Abstract
Key steps of essential metabolic pathways are housed in plant peroxisomes. We conducted a microscopy-based screen for anomalous distribution of peroxisomally targeted fluorescence in Arabidopsis thaliana. This screen uncovered 34 novel alleles in 15 genes affecting oil body mobilization, fatty acid β-oxidation, the glyoxylate cycle, peroxisome fission, and pexophagy. Partial loss-of-function of lipid-mobilization enzymes conferred peroxisomes clustered around retained oil bodies without other notable defects, suggesting that this microscopy-based approach was sensitive to minor perturbations, and that fatty acid β-oxidation rates in wild type are higher than required for normal growth. We recovered three mutants defective in PECTIN METHYLESTERASE31, revealing an unanticipated role in lipid mobilization for this cytosolic enzyme. Whereas mutations reducing fatty acid import had peroxisomes of wild-type size, mutations impairing fatty acid β-oxidation displayed enlarged peroxisomes, possibly caused by excess fatty acid β-oxidation intermediates in the peroxisome. Several fatty acid β-oxidation mutants also displayed defects in peroxisomal matrix protein import. Impairing fatty acid import reduced the large size of peroxisomes in a mutant defective in the PEROXISOMAL NAD+ TRANSPORTER (PXN), supporting the hypothesis that fatty acid accumulation causes pxn peroxisome enlargement. The diverse mutants isolated in this screen will aid future investigations of the roles of β-oxidation and peroxisomal cofactor homeostasis in plant development.
Keywords: peroxisome, lipid mobilization, fatty acid β-oxidation, PXN, PME31
PEROXISOMES are dynamic single lipid bilayer-bound organelles present in most eukaryotes that compartmentalize crucial steps of many metabolic pathways (Gabaldon 2010). Plant peroxisomes house β-oxidation and key steps of photorespiration, nitrogen metabolism, and plant hormone biosynthesis (reviewed in Hu et al. 2012).
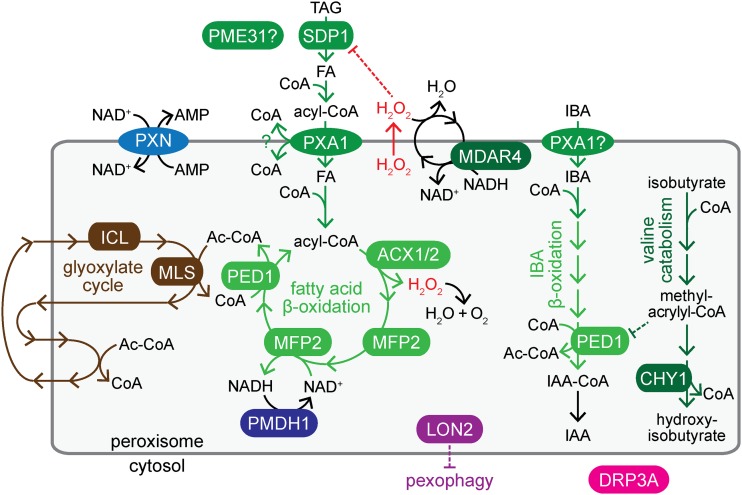
One conserved function of peroxisomes is fatty acid β-oxidation (Figure 1), which is exclusively peroxisomal in plants. Oilseed plants, including Arabidopsis thaliana, obtain energy for postgerminative development by β-oxidizing fats stored as triacylglycerol (TAG) in oil bodies (reviewed in Chapman et al. 2012). The lipase SUGAR-DEPENDENT1 (SDP1) hydrolyzes oil body TAG (Figure 1; Eastmond 2006). The resultant fatty acids are esterified with CoA to enter peroxisomes through PEROXISOMAL ABC TRANSPORTER1 (PXA1; Figure 1; Zolman et al. 2001b), also known as COMATOSE (Footitt et al. 2002) or PEROXISOME DEFECTIVE3 (Hayashi et al. 2002), which cleaves the CoA moiety upon transport (De Marcos Lousa et al. 2013). Inside peroxisomes, fatty acids are reactivated by LONG CHAIN ACYL-COA SYNTHETASE (LACS) 6 and 7, which add the CoA moiety necessary for entry into β-oxidation (Fulda et al. 2002). The four-step β-oxidation cycle starts with oxidation by one of five ACYL-COA OXIDASE (ACX) isozymes with overlapping specificities (Eastmond et al. 2000b; Adham et al. 2005). The next two steps are performed by the partially redundant MULTIFUNCTIONAL PROTEIN2 (MFP2) and ABNORMAL INFLORESCENCE MERISTEM1 (AIM1), which both have 2-trans-enoyl-CoA hydratase and l-3-hydroxyacyl-CoA dehydrogenase activities (Richmond and Bleecker 1999; Rylott et al. 2006). Finally, a 3-ketoacyl-CoA thiolase such as PEROXISOME DEFECTIVE1 (PED1) releases acetyl-CoA and a shortened fatty acyl-CoA, which can undergo further rounds of β-oxidation (Figure 1; Hayashi et al. 1998; Germain et al. 2001). The glyoxylate cycle processes the liberated acetyl-CoA for the eventual production of sugars (reviewed in Graham 2008). ISOCITRATE LYASE (ICL) and MALATE SYNTHASE (MLS) catalyze peroxisomal steps of the glyoxylate cycle (Eastmond et al. 2000a; Cornah et al. 2004). Fatty acid β-oxidation defects ensue when the participating enzymes are dysfunctional, and also when valine catabolism is disrupted. β-HYDROXYISOBUTYRYL-COA HYDROLASE1 (CHY1) catalyzes a late step in valine catabolism (Figure 1; Zolman et al. 2001a). CHY1 impairment leads to accumulation of a toxic intermediate, methacrylyl-CoA, which inactivates the PED1 thiolase (Zolman et al. 2001a; Lange et al. 2004). Mutants with fatty acid β-oxidation defects inefficiently mobilize energy stores, resulting in growth defects that can be partially alleviated by providing an external carbon source such as sucrose (Hayashi et al. 1998; Zolman et al. 2000). These β-oxidation mutants also display additional defects that have not been completely explained (Rylott et al. 2003; Pinfield-Wells et al. 2005; Rylott et al. 2006; Footitt et al. 2007; Khan et al. 2012).
Figure 1.
Peroxisomes house steps of key metabolic pathways; enzymes and transporters disrupted in mutants identified in this work are shown. Conversion of oil body triacylglycerol (TAG) to fatty acids (FA) requires SDP1. Fatty acids are activated with CoA in the cytosol and imported into peroxisomes by PXA1, which hydrolyzes the CoA upon transport. In the peroxisome, fatty acids are reactivated and catabolized to acetyl-CoA through β-oxidation (green), which requires ACX1/2, MFP2, and PED1 enzymes. ACX enzymes generate H2O2, which is detoxified by catalase (not shown), or by an MDAR4-dependent ascorbate peroxidase that inactivates H2O2 escaping from the peroxisome. CHY1 acts in valine catabolism downstream of methacrylyl-CoA, which can inactivate PED1. PME31 might aid in lipid mobilization. PXA1 is also implicated in peroxisomal import of IBA, and IBA-to-IAA β-oxidation likely involves dedicated enzymes (not shown) and PED1, which also acts in fatty acid β-oxidation. Carbon from β-oxidation-derived acetyl-CoA is converted to forms that can be shuttled out of the peroxisome via the glyoxylate cycle (brown), which includes ICL and MLS enzymes. PXN imports NAD+, which is recycled by PMDH1/2; CoA is recycled in the glyoxylate cycle. LON2 is an ATP-dependent protease that restrains pexophagy. DRP3A is a peroxisome division factor.
NAD+ and CoA are key cofactors for β-oxidation (Figure 1). The peroxisomal membrane is permeable to small molecules up to 300–400 Da, but excludes bulkier cofactors such as ATP, NAD+, and CoA (Antonenkov and Hiltunen 2012). PEROXISOMAL MALATE DEHYDROGENASE (PMDH) 1 and 2 recycle NADH to NAD+ inside peroxisomes (Pracharoenwattana et al. 2007), and the glyoxylate cycle recycles CoA (reviewed in Graham 2008). Arabidopsis PEROXISOMAL NAD+ CARRIER (PXN), also known as ABERRANT PEROXISOME MORPHOLOGY3 (Mano et al. 2011), transports NAD+ (Bernhardt et al. 2012) and CoA (Agrimi et al. 2012) in vitro, and NAD+ when expressed in yeast (van Roermund et al. 2016). Defects in PXN lead to minor lipid mobilization defects (Bernhardt et al. 2012) and enlarged peroxisomes (Mano et al. 2011). It is not understood why peroxisomes are enlarged when the PXN transporter is dysfunctional.
The auxin indole-3-acetic acid (IAA) is a vital plant hormone that regulates a plethora of developmental processes (reviewed in Woodward and Bartel 2005; Enders and Strader 2015). IAA can be obtained from the peroxisomal β-oxidation of the precursor indole-3-butyric acid (IBA; Figure 1; Strader and Bartel 2011). Similarly, a synthetic IBA analog, 2,4-dichlorophenoxybutyric acid (2,4-DB), is β-oxidized to the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D; Wain and Wightman 1954; Hayashi et al. 1998). PXA1 is implicated in IBA transport into peroxisomes (Zolman et al. 2001b), and IBA-to-IAA conversion requires a set of β-oxidation enzymes that is partially distinct from those used in fatty acid β-oxidation (Zolman et al. 2007, 2008; Strader et al. 2011).
Peroxisomal matrix proteins are synthesized in the cytosol and imported post-translationally via a peroxisome targeting signal (PTS). The PTS1 is a C-terminal tripeptide (Gould et al. 1989), whereas PTS2 is a nonapeptide in the N-terminal region (Swinkels et al. 1991) that is removed inside the peroxisome (Helm et al. 2007). Mislocalization of peroxisomally targeted proteins to the cytosol can be observed when genes important for peroxisome formation are disabled (Mano et al. 2006; Ramón and Bartel 2010; Goto et al. 2011; Monroe-Augustus et al. 2011; Farmer et al. 2013; Burkhart et al. 2014; Goto-Yamada et al. 2014; Woodward et al. 2014). Mature peroxisomes can divide by fission, and defects in this process can lead to fewer peroxisomes, larger peroxisomes, clustered peroxisomes, increased peroxisomal prolongations called peroxules, or a combination of these phenotypes (Mano et al. 2004; Zhang and Hu 2008; Aung and Hu 2009; Fujimoto et al. 2009; Zhang and Hu, 2009, 2010).
To further our understanding of peroxisomal processes, we performed a microscopy-based forward-genetic screen for altered green fluorescent protein targeted to the peroxisome (GFP-PTS1) distribution in Arabidopsis seedlings. We identified 34 novel mutations in 15 genes involved in a variety of peroxisomal processes, including one gene, PECTIN METHYLESTERASE31 (PME31), not previously implicated in peroxisome function. Most of the mutants displayed clustered peroxisomes around retained oil bodies and disrupted proteins involved in fatty acid utilization. Analyses of these mutants contributed to the understanding of cofactor balance inside peroxisomes and uncovered unexpected peroxisomal defects, such as peroxisomal matrix protein import defects, in several fatty acid β-oxidation mutants.
Materials and Methods
Plant materials
Wild type and mutants were in the Columbia-0 (Col-0) accession. The line expressing GFP-PTS1 driven by the constitutive cauliflower mosaic virus 35S promoter (35S:GFP-PTS1) was described previously (Zolman and Bartel 2004). Some reference mutants were from the Arabidopsis Biological Resource Center (ABRC) at Ohio State University: drp3a-3 (SALK_066958), mdar4-4 (SALK_068667), mls-3 (SALK_002289), and pxn-3 (SAIL_636F12). Other reference mutants were described previously: acx1-2 (SALK_041464; Adham et al. 2005), acx2-1 (SALK_006486; Adham et al. 2005), acx1-2 acx2-1 (Adham et al. 2005), chy1-1 (Zolman et al. 2001a), icl-1 (Eastmond et al. 2000a), lon2-2 (SALK_043857; Lingard and Bartel 2009), pmdh1-1 (Pracharoenwattana et al. 2007), pmdh1-1 pmdh2-1 (Pracharoenwattana et al. 2007), pxa1-1 (Zolman et al. 2001b), ped1-96 (Lingard and Bartel 2009), pex14-1 (Monroe-Augustus et al. 2011), and lon2-2, atg7-3, and lon2-2 atg7-6 expressing 35S:PTS2-GFP (Farmer et al. 2013). We crossed our 35S:GFP-PTS1 line to pxa1-1 and ped1-96, and obtained pxa1-1 35S:GFP-PTS1 and ped1-96 35S:GFP-PTS1, respectively, by following the construct and mutation in progeny by using fluorescence and PCR-based genotyping (Supplemental Material, Table S1).
For newly identified mutants, assays were conducted with twice-backcrossed lines (pxn-4 and mfp2-6), once-backcrossed lines (sdp1-7, mdar4-5, mdar4-6/R541, mdar4-7, pmdh1-2, acx1-4, acx2-3, mfp2-7, mfp2-8, mfp2-9, chy1-5, chy1-6, mls-4, lon2-8, lon2-10, pxn-4, drp3a-4, drp3a-5, and pme31-1), or progeny from the original mutants (mdar4-6/R577, pxa1-4, acx2-2, ped1-4, ped1-5, chy1-7, icl-3, icl-4, icl-5, pxn-4 lon2-8, lon2-9, pxn-5, pxn-6, pxn-7, and pme31-3). For pme31-2, a backcrossed line that did not harbor the background mutations in ACX2 and IBA RESPONSE3 found through whole-genome sequencing was used for assays.
Growth conditions and phenotypic assays
Unless otherwise noted, surface-sterilized seeds were stratified and plated on plant nutrient medium (Haughn and Somerville 1986) solidified with 0.6% (w/v) agar and supplemented with 0.5% (w/v) sucrose in plates sealed with gas-permeable tape in growth chambers at 22° under continuous white fluorescent light. When treating with hormones dissolved in ethanol, control medium was normalized to the same ethanol content, and light was filtered through yellow long-pass filters to slow indolic compound breakdown (Stasinopoulos and Hangarter 1990). For hypocotyl elongation assays, plates were incubated under light for 1 day and then subjected to darkness by covering with two layers of aluminum foil. Root measurements of light-grown seedlings were performed at 8 days, and hypocotyl measurements of dark-grown seedlings were performed at 5 days. To assess lateral root production, seeds were sown on media and incubated for 4 days, then transferred to either media supplemented with hormones or media with no hormone for an additional 4 days. The number of lateral roots that protruded from the root epidermis was counted, root length was measured, and a ratio of these values was calculated.
