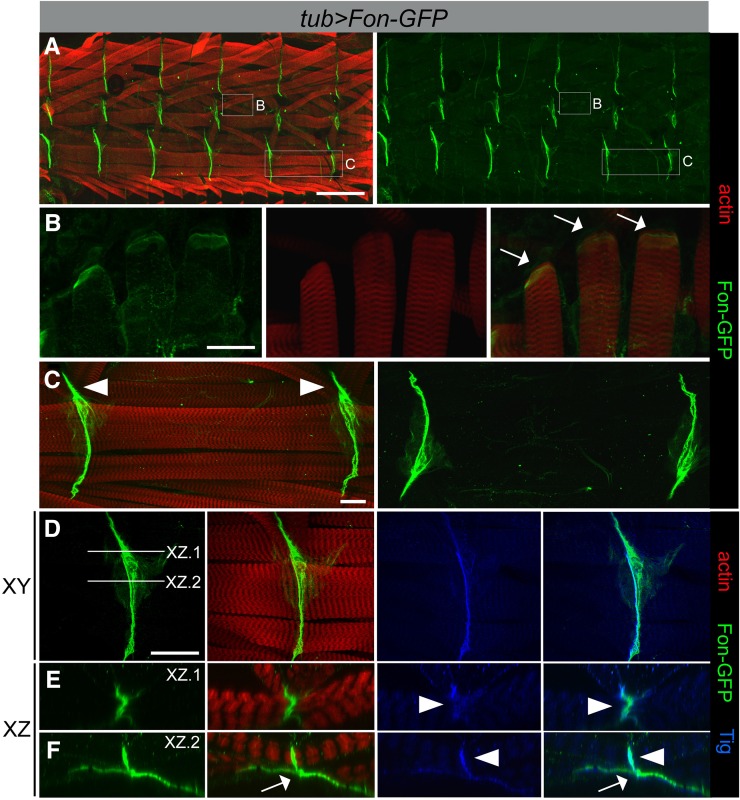
Fon protein accumulates at MASs
Photomicrographs of larvae expressing a Fon–GFP fusion construct driven by da-Gal4 were described as having a banded pattern of cuticular fluorescence (Lindgren et al. 2008; Bajzek et al. 2012). We confirmed that this striped pattern corresponded to MASs between adjacent hemisegments. Dissection of L3 larval pelts from animals expressing Fon–GFP driven by the ubiquitous tubulin (tub)-Gal4 [or fat body and salivary gland pumpless (ppl)-Gal4] driver revealed an accumulation of Fon–GFP at the distal ends of muscles (Figure 3, A–F), although the amount of Fon varied depending on whether the muscle subsets were directly or indirectly linked to the cuticle. In general, Fon weakly localized to the ends of all muscles that were directly attached through tendon cells to the cuticle. Figure 3B shows an example of Fon accumulation at the distal ends of one such set of directly attached muscles (lateral muscles 21–23). Strong accumulation of Fon–GFP was evident between adjacent muscles in each hemisegment at indirect muscle attachment sites (Figure 3, C–F). Views of Fon–GFP accumulation in XY (Figure 3D) or XZ (Figure 3, E and F) planes revealed a heavy localization of Fon–GFP between adjacent muscles and an accumulation under the muscles, likely responsible for the cuticle attachment. Notably, Fon also colocalized with the extracellular integrin ligand Tig at indirect attachment sites (Figure 3E, arrowheads), further supporting Fon as an ECM protein. Interestingly, larvae in which one copy of both fon and Tig were removed showed an increase in the gap distance between adjacent muscles, suggesting that fon and Tig exhibit dominant interactions and may function together in the ECM (Figure S3). Note that the accumulation of Fon–GFP protein was not altered in Tig mutant muscles (Figure S1).
Figure 3.
Fon accumulates at muscle attachment sites. (A–F) Ubiquitous expression of a Fon–GFP fusion protein (green) using the tubulin (tub)-Gal4 driver. Muscles are labeled with phalloidin (F-actin; red). Fon–GFP is enriched at the ends of attached myofibers at direct (muscle–cuticle; white arrows) and indirect (muscle–muscle; white arrowheads) attachments shown in a low magnification view of half a larval fillet (A) or in high magnification views (B–F). Fon–GFP weakly accumulates at the ends of lateral muscles at direct attachments (B) and is found at high levels between muscles at indirect attachment sites, such as the ventral muscles 6 and 7 (C–F). (D–F) The photographs in E and F represent the XZ plane of the lines indicated in D. Fon–GFP colocalizes with anti-Tig immunostaining (blue) at indirect attachments in regions of muscle-to-muscle contacts (E, arrowheads), but weakly within sites where muscles associate with the tendon cell (F, arrows), where Fon–GFP is more prominent. Bars, 200 µm for A; 50 µm for B and D; and 25 µm for C.