Mutant isolation and identification
Wild-type (Col-0) 35S:GFP-PTS1 seeds were mutagenized with 0.24% ethyl methanesulfonate (EMS; Normanly 1997) for 16 hr. For screening, ∼5000 M2 seeds from each pool of 625 M1 plants were placed in ∼12 rows on five or six 100 × 100 mm square plates, and the plates were incubated horizontally for 1 day under constant white light before standing the plates nearly vertically. After 5 days, seedlings on plates were examined using a dissecting microscope equipped for GFP detection. We screened hypocotyls of ∼160,000 M2 seedlings from 32 M1 pools for fluorescence distribution patterns that differed from wild-type puncta. Putative mutants were numbered, and given the prefix “R.” Putative mutants were moved to soil, and 680 of 1020 produced progeny. We assayed for peroxisomal defects on the M3 and M4 progeny of these isolates by retesting GFP-PTS1 distribution using the dissecting microscope and testing for sucrose-dependent growth and IBA and 2,4-DB resistance. Promising lines were further tested for PTS2 processing using immunoblotting and for GFP-PTS1 localization using confocal microscopy.
Several mutants were backcrossed to Col-0 or Col-0 carrying GFP-PTS1 and/or outcrossed to the A. thaliana Landsberg erecta (Ler) accession. F2 individuals from segregating populations were selected for either abnormal fluorescence or the strongest peroxisomal phenotype observed during the secondary screen (e.g., IBA resistance and sucrose dependence). F3 progeny from F2 individuals displaying the phenotype of interest were tested for homogeneity of the selection phenotype as a proxy for homozygosity of the causal mutation.
Analyzing fluorescence in outcrossed populations revealed that the construct in our 35S:GFP-PTS1 line (Zolman and Bartel 2004) was located at the top of chromosome 5, likely between genes At5g13000 and At5g19000. Causal mutations located in this region were identified through whole-genome sequencing (Thole and Strader 2015).
DNA analysis
DNA was prepared for PCR analysis as described (Celenza et al. 1995). For recombination mapping, progeny from mutant outcrosses to Ler were genotyped using PCR-based markers that exploit polymorphisms to differentiate between Col-0 and Ler DNA (Table S2). Overlapping PCR amplicons covering candidate genes of interest (Table S3) were sequenced by Lone Star Labs (Houston, TX). Nucleotide positions are reported relative to the first nucleotide in the first codon of the annotated gene.
Genomic DNA for whole-genome sequencing was prepared as previously described (Thole et al. 2014). DNA was sequenced with Illumina HiSequation 2000 sequencers by the Genome Technology Access Center at Washington University in St. Louis. Sequences were aligned with the Arabidopsis Col-0 genome from The Arabidopsis Information Resource (TAIR build 10) using Novoalign (Novocraft; http://novocraft.com), and mutations were identified using SAMtools (Li et al. 2009) and snpEFF (Cingolani et al. 2012). Mutations were filtered to obtain homozygous G-to-A or C-to-T transitions produced by EMS that resulted in nonsynonymous amino acid changes or altered splice sites in coding regions that are absent in our lab Col-0 line as described (Farmer et al. 2013).
Immunoblotting
Frozen tissue was homogenized in two volumes of 2× NuPAGE sample buffer (Invitrogen, Carlsbad, CA) and centrifuged at 16,100 × g for 5 min. Supernatants were heated at 100° for 5 min with 50 mM dithiothreitol. Samples were electrophoresed alongside prestained protein markers (P7708; New England Biolabs, Beverly, MA) and Cruz molecular mass markers (sc-2035; Santa Cruz Biotechnology, Santa Cruz, CA) on NuPAGE or Bolt 10% Bis-Tris gels (Invitrogen) using 1× MOPS running buffer [50 mM 3-(N-morpholino)propanesulfonic acid, 50 mM Tris base, 0.1% sodium dodecyl sulfate, 1 mM EDTA] and transferred to Hybond Nitrocellulose membrane (Amersham Pharmacia Biotech) using NuPAGE transfer buffer (Invitrogen). Membranes were incubated for 1 hr at 4° in blocking solution (8% nonfat dry milk, 20 mM Tris pH 7.5, 150 mM NaCl, 0.1% Tween 20) and then incubated overnight at 4° with rabbit antibodies against PMDH2 (1:2000; Pracharoenwattana et al. 2007), thiolase (1:5000; Lingard et al. 2009), ICL (1:1000; Maeshima et al. 1988), or MLS (1:10,000; Olsen et al. 1993), or mouse antibodies against HSC70 (1:50,000; Stressgen SPA-817), diluted as indicated in blocking solution with 0.1% sodium azide. Membranes were incubated for 4 hr with secondary horseradish peroxidase-linked goat antibodies against rabbit or mouse IgG (1:5000; Santa Cruz Biotechnology; sc-2030 and sc-2031, respectively) diluted in blocking solution. Horseradish peroxidase was visualized using WesternBright ECL (Advansta) and exposing to autoradiography film. Films were imaged using a scanner. Membranes were incubated sequentially with different primary antibodies without stripping.
Microscopy
A Leica ZM10 F dissecting microscope equipped with filters for GFP detection was used to screen and retest hypocotyl GFP-PTS1 patterns at low (80×) magnification in 5-day-old light-grown seedlings.
For confocal microscopy, cotyledons from light-grown seedlings were excised and mounted in water. For Nile red staining, cotyledons were submerged in 5 µg/ml Nile red for at least 5 min. A Carl Zeiss LSM 710 laser scanning confocal microscope equipped with a 63× oil immersion objective and a Meta detector was used to image fluorescence. Tissue was excited with a 488-nm argon laser, and emission was collected between 493 and 526 nm for GFP, between 587 and 643 nm for Nile red, and between 688 and 757 nm for chlorophyll. Each image is an average of four exposures using a 24-µm pinhole, corresponding to a 1-µm optical slice. Epidermal cells were sometimes imaged at two points: midcell, where cytosolic fluorescence outlines the cell, and beneath the plasma membrane (subcortical), where cytosolic fluorescence has a diffuse pattern.
To estimate peroxisome size, fluorescent puncta in the GFP channel with cross-sectional areas > 1 µm2 were measured using the “Analyze Particles” tool of ImageJ (http://imagej.nih.gov/ij/) from three 135 × 135 µm images. For each time point, all puncta detected in three images were plotted for the line (wild type or pxn) with the smaller number of puncta detected, and the same number of puncta (from one to three images; gathered by position in the image rather than size) were plotted for the other sample.
Data and reagent availability
Strains are available upon request. Sequence data can be found in the Arabidopsis Genome Initiative or GenBank/European Molecular Biology Laboratory databases using accession numbers in Table 1 and Table S4. Table S1 lists PCR-based markers used to genotype mutants and constructs. Table S2 lists PCR-based markers used for recombination mapping. Table S3 lists primers used to amplify and sequence candidate genes. Table S4 lists accession numbers of proteins used for alignments. Table S5 lists whole-genome sequencing strategies and results. Table S6 lists PCR-based genotyping markers used for recombination mapping in the backcross for R79 (pme31-1) and R363 (pme31-2). Table S7 summarizes the key phenotypes of the identified mutants. File S1 lists mutations identified through whole-genome sequencing.
Table 1. Mutants identified in a screen for altered peroxisomally-targeted fluorescence.
| Gene (accession number) | Function of protein | Allele | Alias | Nucleotide change | Protein or transcript change | Phenotypes vs. reference allele |
|---|---|---|---|---|---|---|
| Fatty acid β-oxidation | ||||||
| SDP1 (At5g04040) | Triacylglycerol lipase | sdp1-7 | R330 | c1075t | Conserved R359W | Weakera |
| PXA1 (At4g39850) | ATP binding cassette transporter | pxa1-4 | R751 | g1585a | G307E in EAA-like motif | Similar |
| ACX1 (At4g16760) | Acyl-CoA oxidase | acx1-4 | R574 | g1146a | Conserved G226R | Similar |
| ACX2 (At5g65110) | Acyl-CoA oxidase | acx2-2 | R233 | g603a | W154stop | Similar |
| acx2-3 | R737 | g604a | Conserved G155R | Similar | ||
| MFP2 (At3g06860) | 2-trans-Enoyl-CoA hydratase and l-3-hydroxyacyl-CoA dehydrogenase | mfp2-6 | R281 | g3737a | Conserved G670R | Similara |
| mfp2-7 | R778 | g2736a | Conserved A454T | Weakera | ||
| mfp2-8 | R794/809 | g2051a | Conserved G320D | Similara | ||
| mfp2-9 | R1009 | g2116a | Conserved E342K | Similara | ||
| PED1 (At2g33150) | 3-Ketoacyl-CoA thiolase | ped1-4 | R814 | g928a | Conserved G109E | Weaker |
| ped1-5 | R883 | g1113a | Conserved G141E | Similar | ||
| Indirect fatty acid β-oxidation and glyoxylate cycle | ||||||
| MLS (At5g03860) | Malate synthase (glyoxylate cycle) | mls-4 | R332 | g1595a | Intron 3 splice acceptor | Similar |
| ICL (At3g21720) | Isocitrate lyase (glyoxylate cycle) | icl-3 | R291 | g177a | Conserved E21K | Weaker |
| icl-4 | R601 | g366a | Conserved G84R | Weaker | ||
| icl-5 | R951 | g2128a | W371stop | Similar | ||
| PMDH1 (At2g22780) | Peroxisomal malate dehydrogenase (fatty acid β-oxidation) | pmdh1-2 | R92 | c643t | Q129stop | Similar |
| CHY1 (At5g65940) | β-Hydroxyisobutyryl-CoA hydrolase (valine catabolism) | chy1-5 | R189 | g700a | Conserved G119D | Similar |
| chy1-6 | R499/506 | g871a | Conserved D150N | Similar | ||
| chy1-7 | R728 | g988a | Conserved G165E | Similar | ||
| MDAR4 (At3g27820) | Peroxisomal monodehydroascorbate reductase (hydrogen peroxide detoxification) | mdar4-5 | R340/343 | g942a | Conserved G122D | Similar |
| mdar4-6 | R541/577 | g31a | Conserved G11R | Similar | ||
| mdar4-7 | R923 | g659a | Intron 2 splice acceptor | Similar | ||
| Large GFP-PTS1 puncta | ||||||
| LON2 (At5g47040) | Peroxisomal protease | lon2-8 | R109 | g650a | Q102stop | Similar |
| lon2-9 | R498 | g3610a | Conserved R537K in arginine finger of AAA+ domain | Weaker | ||
| lon2-10 | R973 | g400a | W45stop | Similar | ||
| PXN (At2g39970) | Peroxisomal NAD+ and CoA transporter | pxn-4 | R109 | g2194a | Intron 9 splice acceptor | Similar |
| pxn-5 | R162 | c2060t | R258stop | Similar | ||
| pxn-6 | R986 | g1629a | Intron 6 splice acceptor | Similar | ||
| pxn-7 | R987/995 | g2111a | Conserved G275E | Similar | ||
| Miscellanea | ||||||
| DRP3A (At4g33650) | Dynamin-related protein (peroxisome division) | drp3a-4 | R224 | c755t | Q224stop | Similar |
| drp3a-5 | R402 | g281a | Conserved R94H | Similar | ||
| PME31 (At3g29090) | Pectin methylesterase | pme31-1 | R79 | g701c | Conserved G141D | Not applicable |
| pme31-2 | R363 | g182a | Conserved G61E | Not applicable | ||
| pme31-3 | R922 | g1023a | Conserved G201D | Not applicable | ||
Comparison to description of previously published alleles.
Results
GFP-PTS1 localizes to distinct puncta in wild-type plant cells (Figure 2). To obtain new mutations in genes important for peroxisomal functions, we mutagenized a line expressing GFP-PTS1 and screened 5-day-old seedling hypocotyls for fluorescence distributions that differed from wild type. We examined the progeny of these mutants for additional peroxisome-related defects and identified the causal mutations in 39 mutant lines, revealing 34 novel alleles in 15 genes (Table 1).
Figure 2.
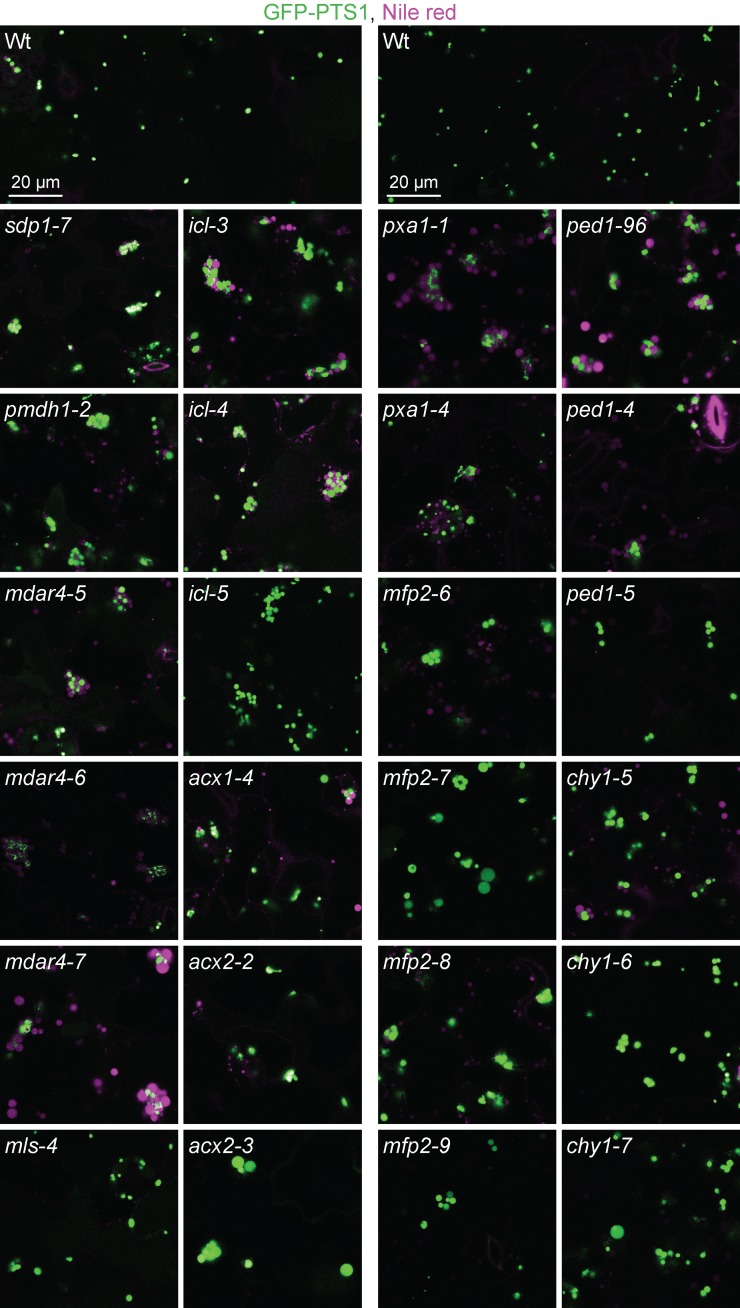
Mutants impaired in lipid mobilization display peroxisomes clustered around retained oil bodies. Confocal micrographs were taken of cotyledon epidermal cells of 4-day-old seedlings expressing peroxisomally targeted fluorescence (GFP-PTS1; green) and stained with Nile red for oil body visualization (magenta). Data are representative of three replicates. Bar, 20 µm.
Mutations in DRP3A, which encodes a peroxisome division protein
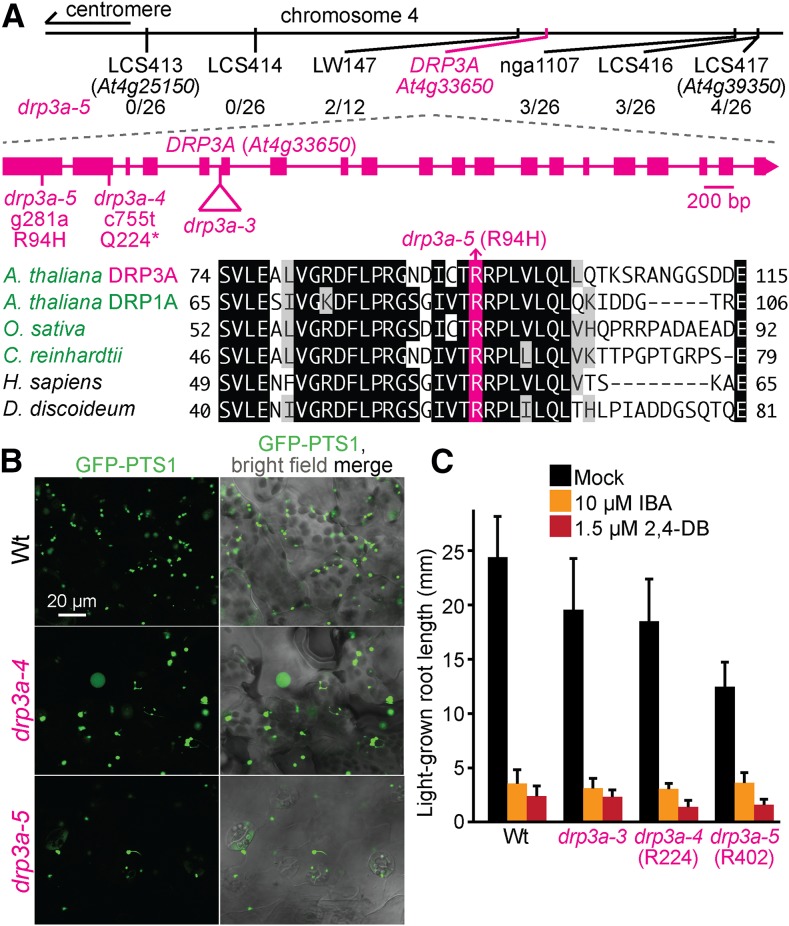
In Arabidopsis, three DYNAMIN-RELATED PROTEIN (DRP) GTPases are implicated in peroxisome division: DRP3A (Mano et al. 2004), DRP3B (Zhang and Hu 2009), and DRP5B (Zhang and Hu 2010). We recovered two DRP3A mutants, drp3a-4 (R224) and drp3a-5 (R402), that displayed enlarged peroxisomes and peroxules (Figure 3B). Sequencing DRP3A revealed a nonsense mutation at Gln224 in drp3a-4 (Figure 3A). Recombination mapping of drp3a-5 indicated linkage at chromosome 4 (Figure 3A), and whole-genome sequencing (Figure 4M, Table S5, and File S1) revealed a DRP3A mutation that changed Arg94 to His (Figure 3A). Arg94 is located in the GTPase domain that is conserved in Arabidopsis DRPs (Mano et al. 2004), plant DRP3 proteins (Hamada et al. 2006), Saccharomyces cerevisiae Dnm1 (Zhang and Hu 2009), and in close homologs in other organisms (Figure 3A). Previously described mutants in DRP3A have enlarged and elongated peroxisomes with numerous peroxules, resulting in a beads-on-a-string pattern (Mano et al. 2004; Zhang and Hu 2008; Aung and Hu 2009; Fujimoto et al. 2009). Our drp3a-4 and drp3a-5 mutants presented enlarged peroxisomes and some peroxules (Figure 3B), but did not show dramatic peroxule overabundance in our growth conditions or examined tissues. drp3a-4 and drp3a-5 lacked peroxisome-related physiological defects in IBA or 2,4-DB responsiveness (Figure 3C), similar to previously characterized drp3a mutants (Mano et al. 2004; Zhang and Hu 2008; Aung and Hu 2009; Fujimoto et al. 2009).
Figure 3.
Mutations in DRP3A confer enlarged peroxisomes. (A) Chromosome map indicates the positions of markers used to map drp3a-5 and ratios of recombinants to the number of chromosomes assessed. A gene diagram indicates the position of drp3a mutations. DRP3A was aligned with related proteins (Table S4); residues are shaded when identical (black or pink) or chemically similar (gray) in at least four sequences. (B) Confocal micrographs were taken of cotyledon epidermal cells of 8-day-old seedlings expressing peroxisomally targeted fluorescence (GFP-PTS1; green). (C) Root lengths of 8-day-old seedlings were measured. Error bars show SD (n = 20). Data (B, C) are representative of three replicates. Bar, 20 µm.
Figure 4.
Mutations identified through whole-genome sequencing in the isolated mutants. Chromosome maps (A–M) indicate the positions and gene identifier numbers of genes with homozygous EMS-consistent changes in splice sites or nonsynonymous amino acid changes in coding regions. Chromosomes that are not shown lacked such mutations. Presumptive causal mutations are denoted in color. Results for mdar4-6 (R541) are not shown because only a single homozygous EMS-consistent mutation (mdar4-6) was found (File S1).
Numerous lipid mobilization mutants show peroxisome clustering around oil bodies
Unlike wild-type seedlings, which display evenly distributed peroxisomes, most of our mutants displayed clustered peroxisomes (Figure 2). Cloning the defective genes revealed that these mutants were directly or indirectly disrupted in fatty acid β-oxidation.
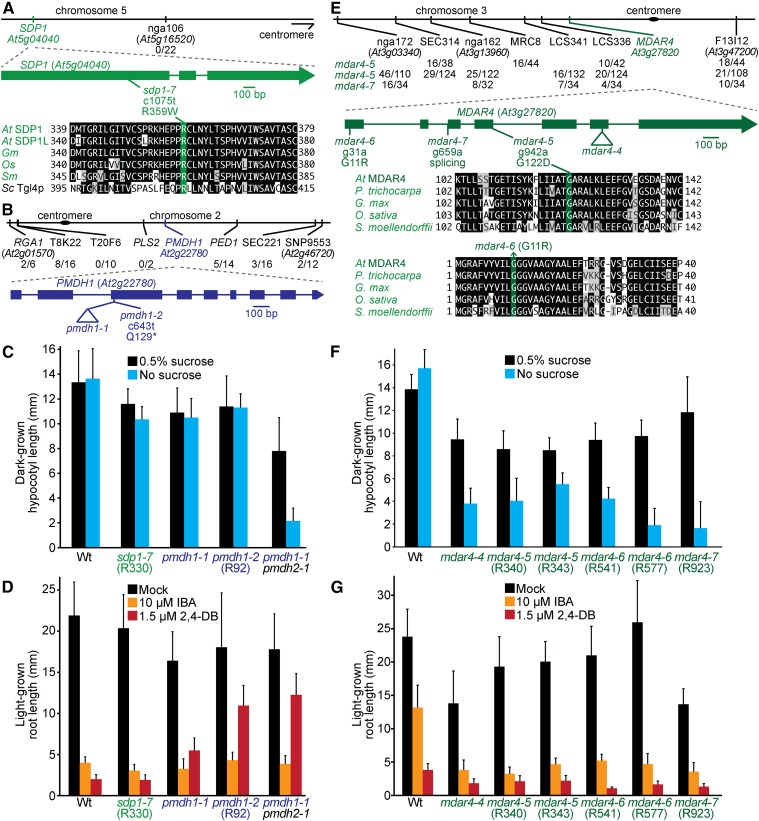
SDP1 is the primary TAG lipase in Arabidopsis seedlings (Figure 1; Eastmond 2006). Recombination mapping of the R330 mutant indicated linkage at the top of chromosome 5 (Figure 5A), and whole-genome sequencing (Figure 4A, Table S5, and File S1) revealed a mutation in SDP1 that changed Arg359 to Trp (sdp1-7). Arg359 is conserved in other plants and the yeast TAG lipase Tgl4p (Figure 5A). In addition to having clustered peroxisomes (Figure 2), sdp1-7 was sensitive to IBA and 2,4-DB (Figure 5D) and retained oil bodies longer than wild type (Figure 2), similar to previously isolated sdp1 mutants (Eastmond 2006). However, sdp1-7 was sucrose independent (Figure 5C), unlike previously isolated sdp1 mutants, which were isolated based on sucrose-dependent growth (Eastmond 2006), suggesting that sdp1-7 is a partial loss-of-function allele.
Figure 5.
A mutation in SDP1 that does not cause physiological defects, mutations in MDAR4 confer sucrose dependence, and mutations in PMDH1 confer 2,4-DB resistance. (A, B) Chromosome maps indicate the positions of markers used to map sdp1-7 (A) and pmdh1-2 (B) mutants and ratios of recombinants to the number of chromosomes assessed. Gene diagrams indicate the positions of the sdp1-7 (A) and pmdh1 (B) mutations. SDP1 was aligned (A) with related proteins (Table S4); residues are shaded when identical (black or green) or chemically similar (gray) in at least four sequences. (C) Hypocotyl lengths of 5-day-old dark-grown seedlings were measured. Error bars show SD (n = 20). (D) Root lengths of 8-day-old light-grown seedlings were measured. Error bars show SD (n = 20). (E) Chromosome map indicates the positions of markers used to map mdar4 mutations and ratios of recombinants to the number of chromosomes assessed. A gene diagram indicates the positions of mdar4 mutations. MDAR4 was aligned with related proteins (Table S4); residues are shaded when identical (black or green) or chemically similar (gray) in at least three sequences. (F) Hypocotyl lengths of 5-day-old dark-grown seedlings were measured. Error bars show SD (n = 20). (G) Root lengths of 8-day-old light-grown seedlings were measured. Error bars show SD (n = 20). Data are representative of two (C, F) or three (D, G) replicates.
Another protein important for oil body mobilization is MONODEHYDROASCORBATE REDUCTASE4 (MDAR4), also known as SDP2, which is part of the membrane-associated ascorbate-dependent electron transfer system that helps detoxify H2O2 at the peroxisome surface (Figure 1; Eastmond 2007). Mutations in MDAR4 lead to increased H2O2 levels, decreased SDP1 activity, and sucrose-dependent growth, perhaps because H2O2 escapes the peroxisome and inactivates nearby SDP1 (Eastmond 2007). We isolated three novel mdar4 alleles: mdar4-5 (R340 and R343), mdar4-6 (R541 and R577), and mdar4-7 (R923). Recombination mapping of mdar4-5 and mdar4-7 indicated linkage on chromosome 3 (Figure 5E); sequencing MDAR4 revealed a mutation that changed the conserved Gly122 to Asp in mdar4-5 and a mutation in the splice acceptor site of the second intron in mdar4-7 (Figure 5E). Whole-genome sequencing of mdar4-6 (Figure 4B, Table S5, and File S1) revealed an MDAR4 mutation that changed conserved Gly11 to Arg (Figure 5E). The mdar4-6 mutation is in the same codon as the sdp2-2 mutation that changes Gly11 to Glu (Eastmond 2007). The mdar4 mutants had clustered peroxisomes around retained oil bodies (Figure 2) and were sucrose dependent (Figure 5F), similar to the previously described mdar4-4 and other mdar4 mutants (Eastmond 2007). Additionally, mdar4 mutants had wild-type IBA and 2,4-DB responsiveness (Figure 5G).
PMDH1 is one of two partially redundant enzymes and accounts for most of the peroxisomal recycling of NADH from fatty acid β-oxidation to NAD+ (Figure 1; Pracharoenwattana et al. 2007). Recombination mapping of the R92 mutant revealed linkage at chromosome 2 (Figure 5B), and whole-genome sequencing (Figure 4C, Table S5, and File S1) revealed a PMDH1 nonsense mutation (pmdh1-2) at Gln129 (Figure 5B). Although pmdh1-1 was previously reported to be sucrose dependent (Pracharoenwattana et al. 2007), pmdh1-1 and pmdh1-2 were sucrose independent in our growth conditions (Figure 5C). pmdh1-2 was 2,4-DB resistant (Figure 5D), similar to pmdh1-1 (Figure 5D; Pracharoenwattana et al. 2007), whereas neither pmdh1-1 nor pmdh1-2 displayed IBA resistance (Figure 5D). Additionally, the pmdh1-2 single mutant retained oil bodies (Figure 2) as reported for the pmdh1-1 pmdh2-1 double mutant (Pracharoenwattana et al. 2007).