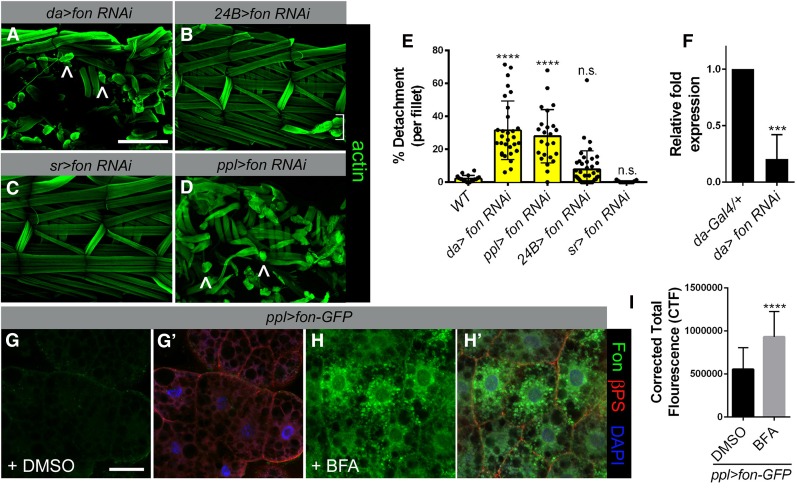
To determine whether Fon accumulation at MASs is produced locally or secreted from the fat body to circulate in the hemolymph as in coagulation (Scherfer et al. 2006), we utilized an RNAi strategy using tissue-specific Gal4 drivers. A ubiquitous decrease in Fon levels (da-Gal4) led to drastic muscle detachment (Figure 4, A and E, carets), demonstrating the efficacy of our fon RNAi approach. The myofibers in muscle-specific 24B > fon RNAi larvae remained attached, but occasionally were stripped away during dissection or missing, possibly resulting from specification or patterning defects in embryonic myogenesis (Figure 4B, bracket, and E). Expression of fon RNAi by the tendon cell promoter stripe (sr) did not produce detached muscles (Figure 4, C and E), while use of the fat body driver ppl-Gal4 resulted in severe myofiber detachment (Figure 4, D and E, carets). Effective knockdown of fon by RNAi was confirmed using quantitative PCR (qPCR) (Figure 4F).
Figure 4.
Fat body-produced Fon is required for stable muscle attachments. (A–D) Visualization of larval muscles (F-actin; green) after fon RNAi knockdown using the indicated Gal4 drivers. Driving fon RNAi under expression of the ubiquitous da (A) or the fat body driver ppl (D) regulatory sequences results in muscle attachment defects (carets). Knockdown of fon RNAi in the muscle (24B-Gal4) occasionally results in missing muscles (B, bracket), while knockdown in tendon cells [C; stripe (sr)-Gal4] does not alter muscle attachment stability. (E) Percent muscle detachment in the indicated genotypes. Muscles within each fillet were scored as being detached or intact (n ≥ 19 for each genotype). (F) qPCR reveals that fon mRNA levels are decreased upon fon RNAi knockdown (da > fon RNAi) compared to control (da-Gal4) L3 larvae. (G–H′) βPS integrin (red) and DAPI (blue) label fat body tissue from ppl > fon-GFP L3 larvae fed control DMSO or BFA. BFA treatment blocks efficient Fon–GFP (green) transport out of fat body cells. (I) Quantitation of mean fluorescence intensity within individual fat body cells (n = 12 for each untreated and BFA-treated samples). Mean ± SD; P-values: **** P < 0.001, *** P < 0.005. Bars, 200 µm for A–D; 50 µm for F and G.
To further test the idea that Fon is produced in fat body, we examined the consequences of blocking Fon protein secretion in this tissue. Larvae expressing Fon–GFP in fat body cells under control of the ppl driver were dissected live and incubated in a solution of either DMSO or the ER → Golgi inhibitor BFA dissolved in DMSO. Dissection and imaging of control DMSO-treated fat body tissue revealed a low level of Fon–GFP inside cells (Figure 4, G and G′) with increased internal Fon–GFP accumulation upon treatment with BFA (Figure 4, H and H′). This block in Fon secretion after BFA treatment was confirmed by quantitation of the relative fluorescence intensity of Fon–GFP (Figure 4I). These data demonstrate that Fon is secreted from the fat body and is incorporated into MASs.