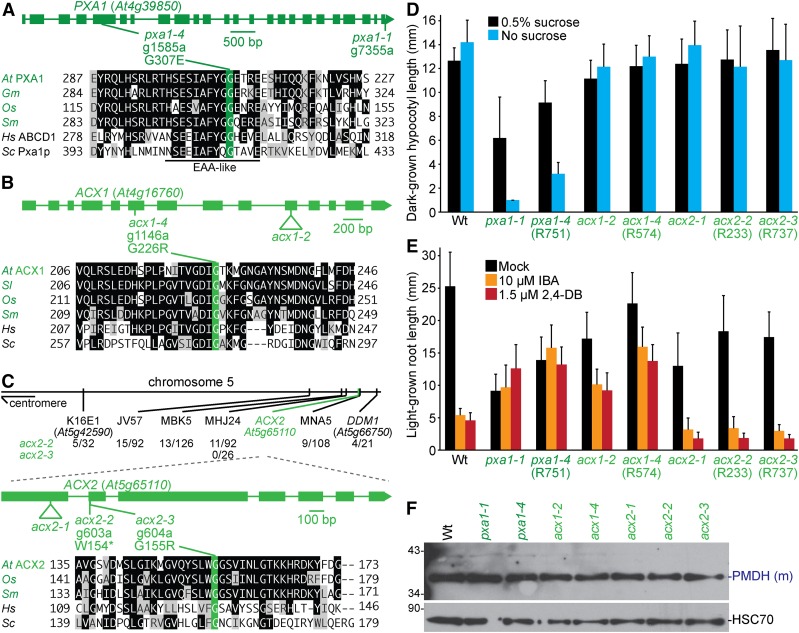
PXA1 imports fatty acids into the peroxisome (Figure 1). Whole-genome sequencing of the R751 mutant (Figure 4H and Table S5) revealed a PXA1 mutation (pxa1-4) that changed Gly307 to Glu. Gly307 is located within an EAA-like motif (EAAX3GX9IXLP) that is similar to fatty-acid-binding domains (Shani et al. 1995) and is conserved in plants, yeast, and humans (Figure 6A; Zolman et al. 2001b). In S. cerevisiae, a mutation in the equivalent Gly413 in Pxa1p (Figure 6A) impairs fatty acid β-oxidation (Shani et al. 1996). Mutations in the equivalent Gly298 in a human PXA1 homolog (Figure 6A) cause adrenomyeloneuropathy (Wichers et al. 1999) or Addison’s disease (Lachtermacher et al. 2000). pxa1-4 was sucrose dependent and IBA and 2,4-DB resistant (Figure 6, D and E), resembling pxa1-1 and other pxa1 mutants (Zolman et al. 2001b; Footitt et al. 2002; Hayashi et al. 2002; Dietrich et al. 2009). Like previously described pxa1 mutants (Footitt et al. 2002; Dietrich et al. 2009), pxa1-4 seedlings retained oil bodies longer than wild type (Figure 2). Therefore, Gly307 appeared to be important for import of fatty acids and IBA in Arabidopsis, supporting previous yeast data suggesting that the EAA-like motif is important for substrate recognition (Shani et al. 1995). However, the EAA-like motif did not seem to be critical for germination, because germination defects were not noticed in mutants with alterations in the EAA-like motif (Dietrich et al. 2009), including pxa1-4. In contrast, the strong comatose alleles of pxa1 have clear germination defects (Russell et al. 2000; Footitt et al. 2002; Dietrich et al. 2009). As with previously described alleles, pxa1-4 processed PTS2 proteins like wild type (Figure 6F).
Figure 6.
Mutations in PXA1 confer sucrose dependence and IBA and 2,4-DB resistance, and mutations in ACX1 lead to IBA and 2,4-DB resistance, whereas mutations in ACX2 do not generate notable physiological defects. (A–C) Gene diagrams indicate positions and nature of pxa1 (A), acx1 (B), and acx2 (C) mutations. PXA1 (A), ACX1 (B), and ACX2 (C) were aligned with related proteins (Table S4); residues are shaded when identical (black or green) or chemically similar (gray) in at least four (A, B) or three (C) sequences. Chromosome map indicates the positions of markers used to map acx2 mutants and ratios of recombinants to the number of chromosomes assessed (C). (D) Hypocotyl lengths of 5-day-old dark-grown seedlings were measured. Error bars show SD (n = 20). (E) Root lengths of 8-day-old light-grown seedlings were measured. Error bars show SD (n ≥ 14). Data are representative of two (D) or three (E) replicates. (F) Eight-day-old seedlings were prepared for immunoblot analysis and serially probed with antibodies recognizing the indicated proteins. PMDH is translated as a precursor with a PTS2 that is cleaved in the peroxisome to yield mature (m) protein. HSC70 was used as a loading control. Positions of molecular mass markers (in kDa) are indicated on the left.
ACX1 and ACX2 are partially redundant enzymes that catalyze the first step of fatty acid β-oxidation (Figure 1). Whole-genome sequencing of R574 (Figure 4I, Table S5, and File S1) revealed an ACX1 mutation that changed Gly226 to Arg (acx1-4). Gly226 is conserved in ACX1 homologs in other organisms (Figure 6B). acx1-4 processed PTS2 proteins like wild type (Figure 6F) and was sucrose independent and IBA resistant (Figure 6, D and E), similar to acx1-2 and other previously isolated acx1 mutants (Adham et al. 2005; Pinfield-Wells et al. 2005). acx1-4 and acx1-2 were 2,4-DB resistant in our conditions (Figure 6E), unlike a previous report of acx1-2 having wild-type 2,4-DB responsiveness (Pinfield-Wells et al. 2005). acx1-4 had clustered peroxisomes with some oil body retention (Figure 2), unlike previously characterized acx1 mutants that display wild-type peroxisome distribution (Pinfield-Wells et al. 2005).
We recovered two new acx2 mutations. Recombination mapping of acx2-2 (R233) and acx2-3 (R737) revealed linkage at the bottom of chromosome 5 (Figure 6C). Sequencing ACX2 revealed a mutation in acx2-2 that would convert Trp154 to a premature stop codon and an adjacent mutation in acx2-3 that would change Gly155 to Arg. Gly155 is conserved in ACX2 homologs of other organisms (Figure 6C). acx2-2 and acx2-3 processed PTS2 proteins like wild type (Figure 6F) and were sucrose independent and IBA and 2,4-DB sensitive (Figure 6, D and E), similar to acx2-1 (Adham et al. 2005; Pinfield-Wells et al. 2005). acx2-2 and acx2-3 displayed clustered peroxisomes around retained oil bodies (Figure 2), whereas acx2-1 accumulates acyl-CoA but displays normal peroxisome distribution with no oil body retention (Pinfield-Wells et al. 2005).
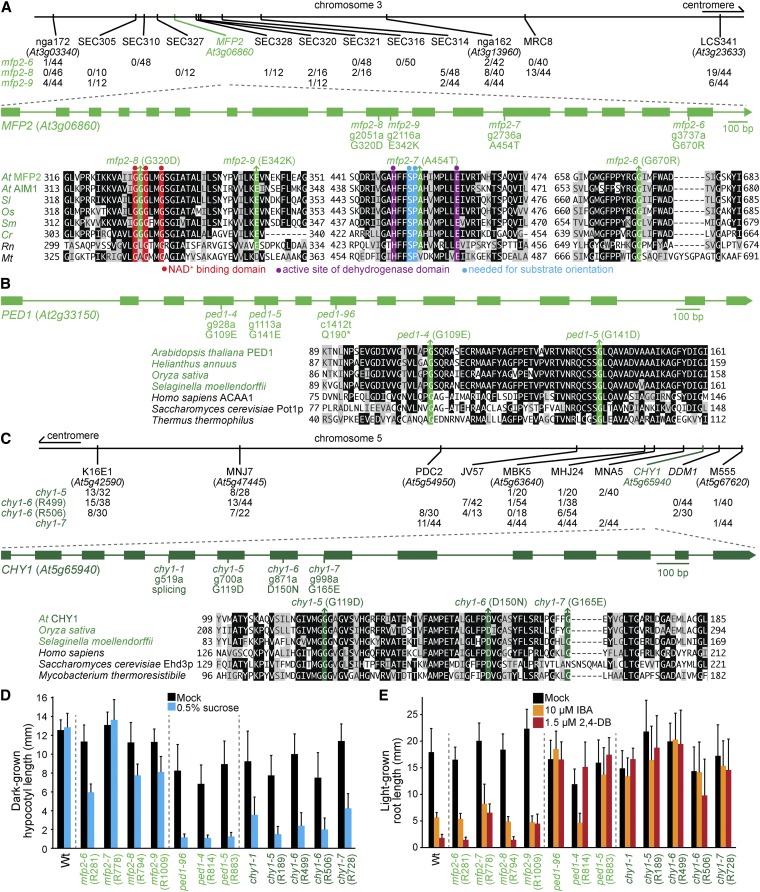
MFP2 catalyzes the second and third steps of fatty acid β-oxidation (Figure 1). We found five mutants carrying four different MFP2 mutations: mfp2-6 (R281), mfp2-7 (R778), mfp2-8 (R794 and R809), and mfp2-9 (R1009). Sequencing MFP2 following recombination mapping to chromosome 3 revealed mutations that changed Gly670 to Arg in mfp2-6, Gly320 to Asp in mfp2-8, and Glu342 to Lys in mfp2-9 (Figure 7A). Whole-genome sequencing revealed the same Gly320Asp mutation in a different mfp2-8 isolate (Figure 4K, Table S5, and File S1) and an Ala454-to-Thr mutation in mfp2-7 (Figure 4J, Table S5, and File S1). All four mutations alter conserved residues in the MFP2 C-terminal dehydrogenase domain (Figure 7A). Gly320 is between two residues important for NAD+ binding (Arent et al. 2010), and Ala454 follows two conserved residues, Ser452 and Pro453, that facilitate substrate orientation in Arabidopsis MFP2 (Arent et al. 2010). mfp2-6, mfp2-8, and mfp2-9 were partially sucrose dependent and IBA and 2,4-DB sensitive (Figure 7, D and E), and displayed clustered peroxisomes around accumulated oil bodies (Figure 2), like previously characterized mfp2 mutants (Rylott et al. 2006). mfp2-7 also showed clustered peroxisomes around retained oil bodies but, in contrast to the other mfp2 alleles, mfp2-7 was sucrose independent and displayed slight 2,4-DB resistance (Figure 7, D and E).
Figure 7.
Mutations in MFP2, PED1, and CHY1 confer sucrose dependence and IBA and 2,4-DB resistance. (A–C) Chromosome map indicates the positions of markers used to map mfp2 (A) and chy1 (C) mutants and ratios of recombinants to the number of chromosomes assessed. Gene diagrams indicate the position of mfp2 (A), ped1 (B), and chy1 (C) mutations. MFP2 (A), PED1 (B), and CHY1 (C) were aligned with related proteins (Table S4); residues are shaded when identical (black or colors) or chemically similar (gray) in at least five (A) or four (B, C) sequences. (D) Hypocotyl lengths of 5-day-old dark-grown seedlings were measured. Error bars show SD (n = 20). (E) Root lengths of 8-day-old light-grown seedlings were measured. Error bars show SD (n ≥ 15). Data are representative of three replicates (D, E).
PED1 is a 3-ketoacyl-CoA thiolase that catalyzes the last step of fatty acid β-oxidation (Figure 1). Sequencing PED1 in several mutants revealed mutations changing Gly109 to Glu in ped1-4 (R814) and Gly141 to Asp in ped1-5 (R883). Gly109 and Gly141 are conserved in plants and homologs in humans and yeast (Figure 7B). ped1-5 displayed reduced levels of protein detected with an anti-thiolase antibody (Figure 11A), peroxisomes clustered around retained oil bodies (Figure 2), sucrose dependence, and IBA- and 2,4-DB resistance (Figure 7, D and E). These phenotypes were similar to those of ped1-96 (Lingard and Bartel 2009) and other previously characterized ped1 mutants (Hayashi et al. 1998; Germain et al. 2001; Lingard and Bartel 2009; Burkhart et al. 2013; Wiszniewski et al. 2014). Although ped1-4 displayed robust sucrose dependence (Figure 7D) and 2,4-DB resistance (Figure 7E), this allele was sensitive to IBA (Figure 7E). The less severe defects observed in ped1-4 suggest that ped1-4 protein, which accumulates at wild-type levels (Figure 11A), retains partial function.
Figure 11.
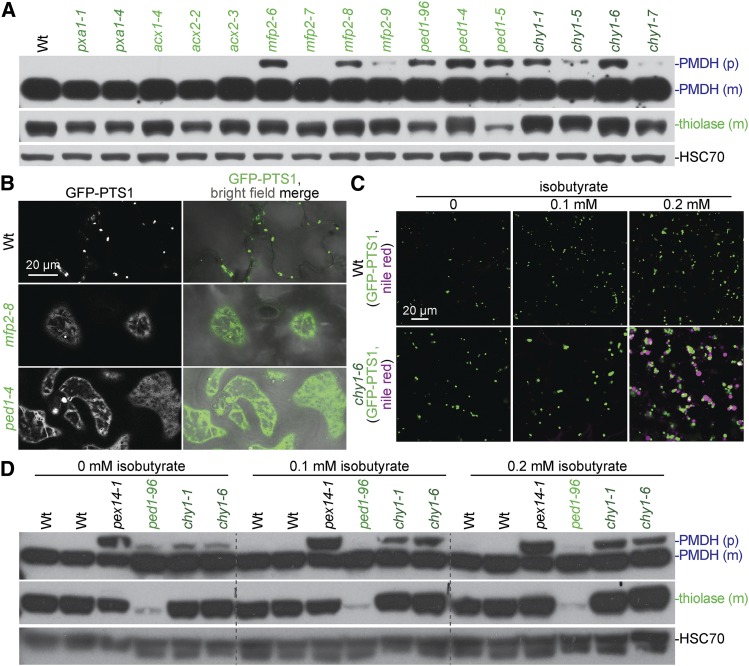
Fatty acid β-oxidation mutants display matrix protein import defects, and chy1 oil body retention and PTS2-processing defects are worsened by isobutyrate supplementation. (A) Nine-day-old seedlings were prepared for immunoblot analysis and serially probed with antibodies recognizing the indicated proteins. PMDH is translated as a precursor (p) with a PTS2 that is cleaved in the peroxisome to yield mature (m) protein. HSC70 was used as a loading control. (B) Confocal micrographs were taken of 8-day-old cotyledon epidermal cells expressing peroxisomally targeted fluorescence (GFP-PTS1; green). The images used for wild type are the same as those used in Figure 9A. (C) Confocal micrographs were taken of cotyledon epidermal cells of 8-day-old seedlings grown on the indicated concentration of isobutyrate, expressing peroxisomally-targeted fluorescence (GFP-PTS1; white in left panels), and stained with Nile red for oil body visualization (magenta). (D) 8-day-old seedlings grown on the indicated concentration of isobutyrate were prepared for immunoblot analysis and serially probed with antibodies recognizing the indicated proteins. pex14-1 is a PTS2-processing defective control. Data are representative of two (C) or three (A, B, and D) replicates. Bars, 20 µm.