Fon is a critical regulator of MAS architecture
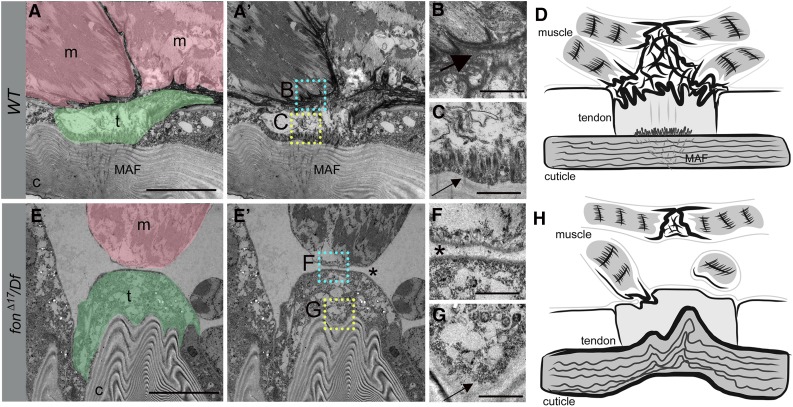
We next utilized TEM to analyze the muscle, tendon, and cuticular structures present in sagittal sections of WT or fon mutant L3 larvae to explore how loss of Fon alters the ultrastructure of indirect attachment sites. Consistent with previous reports (Prokop et al. 1998; Alves-Silva et al. 2008), TEM images revealed a regular arrangement of horizontally oriented cuticular (c) laminae underneath epidermal and tendon (t) cells (Figure 5, A and A′). Extracellular electron-dense material accumulated between the interdigitating sarcolemma of the incoming myofibers (m) and the tendon cell membranes (Figure 5B, large black arrow). Apical junctions at the base of the tendon cell (Figure 5C, small black arrow) connect muscle attachment fibers (MAFs) (Caveney 1969), also called tonofilaments (Prokop et al. 1998), located within cuticle pore canals to the muscle–tendon interface at basal cell junctions. In stark contrast, loss of Fon revealed an unorganized and highly convoluted cuticular structure (Figure 5, E and E′) and a loss of apical junctional complexes in tendon cells (Figure 5G, small black arrow). Most significantly, while the muscle and tendon cell membranes were adjacent to one another, there was a complete loss of electron-dense ECM components and cell interdigitation (Figure S4 and Figure 5F, asterisk). These data show that Fon is essential in the organization of ECM components at the MTJ.
Figure 5.
Loss of Fon alters cuticle integrity, tendon cell cytoarchitecture, and ECM accumulation. (A–C and E–G) TEMs of an indirect muscle–tendon attachment site in WT (A–C) or fonΔ17/Df mutants (E–G). (A and A′) Muscles (m; pink) are interlaced with tendon cells (t; green) at control MTJs. The insets correspond to high magnification images in B (cyan) and C (yellow). (B) An electron-dense ECM matrix is observed between the sarcolemma and tendon cell membranes (large arrow). (C) Apical junctions present at the base of tendon cells (small arrow) are associated with MAFs (A and A′) that extend into the cuticle. (D) Generalized schematic representation of WT muscle attachment. (E and E′) The detached muscle (m; pink) in this fon mutant remains close to the tendon cell (t; green), which is attached to a highly convoluted cuticle (c). The insets correspond to close-up images in F (cyan) and G (yellow). (F) There is a loss of electron-dense ECM material and membrane interdigitation between the muscle and tendon cell (asterisk). (G) Note the absence of tendon cell junctional complexes at the base of the tendon cell (G, small arrow). (H) Illustration reveals the dramatic loss of muscle attachment, ECM accumulation, and morphological abnormalities associated with mutations in fon. Bars, 10 µm for A and E; 1 µm for B, C, F, and G.
A set of conserved clotting proteins are required at MASs
Fon and Tig are present in the hemolymph clot (Karlsson et al. 2004; Scherfer et al. 2004). Thus, we wondered whether other ECM and/or clotting factor proteins are necessary for Drosophila muscle attachment. We chose a fon-sensitized RNAi approach for two reasons: (1) to target genes that may interact with fon in the ECM and (2) because characterized mutations were not available for many of the candidate clotting genes.
To examine the contribution of RGD- and KGD-containing integrin ligands in larval myofiber attachment stability, we performed candidate RNAi knockdown of Tig and Tsp in a genetically sensitized fon mutant (fonΔ24/+; da-Gal4) background. Ubiquitous knockdown of Tig RNAi (Figure 6B), Tsp RNAi (Figure 6C), or fonΔ24/+; da-Gal4 (Figure 6E) alone resulted in a low level of detached muscles (Figure 6I). In contrast, a significant increase in the number of detached muscles (Figure 6I) was observed upon a 50% reduction in fon copy number in a Tig (Figure 6F) or Tsp (Figure 6G) RNAi genetic background. Since both Tig and Tsp are extracellular proteins, we utilized secreted Gelsolin (Gel), a protein that also circulates in the hemolymph (Guedes et al. 2003; Karlsson et al. 2004) as a negative control. RNAi knockdown in two independent lines of Gel alone (Figure 6D), or in combination with a reduction in fon (Figure 6H), did not affect the ability of muscles to remain attached (Figure 6I). qPCR results demonstrating RNAi knockdown are shown in Figure 6J.
Figure 6.
RNAi knockdown of Tig or Tsp enhances fon-mediated muscle detachment. (A–H) Two hemisegments of the larval musculature stained for F-actin in a WT control, the indicated RNAi lines alone (B–D) or in a sensitized fon (fonΔ24/+) genetic background (E–H). (B) RNAi knockdown of Tig results in missing muscles 6 and 7 (bracket) or muscles that lift off of the cuticle (indented arrowheads). (C and D) Loss of Tsp (C) mildly affects muscle attachment, while Gel (D) alone has no effect. Compared to the WT appearance of fonΔ24/+, da-Gal4 alone (E), an enhancement of detached muscles (arrows) is observed upon concurrent expression of Tig RNAi (F) or Tsp RNAi (G), but not Gel RNAi (H). (I) Quantitation of muscle detachment in the indicated genotypes in a fon-sensitized background (14 ≤ n ≤ 29 for each genotype). The purple lines illustrate the predicted additive effects of each individual contribution. (J) qPCR results showing that the indicated RNAi lines effectively knockdown Tig, Tsp, and Gel transcripts. Mean ± SD; P-values: *** P < 0.005, ** P < 0.01, * P < 0.05; n.s. = not significant. Notations in purple indicate comparisons to fonΔ24/+; da-Gal4 alone, P-values: #### P < 0.001, n.s. = not significant. Bars, 100 µm for A–G.
We utilized our genetic interaction assay to test the requirement for multiple candidate proteins found in the hemolymph clot, including Imaginal disc growth factor 4 (Idgf4), Retinoid- and fatty acid-binding glycoprotein (RfaBp/ApoL1), Larval serum protein 1γ (Lsp1γ), and Larval serum protein 2 (Lsp2) (data not shown). Only RNAi knockdown of Lsp1γ (Figure 7D) resulted in detached muscles (Figure 7, B and C, carets) although penetrance of the phenotype was not increased in a heterozygous fon (fonΔ24/+) background (Figure 7C). To further assess how Lsp1γ may contribute to larval muscle attachment, we expressed Lsp1γ–GFP transgenic flies under UAS control. As Lsp1γ is secreted from fat body tissue (Deutsch et al. 1989), we expressed this fluorescently tagged fusion protein with ppl-Gal4 (Figure 7, E and E′). Lsp1γ–GFP accumulated at junctions where muscles were directly attached to the cuticle, such as the lateral transverse muscles 21–23 (Figure 7, E and F, small arrows). Lsp1γ–GFP was also observed at indirect muscle sites that meet at the hemisegmental border (Figure 7, E and E′, white arrowheads). Closer examination revealed a block-like appearance of Lsp1γ–GFP accumulation (Figure 7, G and G′), consistent with the location of tendon cells along the cuticle as visualized by the tendon cell marker Sr (Figure 7, I and I′). Scanning from the top of the muscle toward the cuticle revealed an accumulation of Lsp1γ–GFP underneath (Figure 7, H and H ′, white arrow), but not between muscles in adjoining hemisegments (white arrowhead), further demonstrating that Lsp1γ is located near tendon cells. This is in contrast to Tig, which primarily localizes to the junctions between muscles (Figure 3, D–F).
Figure 7.