Mutations in CHY1 confer ped1-like phenotypes (Zolman et al. 2001a). We recovered three chy1 mutants that displayed peroxisomes clustered around retained oil bodies (Figure 2). Recombination mapping indicated linkage at the bottom of chromosome 5 (Figure 7C), and sequencing CHY1 revealed mutations that changed Gly119 to Asp in chy1-5 (R189), Asp150 to Asn in chy1-6 (R499 and R506), and Gly165 to Glu in chy1-7 (R728). Asp150 is needed for in vitro CHY1 enzymatic activity (Zolman et al. 2001a), and all three mutations altered residues conserved among closely related genes in Arabidopsis and humans (Zolman et al. 2001a) and other species (Figure 7C). chy1-5, chy1-6, and chy1-7 were sucrose dependent, IBA resistant, and 2,4-DB resistant (Figure 7, D and E), similar to chy1-1 and other previously characterized chy1 mutants (Zolman et al. 2001a; Lange et al. 2004).
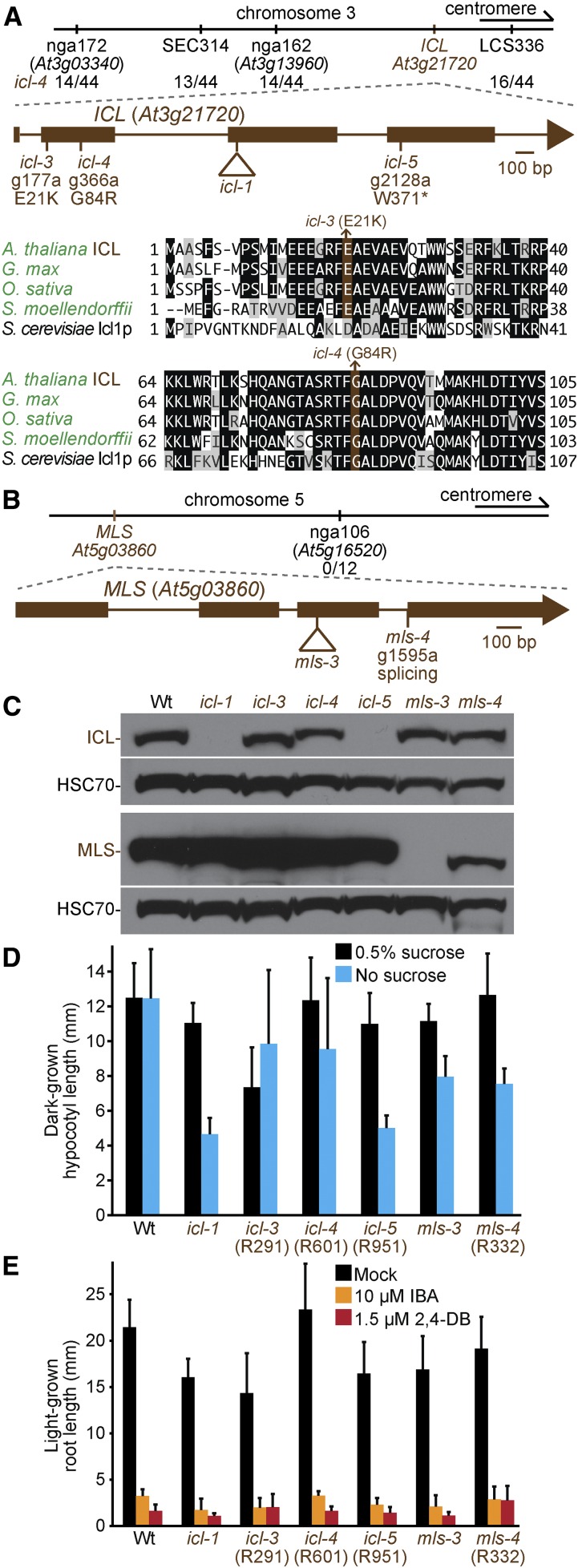
We also recovered several mutants disrupted in the glyoxylate cycle. We isolated three icl mutants with peroxisomes clustered around retained oil bodies (Figure 2). Recombination mapping of icl-4 (R601) indicated linkage at chromosome 3 (Figure 8A), and whole-genome sequencing of icl-3 (R291) and icl-4 (Figure 4, D and E, Table S5, and File S1) revealed ICL mutations that changed Glu21 to Lys in icl-3 and Gly84 to Arg in icl-4 (Figure 8A). Glu21 is conserved in plant ICL homologs, and Gly84 is conserved in plants and yeast (Figure 8A). Whole-genome sequencing of icl-5 (R951) (Figure 4F, Table S5, and File S1) revealed an ICL nonsense mutation at Trp371 (Figure 8A). Like icl-1, ICL protein was not detected in the icl-5 nonsense allele. In contrast, both missense alleles accumulated icl protein, although icl-3 had a slight shift in electrophoretic mobility (Figure 8C). Like icl-1 (Eastmond et al. 2000a), all icl alleles responded like wild type to IBA and 2,4-DB (Figure 8E), and icl-5 was sucrose-dependent in the dark (Figure 8D). In contrast, icl-3 and icl-4 were sucrose independent (Figure 8D). The sucrose independence of icl-3 and icl-4 suggests that the icl protein that accumulates in these mutants (Figure 8C) retains partial function.
Figure 8.
Mutations in ICL and MLS confer sucrose dependence. (A, B) Chromosome maps indicate the positions of markers used to map icl-4 (A) or mls-4 (B) and ratios of recombinants to the number of chromosomes assessed. Gene diagrams indicate the positions of icl (A) or mls (B) mutations. ICL was aligned (A) with related proteins (Table S4); residues are shaded when identical (black or brown) or chemically similar (gray) in at least three sequences. (C) Eight-day-old seedlings were used for immunoblot analysis and serially probed with antibodies recognizing the indicated proteins. HSC70 was used as a loading control. (D) Hypocotyl lengths of 5-day-old dark-grown seedlings were measured. Error bars show SD (n = 20). (E) Root lengths of 8-day-old light-grown seedlings were measured. Error bars show SD (n = 20). Data are representative of two replicates (C, D).
We recovered a mutant in MLS, mls-4 (R332), with clustered peroxisomes (Figure 2). Recombination mapping indicated linkage at chromosome 5 (Figure 8A), and whole-genome sequencing (Figure 4G, Table S5, and File S1) revealed an MLS mutation in the splice acceptor site of the third intron (Figure 8B). Immunoblot analysis of MLS protein revealed reduced levels and faster electrophoretic mobility of mls-4 (Figure 8C). mls-4 was slightly sucrose dependent, similar to the null mls-3 allele (Figure 8D) and other mls mutants (Cornah et al. 2004). mls-3 and mls-4 were IBA and 2,4-DB sensitive (Figure 8E).
These sdp1, pmdh1, mdar4, pxa1, acx1, acx2, mfp2, ped1, chy1, icl, and mls mutants were originally selected for clustered GFP-PTS1 puncta, and confocal microscopy revealed that peroxisomes were clustered around retained oil bodies (Figure 2). Because the mutations identified are in genes directly or indirectly implicated in lipid mobilization, we concluded that these mutants were isolated due to delayed lipid mobilization, which led to peroxisomes clustering around retained oil bodies.
Fatty acid β-oxidation mutants have defects in peroxisome size and matrix protein import
Several fatty acid β-oxidation mutants displayed peroxisomal defects beyond the physiological phenotypes expected from impaired β-oxidation (sucrose dependence and IBA or 2,4-DB resistance). For example, acx1-4, acx2-2, and acx2-3 displayed some enlarged peroxisomes (Figure 9, C and D) similar to the acx1-2 acx2-1 double mutant (Pinfield-Wells et al. 2005), but unlike previously characterized acx1 and acx2 single mutants, which have wild-type-sized peroxisomes (Pinfield-Wells et al. 2005). Additionally, some peroxisomes were enlarged in mfp2-6, mfp2-7, mfp2-8, mfp2-9, ped1-4, and ped1-5 (Figure 9, E and F), similar to previously isolated mfp2 (Rylott et al. 2006) and ped1 (Hayashi et al. 1998; Germain et al. 2001) mutants. Moreover, chy1-5, chy1-6, and chy1-7 had some enlarged peroxisomes (Figure 9G), which had not been reported previously.
Figure 9.
A subset of fatty acid β-oxidation mutants display enlarged peroxisomes and matrix protein import defects. (A–G) Wild type (A) and pxa1 mutants (B) display small peroxisomes, whereas mutations in ACX1 (C), ACX2 (D), MFP2 (E), PED1 (F), and CHY1 (G) lead to enlarged peroxisomes and some GFP-PTS1 mislocalization to the cytosol. Confocal micrographs were taken of cotyledon epidermal cells of 5- or 8-day-old seedlings expressing peroxisomally targeted fluorescence (GFP-PTS1; white in left panels; green in merged images). Data are representative of three replicates. Bar, 20 µm.
Unlike in mutants with impaired fatty acid β-oxidation enzymes, peroxisome size resembles wild type in pxa1 mutants (Footitt et al. 2002), including our pxa1-4 allele (Figure 9B), and in lacs6-1 lacs7-1 (Fulda et al. 2004). PXA1 interacts physically and functionally with LACS6/7 in yeast (De Marcos Lousa et al. 2013), so PXA1 transport may be reduced in the lacs6-1 lacs7-1 mutant. Other mutants blocked early in lipid mobilization, such as sdp1 and mdar4, which indirectly reduces SDP1 activity (Eastmond 2007), also displayed wild-type-sized peroxisomes (Figure 2). Thus, fatty acid mobilization defects upstream of fatty acid import appear not to cause peroxisome enlargement. Fatty acid mobilization defects downstream of fatty acid import do lead to enlarged peroxisomes (Table 2), suggesting that excessive peroxisomal fatty acids or their β-oxidation intermediates cause peroxisome enlargement in β-oxidation mutants (Graham et al. 2002).
Table 2. Summary of peroxisome size and import defects in fatty acid β-oxidation mutants.
| Mutant | Defect | Peroxisome size | PTS2 processing | PTS1 import |
|---|---|---|---|---|
| pxa1 | Fatty acid import into peroxisomes | Wta | Wtb | Wtb |
| lacs6-1 lacs7-1 | Fatty acid import and activation | Wtc | Not determined | Not determined |
| acx1-2 acx2-1 | Fatty acid β-oxidation | Larged | Not determined | Not determined |
| mfp2 | Fatty acid β-oxidation | Largee | Defectb | Defectb |
| ped1 | Fatty acid β-oxidation | Largef | Defectg | Defectg |
| pxn | NAD+ and CoA import into peroxisomes | Largeh | Wtb | Wtb |
| pxa1 pxn | Fatty acid, NAD+, and CoA import into peroxisomes | Wtb | Wtb | Wtb |
| pxn ped1 | NAD+ and CoA import into peroxisomes and fatty acid β-oxidation | Largeb | Enhanced defectb | Enhanced defectb |
This work and Hayashi et al. (2002).
This work.
This work and Rylott et al. (2006).
This work and Hayashi et al. (1998).
This work and Burkhart et al. (2013).
This work and Mano et al. (2011).
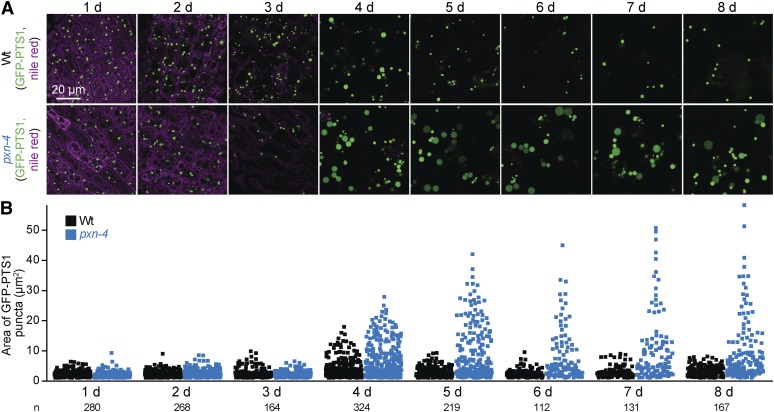
We also observed that a subset of peroxisomes in wild type cotyledons became transiently enlarged around 4 days after plating (Figure 10). The numerous oil bodies in germinating cotyledons became less abundant during the first 4 days of development as peroxisomes enlarged (Figure 10). By day 5, few oil bodies remained, and peroxisomes returned to the original size (Figure 10). This transient enlargement of a subset of peroxisome in wild type during oil body consumption is consistent with the hypothesis that fatty acids can overwhelm the β-oxidation machinery, leading to peroxisomal accumulation of fatty acids or β-oxidation intermediates, a state similar to the one expected in fatty acid β-oxidation mutants with enlarged peroxisomes.
Figure 10.
Peroxisomes expand transiently during wild-type seedling development but remain large in pxn seedlings. (A) Confocal micrographs were taken of cotyledon epidermal cells of wild-type and pxn-4 seedlings expressing peroxisomally targeted fluorescence (GFP-PTS1; green) and stained with Nile red for oil body visualization (magenta). (B) GFP-PTS1 puncta areas from one to three 135 × 135 µm images including those shown in (A) are plotted; the number of puncta measured for each genotype is indicated below the y-axis labels. The number of puncta per cell was not quantified. Bar, 20 µm.
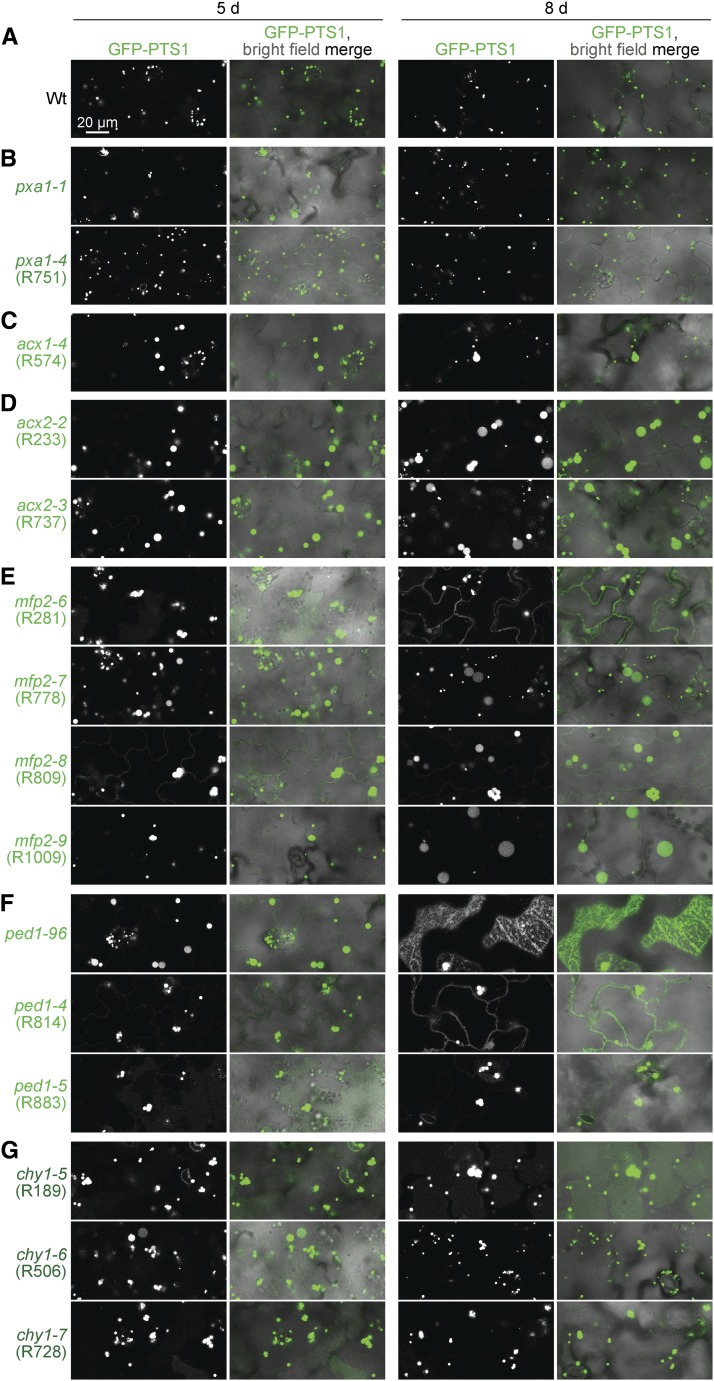
Fatty acid β-oxidation mutants also displayed peroxisomal matrix protein import defects. mfp2-6, mfp2-8, and ped1-4 mislocalized some GFP-PTS1 to the cytosol (Figure 9, E and F) similarly to ped1-96 (Figure 9F), which also mislocalizes GFP-ICL (Burkhart et al. 2013). Furthermore, immunoblot analysis revealed that mfp2-6, mfp2-8, ped1-4, ped1-5, chy1-1, and chy1-5 seedlings displayed partial PTS2-processing defects (Figure 11A) similar to ped1-96 (Burkhart et al. 2013). In contrast, pxa1, acx1, and acx2 mutants displayed wild-type PTS2-processing (Figure 11A).
Like ped1 mutants, chy1 mutants displayed enlarged peroxisomes, cytosolic GFP-PTS1 mislocalization, and PTS2-processing defects (Figure 9G and Figure 11, A and D). In chy1 mutants, methacrylyl-CoA is thought to accumulate and inactivate the PED1 thiolase, yielding ped1-like defects (Zolman et al. 2001a; Lange et al. 2004). Isobutyrate is an intermediate upstream of methacrylyl-CoA (Figure 1), and chy1 root elongation is hypersensitive to isobutyrate (Lucas et al. 2007), perhaps because isobutyrate supplementation further increases methacrylyl-CoA levels. To determine if PED1 inhibition by methacrylyl-CoA was solely responsible for chy1 mutant defects, we supplemented the growth medium with isobutyrate. We found that growth on isobutyrate appeared to slightly increase peroxisome size in 8-day-old wild-type seedlings while markedly enhancing peroxisome enlargement, oil body retention, and peroxisome clustering around retained oil bodies in the chy1-6 mutant (Figure 11C). Isobutyrate enhanced the PTS2-processing defect in chy1-1 and chy1-6 without impairing PTS2 processing in wild type (Figure 11D). Interestingly, isobutyrate conferred a more severe PTS2-processing defect on chy1 than on the ped1-96 null mutant (Figure 11D), suggesting that methacrylyl-CoA has additional targets beyond PED1 that affect PTS2 processing.
LON PROTEASE2 AAA+ ATPase domain is needed for function
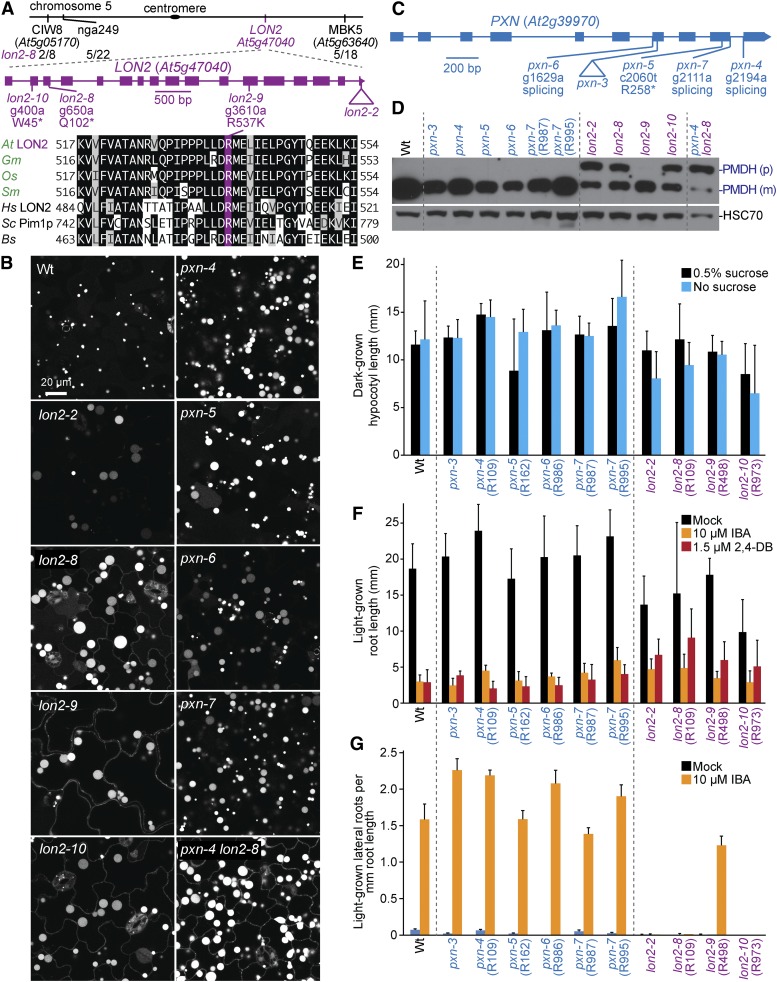
LON PROTEASE2 (LON2) is a peroxisomal protein that belongs to a family of ATPases associated with diverse cellular activities+ (AAA+) and is needed for sustained peroxisome function during seedling development (Lingard and Bartel 2009). We recovered three mutants with enlarged GFP-PTS1 puncta accompanied by some cytosolic GFP-PTS1 fluorescence reminiscent of lon2 mutants (Farmer et al. 2013; Goto-Yamada et al. 2014). Sequencing LON2 revealed a nonsense mutation at Trp45 in lon2-10 (R973) and a mutation that changed Arg537 to Lys in lon2-9 (R498; Figure 12A). Recombination mapping of R109 using the enlarged puncta phenotype failed to identify a clear linkage. Because the enlarged GFP-PTS1 puncta could be due to a PXN mutation (Mano et al. 2011), we sequenced PXN and discovered pxn-4. However, R109 also presented IBA resistance, cytosolic GFP-PTS1, and a PTS2-processing defect, phenotypes not associated with other pxn alleles. Recombination mapping using PTS2-processing defects revealed a linkage at the bottom of chromosome 5 (Figure 12A). Whole-genome sequencing of pooled backcrossed lines with PTS2-processing defects (Figure 4L, Table S5, and File S1) revealed lon2-8, a nonsense mutation at Gln102 (Figure 12A). A backcrossed lon2-8 line homozygous for wild-type PXN was used for lon2-8 assays.
Figure 12.
Mutations in LON2 and PXN lead to enlarged GFP-PTS1 puncta, and a subset of lon2 mutants display GFP-PTS1 mislocalization to the cytosol, PTS2-processing defects, and IBA resistance in lateral root production. (A) Chromosome map indicates the positions of markers used to map lon2-8 and ratio of recombinants to the number of chromosomes assessed. A gene diagram indicates the positions of lon2 mutations. LON2 was aligned with related proteins (Table S4); residues are shaded when identical (black or purple) or chemically similar (gray) in at least four sequences. (B) Confocal micrographs were taken of cotyledon epidermal cells of 8-day-old seedlings expressing peroxisomally targeted fluorescence (GFP-PTS1; white). (C) A gene diagram indicates the positions of pxn mutations. (D) Eight-day-old seedlings were prepared for immunoblot analysis and serially probed with antibodies recognizing the indicated proteins. PMDH is translated as a precursor (p) with a PTS2 that is cleaved in the peroxisome to yield mature (m) protein. HSC70 was used as a loading control. (E) Hypocotyl lengths of 5-day-old dark-grown seedlings were measured. Error bars show SD (n ≥ 15). (F) Root lengths of 8-day-old light-grown seedlings were measured. Error bars show SD (n ≥ 17). (G) Seedlings were grown in the absence of hormone for 4 days and then transferred to either media without hormone or supplemented with IBA for an additional 4 days. The number of lateral roots and root lengths were measured, and the ratio is shown. Error bars show SD (n = 20). Data are representative of two (B, D, E, and G) or three (F) replicates. Bar, 20 µm.
Previously isolated lon2 mutants display slight sucrose dependence and IBA resistance in root elongation, strong IBA resistance in lateral root promotion, PTS2-processing defects, partially cytosolic localization of peroxisomal matrix proteins (Lingard and Bartel 2009), and dramatically enlarged peroxisomes (Farmer et al. 2013; Goto-Yamada et al. 2014). Similarly, lon2-8 and lon2-10, which are expected to result in early truncations of LON2 protein, displayed GFP-PTS1 in enlarged puncta and in cytosolic-like distribution (Figure 12B), PTS2-processing defects (Figure 12D), and IBA resistance in lateral root production (Figure 12G), but only slight sucrose dependence (Figure 12E) and minor IBA and 2,4-DB resistance in root elongation (Figure 12F). Interestingly, lon2-9 also showed a marked GFP-PTS1 mislocalization and enlarged GFP-PTS1 puncta comparable to lon2 mutants expected to be null alleles (Figure 12B; Goto-Yamada et al. 2014), but, in contrast, appeared to process PTS2 proteins normally (Figure 12D) and displayed only slight IBA resistance in lateral root production (Figure 12G), indicating that this missense allele retains some LON2 function.
Fatty acid metabolism is necessary for enlarged puncta in PXN mutants
We identified five mutants with enlarged GFP-PTS1 puncta similar to pxn mutants (Mano et al. 2011), revealing four new PXN mutations: pxn-4 (R109), pxn-5 (R162), pxn-6 (R986), and pxn-7 (R987 and R995; Figure 12C). Unlike wild type, pxn peroxisomes can be large enough to be visible even using bright field microscopy (Figure 13C). Sequencing PXN revealed that pxn-5 had a nonsense mutation at Arg258, pxn-6 had a mutation in the splice acceptor sequence of the sixth intron, and pxn-7 had a mutation in the splice donor site of the ninth exon. pxn-4 had a mutation in the splice acceptor sequence of the ninth PXN intron, and a backcrossed line without lon2-8 was used for assays. Our pxn mutants lacked noticeable matrix protein import defects; they efficiently processed PTS2 proteins (Figure 12D) and lacked cytosolic GFP-PTS1 fluorescence (Figure 12B). pxn mutants were sucrose independent (Figure 12E) and sensitive to IBA and 2,4-DB (Figure 12, F and G), similar to wild type and previously characterized pxn mutants, which are sucrose independent (Mano et al. 2011; Bernhardt et al. 2012) and only slightly (Bernhardt et al. 2012) or not at all (Mano et al. 2011) 2,4-DB resistant. We did not observe pxn oil body retention in our conditions (Figure 10), unlike previously characterized pxn mutants (Bernhardt et al. 2012).
Figure 13.