The clotting factor Lsp1γ accumulates at MASs. (A and B) Three hemisegments of the larval musculature stained for F-actin. (A) The normal pattern in WT larvae. (B) Ubiquitous induction of Lsp1γ RNAi reveal detached muscles (carets). (C) Quantitation of muscle attachment defects present in Lsp1γ knockdown larvae (16 ≤ n ≤ 40 for each genotype). Note that the penetrance is not increased in a fon-sensitized background. (D) Lsp1γ RNA is decreased upon RNAi knockdown as determined by qPCR. (E–H′) Lsp1γ–GFP fusion protein accumulates at sites consistent with tendon cell localization. (E–F′) The fusion protein is found at the ends of lateral muscles (small arrows in E, E′, and F) and on tendon cells at the segmental borders (arrowheads in E and E′). (G–H′) An XY (G and G′) or XZ (H and H′) scan through the plane shown in G reveals Lsp1γ accumulation on tendon cells (arrows), but not between adjacent muscles (open arrowhead). (I) Confirmation of tendon cell localization at segmental borders as visualized by GFP under control of the sr tendon cell promoter. Mean ± SD; P-values: **** P < 0.001, *** P < 0.005, n.s. = not significant. Bars, 150 µm for A and B; 50 µm for D–F and H.
Discussion
The unexpected lethality of fon mutant alleles led us to the surprising finding that a subset of clotting factors also acts in the independent process of muscle attachment. Further identification of a common suite of secreted proteins (Tig and Lsp1γ) that function in both coagulation and muscle attachment suggests that these ECM proteins share a unique structural role in providing rigidity and strength during wound healing and MTJ attachment, yet remain elastic to withstand forces generated by muscle contraction and organismal movement.
Seminal studies in Drosophila uncovered the role of integrins in muscle attachment over 20 years ago. Since this discovery, the requirement for integrins and integrin-associated proteins in vertebrate muscle attachment has reinforced evolutionarily conserved mechanisms that underlie MTJ structure and function (Volk et al. 1990; Schnorrer and Dickson 2004; Schejter and Baylies 2010). The prevailing notion in muscle biology is that the stable attachment of muscles depends upon integrin-mediated noncovalent interactions that link the internal muscle cell cytoskeleton to the external environment during active muscle contraction. Moreover, the majority of ECM proteins (e.g., Tig, Tsp, and Laminin) identified that function in muscle–tendon attachment directly bind integrin heterodimers through RGD or KGD binding motifs (Ruoslahti 1996). Neither the Fon nor Lsp1γ protein sequences contain these tripeptide sequences, suggesting they are not integrin ligands. Interestingly, the lack of integrin binding motifs implies that these, and possibly other, secreted proteins accumulate in the extracellular space and associate with integrin ligands to maintain MTJ integrity. Whether these proteins physically associate in the extracellular environment remains to be determined.
Based upon the data presented here, our current model suggests that Fon is a key ECM component required for MAS stability. First, the muscle detachment phenotypes in fon mutants appear consistently stronger than loss of Tig, Tsp, or the α-Laminin chain encoded by the wb gene (Martin et al. 1999). More importantly, loss of Fon in TEM studies reveals not just a loss of muscle attachment, but a complete absence of ECM components and a loss of membrane interdigitation between the sarcolemma and tendon cell (Figure 5, D and H). We postulate that the lack of mechanical tension at the MTJ compromises the stability of cytoskeletal arrays attached to apical junctions in tendon cells, leading to a loss of muscle attachment fibers in lamellae-associated pore canals and a loss of cuticle organization in fon mutants.
Surprisingly, we find that Lsp1γ accumulates on tendon cells to mediate the attachment of muscles to the underlying cuticle, adding a novel role to the repertoire of potential Lsp functions. This secreted protein is a member of the insect hexamerin family and is widely regarded as a nutrient storage protein (Burmester and Scheller 1999; Chandrasekar et al. 2009). Since hexamerins accumulate during late larval stages and are not detected in pupae or adults, it was proposed that these proteins store amino acids and thus energy reserves during nonfeeding stages. This idea was supported by experimental evidence in multiple organisms whereby hexamerin storage and degradation are correlated with stage- and sex-specific usage. Other possible roles for hexamerins include ecdysteroid binding and transport, cuticle formation, and humoral immune defense, although it is worth noting that these diverse roles have been demonstrated in insects other than Drosophila. Genetic studies in the fly model reported that null mutations in all three Lsp1 genes (α, β, and γ) are viable (Roberts et al. 1991). However, another study observed Lsp1 proteins in these null animals by electron microscopy, questioning the validity of the null alleles (Markl et al. 1992).