Preventing fatty acid import but not preventing autophagy rescues pxn enlarged GFP-PTS1 puncta, and combining pxn with ped1 rescues enlarged GFP-PTS1 puncta while enhancing ped1 matrix protein import defects. (A) Confocal micrographs were taken of cotyledon epidermal cells of 8-day-old seedlings expressing peroxisomally-targeted fluorescence (GFP-PTS1 in all lines except lon2-2 atg7-6, which carried PTS2-GFP; green), and displaying chlorophyll autofluorescence (red). (B) Confocal micrographs were taken of cotyledon epidermal cells of 8-day-old seedlings expressing peroxisomally targeted fluorescence (GFP-PTS1; white in top rows, green in merged images). Blue arrows point to enlarged GFP-PTS1 puncta in pxn-4 that can be seen in bright field. (C) Digitally enlarged sections of confocal micrographs shown in (B). Blue arrows point to enlarged GFP-PTS1 puncta in pxn-4 that can be seen in bright field. (D) Eight-day-old seedlings were prepared for immunoblot analysis and serially probed with antibodies recognizing the indicated proteins. PMDH is translated as a precursor (p) with a PTS2 that is cleaved to yield mature (m) protein in the peroxisome. HSC70 was used as a loading control. Data are representative of three replicates (B, D). Bars (A, B), 10 µm; (C), 2 µm.
Because one of our mutants, R109, carried both lon2-8 and pxn-4 mutations, we examined the double mutant to see if the effects of these mutations were additive. We found that lon2-8 pxn-4 did not show a further increase in peroxisome size compared to the single mutants (Figure 12B), and that PTS2-processing remained impaired in lon2-8 pxn-4 (Figure 12D).
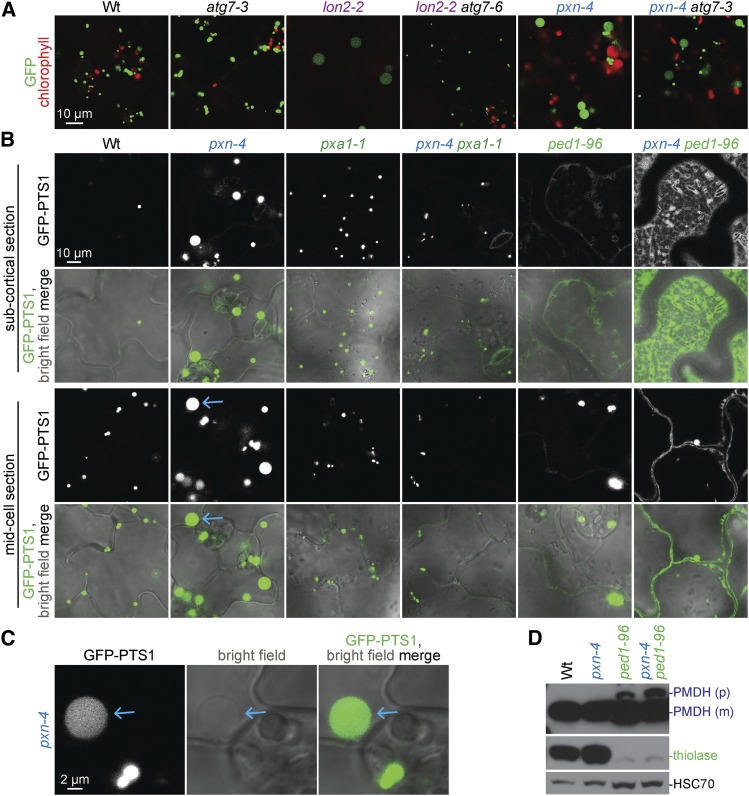
The large GFP-PTS1 puncta in pxn mutants resemble the enlarged GFP-PTS1 puncta observed in lon2 mutants (Figure 12B; Farmer et al. 2013). LON2 research provided the first evidence of peroxisome-specific autophagy (pexophagy) in plants (Farmer et al. 2013). Autophagy is a process that degrades entire organelles and other cellular constituents (reviewed in Li and Vierstra 2012). Combining lon2 with null mutations in AUTOPHAGY RELATED (ATG) 2, ATG3, or ATG7 rescues lon2 defects, improving peroxisomal matrix protein import, returning peroxisomes to a wild-type size, and stabilizing peroxisomal proteins that are more quickly degraded in lon2 (Farmer et al. 2013; Bartel et al. 2014; Goto-Yamada et al. 2014; Young and Bartel 2015). We asked if autophagy was similarly required to generate enlarged peroxisomes in pxn mutants by crossing pxn-4 to atg7-3, a null autophagy mutant (Lai et al. 2011). Whereas lon2-2 atg7 PTS2-GFP puncta resemble peroxisomes in wild type and atg7 mutants (Figure 13A; Farmer et al. 2013), the pxn-4 atg7-3 double mutant displayed both large pxn-like GFP-PTS1 puncta and smaller puncta (Figure 13A). The additional small GFP-PTS1 puncta observed in pxn-4 atg7-3 (Figure 13A) may reflect accumulating peroxisomes caused by autophagy disruption in atg7-3. We concluded that the mechanism by which peroxisomes increase in size in pxn does not involve pexophagy and is distinct from the mechanism that generates large GFP-PTS1 puncta in lon2-2.
The enlarged peroxisomes in pxn mutants might relate to the role of PXN in importing NAD+ (Agrimi et al. 2012; Bernhardt et al. 2012; van Roermund et al. 2016), a key cofactor in fatty acid β-oxidation (Figure 1). Perhaps in pxn mutant peroxisomes, oxidized NAD+ is depleted and fatty acid β-oxidation stalls. This situation would resemble that of fatty acid β-oxidation mutants that show enlarged peroxisomes (Figure 9, C–G). We examined pxn peroxisomes following germination, when fatty acids might accumulate in peroxisomes due to intense lipid mobilization. We found that pxn peroxisomes were similar in size to wild-type peroxisomes during the first few days after germination, starting small and enlarging by 4 days (Figure 10). However, wild-type peroxisomes returned to a normal size at day 5, when visible oil bodies had been consumed, while numerous pxn peroxisomes remained enlarged (Figure 10). This result is consistent with the possibility that peroxisomes become enlarged in pxn due to fatty acids accumulating in the peroxisome. Perhaps peroxisomes remain enlarged beyond 5 days because once NAD+ is depleted, the remaining peroxisomal fatty acids cannot be fully β-oxidized.
To directly test the connection between fatty acid β-oxidation and enlarged pxn GFP-PTS1 puncta, we impaired fatty acid import in a pxn mutant by crossing to pxa1-1. GFP-PTS1 puncta in pxa1-1 pxn-4 resembled peroxisomes in pxa1-1 and wild type instead of the large GFP-PTS1 puncta present in pxn-4 (Figure 13B; Table 2). This epistasis suggests that import of PXA1 substrates into peroxisomes is necessary to produce the large GFP-PTS1 puncta observed in pxn, and further supports a requirement for fatty acid import for peroxisome enlargement in fatty acid β-oxidation mutants.
In addition, we crossed pxn-4 to ped1-96. Both mutants displayed enlarged GFP-PTS1 puncta (Figure 13B); pxn-4 GFP-PTS1 puncta were more enlarged than ped1-96 puncta (Figure 13B). pxn-4 ped1-96 GFP-PTS1 puncta resembled ped1-96, indicating that removing thiolase activity prevents the extreme enlargement of GFP-PTS1 puncta in pxn-4 (Figure 13B). Surprisingly, pxn-4 appeared to enhance the peroxisomal protein import defects of ped1-96; both GFP-PTS1 mislocalization to the cytosol and PTS2-processing defects were exacerbated by pxn-4 (Figure 13, B and D). Given that ped1-96 import defects likely stem from deficient fatty acid β-oxidation, this result suggests that pxn enhances ped1 defects by further impairing fatty acid β-oxidation, presumably because NAD+ become limiting inside the peroxisome. Thus, PXN-dependent cofactor import may contribute to peroxisomal β-oxidation.
Mutations in PECTIN METHYLESTERASE31 lead to defects in oil body mobilization
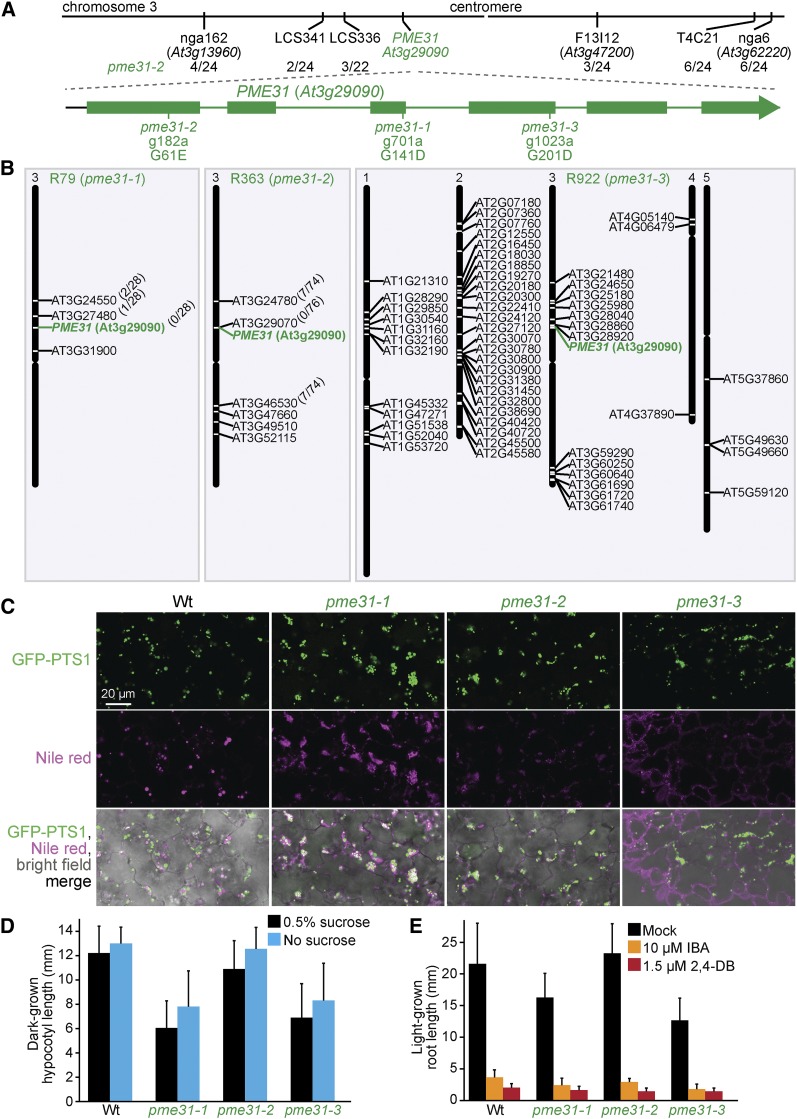
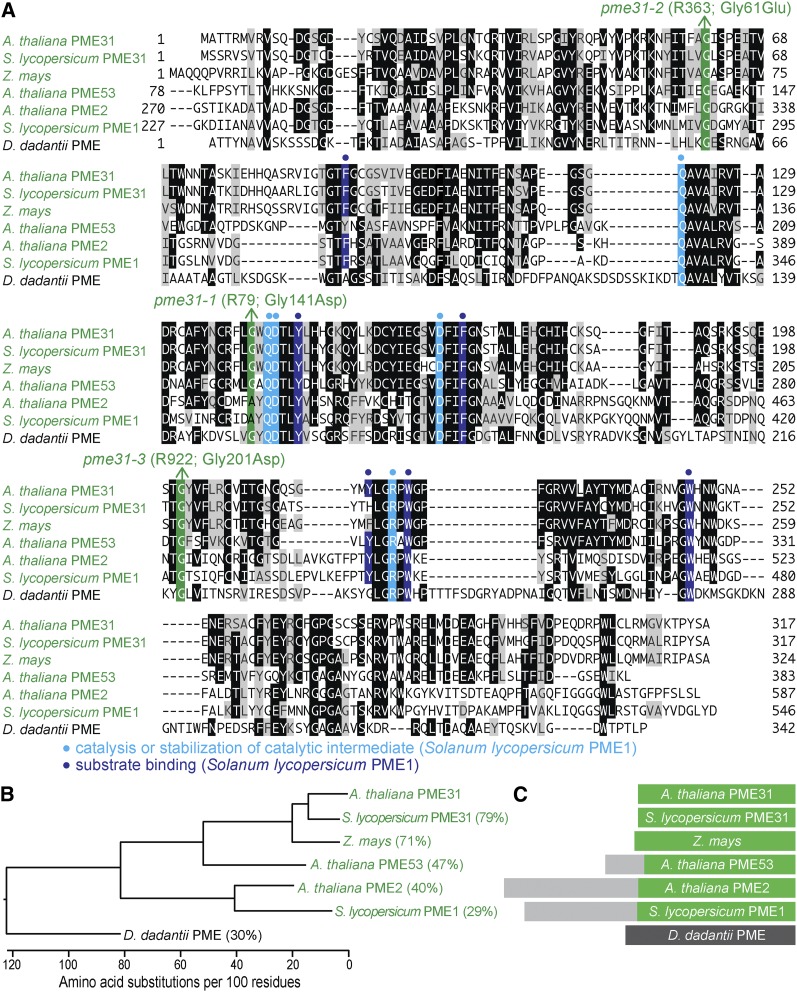
Pectin methylesterases (PME proteins) regulate cell wall structure. Surprisingly, we isolated three independent lines with clustered peroxisomes and mutations in PECTIN METHYLESTERASE31 (PME31): pme31-1 (R79), pme31-2 (R363), and pme31-3 (R922). Whole-genome sequencing of pme31-1 revealed four homozygous mutations, all on chromosome 3 (Figure 14B, Table S5, and File S1), including a PME31 mutation that changed Gly141 to Asp (Figure 14A). Recombination mapping using markers for three of the mutations revealed by whole-genome sequencing (Table S6) indicated closer linkage for pme31-1 than for two other mutations (Figure 14B). Recombination mapping of pme31-2 indicated linkage at chromosome 3 (Figure 14A), and whole-genome sequencing revealed seven homozygous mutations, all on chromosome 3 (Figure 14B, Table S5, and File S1), including a PME31 mutation that modified Gly61 to Glu (Figure 14A). Recombination mapping using markers for the mutations revealed by whole-genome sequencing (Table S6) indicated close linkage to pme31-2 (Figure 14B). Whole-genome sequencing of pme31-3 (Figure 14B, Table S5, and File S1) revealed a PME31 mutation that changed Gly201 to Asp (Figure 14A). These three altered Gly residues at positions 61, 141, and 201 are conserved in Arabidopsis PME proteins (Figure 15A; Dedeurwaerder et al. 2009) and in various PME31-related proteins of plants and bacteria (Figure 15A). Although pme31 mutants were sucrose independent (Figure 14D) and IBA and 2,4-DB sensitive (Figure 14E), they displayed clustered peroxisomes around persisting oil bodies at 4 days (Figure 14C). This phenotype is similar to the one observed for sdp1, pmdh1, acx1, acx2, mfp2, and icl mutants (Figure 2), suggesting that PME31 contributes to lipid mobilization.