In addition to its role in the immune response and energy storage, the fat body serves as a source of secreted protein to regulate the development of multiple organs. Fon, Tig, and Lsp1γ are secreted from fat body tissue and circulate in the hemolymph to reach their final destination. We provide evidence that Fon is secreted via the canonical secretory pathway as treatment with BFA blocks Fon–GFP transport out of fat body cells. Furthermore, knockdown of Fon in the fat body using targeted RNAi causes muscle detachment. There is ample evidence for fat body secretion of proteins into the hemolymph and subsequent localization to target tissues, including Collagen IV (Col IV) into the basement membrane (BM) and the chitinase Serpentine (Serp) into the trachea (Pastor-Pareja and Xu 2011; Dong et al. 2014). While it is not clear how Col IV is targeted to the BM, synthesized Serp is secreted into the hemolymph and is transcytosed across epithelial cells to reach the tracheal inner lumen. This type of mechanism seems unlikely for Fon, Tig, or Lsp1γ transport as reaching MASs does not require crossing cell layers. While it is not yet clear how circulating hemolymph proteins become targeted to other tissues, possibilities include diffusion or lipid/protein-based transport systems.
Over 85% of the Fon residues are polar or hydrophobic, including an abundance of Ser, Ala, and Gly residues not uncommon in extracellular proteins. Fon protein appears to be composed of unstructured regions with no discernible predicted secondary structure or predicted conserved domains. Yet, clearly Fon is crucial for organization of the ECM at MASs. Thus, further insight into Fon structural and/or biochemical properties may shed light on this exciting new role for Fon.
Acknowledgments
We thank Talila Volk, Stephan Baumgartner, and Uli Theopold for contributing reagents; Ravindra Thakkar and Lloyd Willard (Nanotechnology Innovation Center of Kansas State, Kansas State University (KSU) for TEM assistance; and the KSU Integrated Genomics Center for qPCR resources. We also thank the Vienna Drosophila Resource Center and the Bloomington Drosophila Stock Center for stocks used in this study and the Developmental Studies Hybridoma Bank, created by the National Institute of Child Health and Human Development of the National Institutes of Health (NIH) and maintained at the University of Iowa for monoclonal antibodies. We also acknowledge funding from the National Science Foundation for a GK-12 award to N.M.G. (0841414) and from the NIH for grant awards to E.R.G. (RO1AR060788) and K.R.C. (P40OD018537).
Footnotes
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.189787/-/DC1.
Communicating editor: L. Cooley
Literature Cited
- Alves-Silva J., Hahn I., Huber O., Mende M., Reissaus A., et al. , 2008. Prominent actin fiber arrays in Drosophila tendon cells represent architectural elements different from stress fibers. Mol. Biol. Cell 19: 4287–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajzek C., Rice A. M., Andreazza S., Dushay M. S., 2012. Coagulation and survival in Drosophila melanogaster fondue mutants. J. Insect Physiol. 58: 1376–1381. [DOI] [PubMed] [Google Scholar]
- Brower D. L., Bunch T. A., Mukai L., Adamson T. E., Wehrli M., et al. , 1995. Nonequivalent requirements for PS1 and PS2 integrin at cell attachments in Drosophila: genetic analysis of the alpha PS1 integrin subunit. Development 121: 1311–1320. [DOI] [PubMed] [Google Scholar]
- Brown N. H., 1994. Null mutations in the alpha PS2 and beta PS integrin subunit genes have distinct phenotypes. Development 120: 1221–1231. [DOI] [PubMed] [Google Scholar]
- Bulgakova N. A., Klapholz B., Brown N. H., 2012. Cell adhesion in Drosophila: versatility of cadherin and integrin complexes during development. Curr. Opin. Cell Biol. 24: 702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunch T. A., Graner M. W., Fessler L. I., Fessler J. H., Schneider K. D., et al. , 1998. The PS2 integrin ligand tiggrin is required for proper muscle function in Drosophila. Development 125: 1679–1689. [DOI] [PubMed] [Google Scholar]
- Burmester T., Scheller K., 1999. Ligands and receptors: common theme in insect storage protein transport. Naturwissenschaften 86: 468–474. [DOI] [PubMed] [Google Scholar]
- Caveney S., 1969. Muscle attachment related to cuticle architecture in Apterygota. J. Cell Sci. 4: 541–559. [DOI] [PubMed] [Google Scholar]
- Chanana B., Graf R., Koledachkina T., Pflanz R., Vorbrüggen G., 2007. AlphaPS2 integrin-mediated muscle attachment in Drosophila requires the ECM protein thrombospondin. Mech. Dev. 124: 463–475. [DOI] [PubMed] [Google Scholar]
- Chandrasekar R., Muthu Meenaskhi P., Luiz Paulo A. M., 2009. Hexamerin storage proteins: biosynthesis, utilization, and evolution, pp. 49–68 in Short Views on Insect Molecular Biology. International Book Mission, Tamilnadu, South India. [Google Scholar]
- Clark K. A., Bland J. M., Beckerle M. C., 2007. The Drosophila muscle LIM protein, Mlp84B, cooperates with D-titin to maintain muscle structural integrity. J. Cell Sci. 120: 2066–2077. [DOI] [PubMed] [Google Scholar]
- Deutsch J., Laval M., Lepesant J. A., Maschat F., Pourrain F., et al. , 1989. Larval fat body-specific gene expression in D. melanogaster. Dev. Genet. 10: 220–231. [DOI] [PubMed] [Google Scholar]
- Domsch K., Ezzeddine N., Nguyen H. T., 2013. Abba is an essential TRIM/RBCC protein to maintain the integrity of sarcomeric cytoarchitecture. J. Cell Sci. 126: 3314–3323. [DOI] [PubMed] [Google Scholar]
- Dong B., Miao G., Hayashi S., 2014. A fat body-derived apical extracellular matrix enzyme is transported to the tracheal lumen and is required for tube morphogenesis in Drosophila. Development 141: 4104–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J. B., Schoeberl B., Nielsen U. B., Sorger P. K., 2006. Systems biology and combination therapy in the quest for clinical efficacy. Nat. Chem. Biol. 2: 458–466. [DOI] [PubMed] [Google Scholar]
- Fogerty F. J., Fessler L. I., Bunch T. A., Yaron Y., Parker C. G., et al. , 1994. Tiggrin, a novel Drosophila extracellular matrix protein that functions as a ligand for Drosophila alpha PS2 beta PS integrins. Development 120: 1747–1758. [DOI] [PubMed] [Google Scholar]
- Frantz C., Stewart K. M., Weaver V. M., 2010. The extracellular matrix at a glance. J. Cell Sci. 123: 4195–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich M. V., Schneider M., Timpl R., Baumgartner S., 2000. Perlecan domain V of Drosophila melanogaster. Sequence, recombinant analysis and tissue expression. Eur. J. Biochem. 267: 3149–3159. [DOI] [PubMed] [Google Scholar]
- Gotwals P. J., Fessler L. I., Wehrli M., Hynes R. O., 1994. Drosophila PS1 integrin is a laminin receptor and differs in ligand specificity from PS2. Proc. Natl. Acad. Sci. USA 91: 11447–11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graner M. W., Bunch T. A., Baumgartner S., Kerschen A., Brower D. L., 1998. Splice variants of the Drosophila PS2 integrins differentially interact with RGD-containing fragments of the extracellular proteins tiggrin, ten-m, and D-laminin 2. J. Biol. Chem. 273: 18235–18241. [DOI] [PubMed] [Google Scholar]
- Guedes S. M., Vitorino R., Tomer K., Domingues M. R., Correia A. J., et al. , 2003. Drosophila melanogaster larval hemolymph protein mapping. Biochem. Biophys. Res. Commun. 312: 545–554. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110: 673–687. [DOI] [PubMed] [Google Scholar]
- Karlsson C., Korayem A. M., Scherfer C., Loseva O., Dushay M. S., et al. , 2004. Proteomic analysis of the Drosophila larval hemolymph clot. J. Biol. Chem. 279: 52033–52041. [DOI] [PubMed] [Google Scholar]
- LaBeau-DiMenna E. M., Clark K. A., Bauman K. D., Parker D. S., Cripps R. M., et al. , 2012. Thin, a Trim32 ortholog, is essential for myofibril stability and is required for the integrity of the costamere in Drosophila. Proc. Natl. Acad. Sci. USA 109: 17983–17988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leptin M., Bogaert T., Lehmann R., Wilcox M., 1989. The function of PS integrins during Drosophila embryogenesis. Cell 56: 401–408. [DOI] [PubMed] [Google Scholar]
- Lesch C., Goto A., Lindgren M., Bidla G., Dushay M. S., et al. , 2007. A role for Hemolectin in coagulation and immunity in Drosophila melanogaster. Dev. Comp. Immunol. 31: 1255–1263. [DOI] [PubMed] [Google Scholar]
- Lindgren M., Riazi R., Lesch C., Wilhelmsson C., Theopold U., et al. , 2008. Fondue and transglutaminase in the Drosophila larval clot. J. Insect Physiol. 54: 586–592. [DOI] [PubMed] [Google Scholar]
- Maartens A. P., Brown N. H., 2015. The many faces of cell adhesion during Drosophila muscle development. Dev. Biol. 401: 62–74. [DOI] [PubMed] [Google Scholar]
- Markl J., Burmester T., Decker H., Savel-Niemann A., Harris J. R., et al. , 1992. Quaternary and subunit structure of Calliphora arylphorin as deduced from electron microscopy, electrophoresis, and sequence similarities with arthropod hemocyanin. J. Comp. Physiol. B 162: 665–680. [DOI] [PubMed] [Google Scholar]
- Martin D., Zusman S., Li X., Williams E. L., Khare N., et al. , 1999. wing blister, a new Drosophila laminin alpha chain required for cell adhesion and migration during embryonic and imaginal development. J. Cell Biol. 145: 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Bermudo M. D., Brown N. H., 1996. Intracellular signals direct integrin localization to sites of function in embryonic muscles. J. Cell Biol. 134: 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U., Saher G., Fässler R., Bornemann A., Echtermeyer F., et al. , 1997. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat. Genet. 17: 318–323. [DOI] [PubMed] [Google Scholar]
- McCloy R. A., Rogers S., Caldon C. E., Lorca T., Castro A., et al. , 2014. Partial inhibition of Cdk1 in G 2 phase overrides the SAC and decouples mitotic events. Cell Cycle 13: 1400–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin X., Daneman R., Zavortink M., Chia W., 2001. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc. Natl. Acad. Sci. USA 98: 15050–15055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugasu-Oei B., Rodrigues V., Yang X., Chia W., 1995. Masquerade: a novel secreted serine protease-like molecule is required for somatic muscle attachment in the Drosophila embryo. Genes Dev. 9: 139–154. [DOI] [PubMed] [Google Scholar]
- Pastor-Pareja J. C., Xu T., 2011. Shaping cells and organs in Drosophila by opposing roles of fat body-secreted Collagen IV and perlecan. Dev. Cell 21: 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop A., Martín-Bermudo M. D., Bate M., Brown N. H., 1998. Absence of PS integrins or laminin A affects extracellular adhesion, but not intracellular assembly, of hemiadherens and neuromuscular junctions in Drosophila embryos. Dev. Biol. 196: 58–76. [DOI] [PubMed] [Google Scholar]
- Qadota H., Benian G. M., 2010. Molecular structure of sarcomere-to-membrane attachment at M-Lines in C. elegans muscle. J. Biomed. Biotechnol. 2010: 864749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. B., Jowett T., Hughes J., Smith D. F., Glover D. M., 1991. The major serum protein of Drosophila larvae, larval serum protein 1, is dispensable. Eur. J. Biochem. 195: 195–201. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., 1996. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 12: 697–715. [DOI] [PubMed] [Google Scholar]
- Schejter E. D., Baylies M. K., 2010. Born to run: creating the muscle fiber. Curr. Opin. Cell Biol. 22: 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherfer C., Karlsson C., Loseva O., Bidla G., Goto A., et al. , 2004. Isolation and characterization of hemolymph clotting factors in Drosophila melanogaster by a pullout method. Curr. Biol. 14: 625–629. [DOI] [PubMed] [Google Scholar]
- Scherfer C., Qazi M. R., Takahashi K., Ueda R., Dushay M. S., et al. , 2006. The Toll immune-regulated Drosophila protein Fondue is involved in hemolymph clotting and puparium formation. Dev. Biol. 295: 156–163. [DOI] [PubMed] [Google Scholar]
- Schnorrer F., Dickson B. J., 2004. Muscle building; mechanisms of myotube guidance and attachment site selection. Dev. Cell 7: 9–20. [DOI] [PubMed] [Google Scholar]
- Schweitzer R., Zelzer E., Volk T., 2010. Connecting muscles to tendons: tendons and musculoskeletal development in flies and vertebrates. Development 137: 2807–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A., Wayburn B., Bunch T., Volk T., 2007. Thrombospondin-mediated adhesion is essential for the formation of the myotendinous junction in Drosophila. Development 134: 1269–1278. [DOI] [PubMed] [Google Scholar]
- Umemiya T., Takeichi M., Nose A., 1997. M-spondin, a novel ECM protein highly homologous to vertebrate F-spondin, is localized at the muscle attachment sites in the Drosophila embryo. Dev. Biol. 186: 165–176. [DOI] [PubMed] [Google Scholar]
- Vierstraete E., Cerstiaens A., Baggerman G., Van den Bergh G., De Loof A., et al. , 2003. Proteomics in Drosophila melanogaster: first 2D database of larval hemolymph proteins. Biochem. Biophys. Res. Commun. 304: 831–838. [DOI] [PubMed] [Google Scholar]
- Volk T., Fessler L. I., Fessler J. H., 1990. A role for integrin in the formation of sarcomeric cytoarchitecture. Cell 63: 525–536. [DOI] [PubMed] [Google Scholar]
- Wright T. R., 1960. The phenogenetics of the embryonic mutant, lethal myospheroid, in Drosophila melanogaster. J. Exp. Zool. 143: 77–99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.