Figure 14.
Mutations in PME31 lead to peroxisome clustering around retained oil bodies. (A) Chromosome map indicates the positions of markers used to map pme31-2 and ratios of recombinants to the number of chromosomes assessed. A gene diagram indicates the position of pme31 mutations. (B) Chromosome maps indicate the positions and gene identifier numbers of homozygous EMS-consistent changes in splice sites or nonsynonymous amino acid changes in coding regions. For pme31-1 and pme31-2, genotyping markers for the mutations identified through whole-genome sequencing were used to map in the backcross; the number of recombinants (over the number of chromosomes assessed) are indicated in parentheses. (C) Confocal micrographs were taken of cotyledon epidermal cells of 4-day-old seedlings expressing peroxisomally targeted fluorescence (GFP-PTS1; green) and stained with Nile red for oil body visualization (magenta). (D) Hypocotyl lengths of 5-day-old dark-grown seedlings were measured. Error bars show SD (n ≥ 10). (E) Root lengths of 8-day-old light-grown seedlings were measured. Error bars show SD (n = 10). Data are representative of two replicates (C, E). Bar, 20 µm.
Figure 15.
PME31 is closely related to other PME proteins and lacks a predicted signal peptide. (A) PME31 was aligned with related proteins (Table S4); residues are shaded when identical (black or colors) or chemically similar (gray) in at least three sequences. Residues important for catalysis or stabilization of a catalytic intermediate (light blue background and dots) and for substrate binding (dark blue background and dots) in S. lycopersicum PME1 are indicated. Mutations identified in this work are denoted (green). (B) Phylogenetic tree of PME proteins constructed using ClustalW on Lasergene MegAlign (DNAStar). (C) Diagram of PME proteins, showing sections of the protein that align (green and dark gray) and the N-terminal extensions present only in some PME proteins (light gray) that target these PME enzymes for secretion to the cell wall (Dedeurwaerder et al. 2009).
Discussion
Seedling peroxisomes cluster around oil bodies
Screening for mutants with altered GFP-PTS1 fluorescence patterns in Arabidopsis seedlings revealed numerous mutants displaying clustered peroxisomes around retained oil bodies (Figure 2 and Table S7) with mutations in genes directly or indirectly implicated in lipid mobilization. We presumably isolated these mutants because delayed lipid mobilization led to peroxisomes clustering around retained oil bodies. Peroxisome association with oil bodies reduces distance and increases interaction surfaces between the organelles, thereby increasing the efficiency of lipid mobilization. The SDP1 lipase associates both with the cytosolic side of the peroxisomal membrane and with oil bodies (Eastmond 2006; Thazar-Poulot et al. 2015), probably making the released fatty acids more readily available for peroxisomal import. Sucrose supplementation reduces peroxisome–oil body association (Cui et al. 2016), suggesting that the close interaction might be favored when the cell needs energy. The importance of peroxisome–oil body interactions is highlighted by the physical proximity we observed in a variety of mutants with impeded lipid mobilization. Peroxisomal β-oxidation of fatty acids stored in oil bodies is important not only in seedlings, but also provides energy for stomatal opening in leaves (McLachlan et al. 2016), and it will be interesting to learn whether the mutants isolated here display impairments in stomatal opening.
Several mutants, including sdp1-7, pmdh1-2, acx1-4, acx2-2, acx2-3, mfp2-7, icl-3, and icl-4, displayed clustered peroxisomes (Figure 2 and Table S7) without notable sucrose dependence (Figure 5C, Figure 6D, Figure 7D, and Figure 8D). This finding suggests that microscopic visualization of oil bodies and clustered peroxisomes is more sensitive to impaired β-oxidation than the sucrose dependence phenotype. Most oil bodies are consumed during the first 4 days after germination in wild type (Figure 10; Hayashi et al. 1998). By screening 5-day-old seedlings, our screen identified mutants that retained oil bodies (and thus displayed clustered peroxisomes) even slightly longer than wild type. Indeed, this screen was the first forward-genetics screen to isolate mutations in ACX2, which is partially redundant with ACX1. Although acx2 mutants are sucrose independent (Figure 6D; Adham et al. 2005; Pinfield-Wells et al. 2005), acx2-1 accumulates very long chain fatty acids (Pinfield-Wells et al. 2005) because ACX1 and ACX2 have partially distinct substrate specificities (Hooks et al. 1999). acx1-4, acx2-2, and acx2-3 each showed clustered peroxisomes and oil body retention (Figure 2), suggesting that reduced β-oxidation of a subset of acyl-CoA substrates is sufficient to cluster peroxisomes around retained oil bodies. Our observation that some mutants with retained oil bodies were sucrose independent indicates that slight reductions in lipid mobilization do not noticeably impede seedling growth, implying that lipids are normally mobilized faster than necessary to support wild-type growth.
A role for PME31 in lipid mobilization
We were surprised to identify pme31 mutants in a screen for altered peroxisome positioning. Although PME31 has pectin methylesterase activity in vitro (Dedeurwaerder et al. 2009), PME31 lacks a signal peptide to direct it to the secretory pathway (Figure 15C) for access to the pectin in the cell wall; PME31 is instead predicted to be cytosolic (Dedeurwaerder et al. 2009). In this work we report the first mutations in PME31.
We found that mutations in PME31 led to oil body retention (Figure 14C), much like mutants deficient in lipid mobilization (Figure 2). Because PME31 is predicted to be cytosolic (Dedeurwaerder et al. 2009), it is tempting to speculate that PME31 might act as a lipase or otherwise aid in postgerminative lipid mobilization through esterase activity. SDP1 and SDP1L together are responsible for most TAG lipase activity in seedlings, but are less active on diacylglycerol and inactive on monoacylglycerol (Eastmond 2006; Kelly et al. 2011); the lipase responsible for these activities is not known. It would be interesting to examine PME31 activity on TAG, diacylglycerol, and monoacylglycerol. Moreover, the subcellular localization and protein interaction partners of PME31 in germinating seedlings might provide evidence for direct vs. indirect roles for PME31 in β-oxidation. Regardless of the specific role played by PME31 in lipid mobilization, the presence of PME31 homologs that also lack secretion signals (Figure 15) suggests that this role might be conserved in a variety of plants.
Fatty acid β-oxidation influences peroxisome morphology and function
Peroxisome enlargement due to fatty acid β-oxidation defects is a conserved phenomenon. Defects in acyl-CoA oxidase or homologs of the MFP2 multifunctional protein lead to enlarged peroxisomes in yeast (Smith et al. 2000; van Roermund 2000) and mammalian cells (Goldfischer et al. 1986; Poll-The et al. 1988). Similarly, Arabidopsis mfp2 and ped1 single mutants and acx1 acx2 double mutants have enlarged peroxisomes (Figure 9, C–E; Hayashi et al. 1998; Germain et al. 2001; Pinfield-Wells et al. 2005; Rylott et al. 2006). GFP-PTS1 puncta were enlarged in acx2 single mutants (Figure 9D) even though acx2 mutants lack other notable phenotypes except slightly increased fatty acyl-CoA levels (Pinfield-Wells et al. 2005), indicating that peroxisome enlargement can occur when only a subset of fatty acids is inefficiently β-oxidized.
Peroxisomes also become enlarged during the first days of development in wild-type Arabidopsis (Figure 10; Mano et al. 2002) and cotton (Kunce et al. 1984), which is also an oilseed plant. Intense lipid mobilization immediately following germination might saturate the β-oxidation pathway so that fatty acids or β-oxidation intermediates accumulate in peroxisomes. Indeed, supplementation with non-β-oxidizable fatty acids increases peroxisome size in rat hepatocytes (Berge et al. 1989; Kryvi et al. 1990; Froyland et al. 1996).
Fatty acid β-oxidation mutants displayed peroxisomal matrix protein import defects, indicated by partial GFP-PTS1 mislocalization to the cytosol (Figure 9 and Figure 11B), and a defect in removing the PTS2 region from PMDH (Figure 11A). Import defects in fatty acid β-oxidation mutants are also observed in mice; mutations in acyl-CoA oxidase lead to a lack of detectable peroxisomes in some mouse hepatocytes and catalase mislocalization to the cytosol (Fan et al. 1996).
Free fatty acids are toxic (Ho et al. 1995) and their accumulation in peroxisomes might damage enzymes or cofactors, causing size deregulation and matrix protein import defects. In humans, peroxisome biogenesis disorders can manifest when single enzymes in fatty acid β-oxidation are mutated, perhaps due to very long chain fatty acid accumulation (Waterham et al. 2015). Also, fatty acids accumulate in membranes, increasing membrane microviscosity in patients with peroxisome biogenesis disorders (Knazek et al. 1983; Whitcomb et al. 1988). Peroxisomal membrane fluidity might promote movement of membrane proteins and modulate the frequency of interactions of peroxisomal membrane proteins or complexes, such as the import and export peroxins (Fan et al. 1996). pxn mutants do not display noticeable matrix protein import defects despite displaying enlarged peroxisomes (Figure 12, B and D; Mano et al. 2011), contradicting a previous hypothesis that peroxisome enlargement might confer other defects by reducing the surface-to-volume ratio (De Craemer et al. 1991). However, it remains possible that reducing the surface-to-volume ratio might enhance previously existing defects.
Some developmental defects observed in β-oxidation mutants can be explained by reduced β-oxidation of plant hormone precursors. Mutations in fatty acid β-oxidation enzymes can confer IBA resistance (Figure 6E and Figure 7E; Adham et al. 2005; Lingard and Bartel 2009; Burkhart et al. 2013) and additional defects stemming from reduced auxin levels (Strader et al. 2011). β-oxidation also converts the precursor 12-oxo-phytodienoic acid into the plant hormone jasmonic acid. Some developmental defects in fatty acid β-oxidation mutants, such as abnormal inflorescences and flowers and reduced fertility may be caused by reduced jasmonate levels (Richmond and Bleecker 1999; Stintzi and Browse 2000; Delker et al. 2007; Schilmiller et al. 2007; Castillo and Leon 2008). However, additional defects in fatty acid β-oxidation mutants are not fully explained by loss of known β-oxidation products. For example, AIM1 and MFP2 are redundantly required for embryo development (Rylott et al. 2006), as are several ACX enzymes (Rylott et al. 2003; Khan et al. 2012). PED1 contributes to vegetative growth and floral development (Footitt et al. 2007). Defects in ACX proteins and PED1 lead to germination defects that are not rescued by provision of a fixed carbon source (Pinfield-Wells et al. 2005). Perhaps the matrix protein import defects observed in this work contribute to these additional developmental defects.
Peroxisome enlargement in pxn is prevented by impeding peroxisomal import of fatty acids
PXN is a transporter implicated in NAD+ (Agrimi et al. 2012; Bernhardt et al. 2012; van Roermund et al. 2016) and perhaps CoA (Agrimi et al. 2012) import into Arabidopsis peroxisomes. pxn mutants accumulate longer chain fatty acids and display minor oil body retention (Bernhardt et al. 2012) but are not sucrose dependent (Figure 12E), suggesting that pxn peroxisomes have sufficient cofactors to sustain enough fatty acid β-oxidation for growth immediately following germination. Indeed, our observation that weak mutants in fatty acid β-oxidation displayed oil body retention but not sucrose dependence also supports the conclusion that fatty acid β-oxidation happens at a higher rate than needed for normal seedling growth. It has been postulated that pxn β-oxidation defects are mild because seedling peroxisomes synthesized de novo from the ER come preloaded with NAD+ (Bernhardt et al. 2012).
In spite of minor physiological defects, pxn mutants show striking deregulation of peroxisome size (Figure 10 and Figure 12B; Mano et al. 2004). Although NAD+ recycling by PMDH within the peroxisome might compensate for reduced import, NAD+ might be sensitive to destruction by ROS (Antonenkov and Hiltunen 2012) and need to be replaced via PXN import. This necessity would be heightened following the first few days of development when the high rate of fatty acid β-oxidation generates more H2O2. Indeed, we found that decreasing fatty acid import by combining pxn with pxa1 fully rescued the enlarged peroxisome phenotype (Figure 13B). Because mutating PXA1 prevents H2O2 production from fatty acid β-oxidation (Eastmond 2007), the rescue of pxn peroxisome size by pxa1 indicates that fatty acid import, and perhaps H2O2 accumulation, exacerbates pxn defects.
We also examined a double mutant defective in PXN and PED1 and observed peroxisomes the size of ped1 peroxisomes (Figure 13B), again indicating fatty acid β-oxidation is needed to fully enlarge pxn peroxisomes. Interestingly, pxn worsened the matrix protein import defects of ped1-96 (Figure 13, B and D), suggesting that reduced NAD+ availability further impairs fatty acid β-oxidation and contributes to secondary defects. In contrast, blocking autophagy did not rescue the large peroxisome phenotype in pxn-4 (Figure 13A), suggesting that pxn peroxisomes are enlarged in an autophagy-independent mechanism.
Multiple functions for LON2
LON2 is a peroxisomal protease that is needed for peroxisome maintenance during seedling development (Lingard and Bartel 2009); peroxisomes are degraded via pexophagy when LON2 is dysfunctional (Farmer et al. 2013; Goto-Yamada et al. 2014). We isolated two lon2 nonsense alleles and one missense mutation. The lon2-9 missense allele displayed only a subset of phenotypes similar to null lon2 mutants whereas other phenotypes were milder (Figure 12). The altered Arg537 residue in lon2-9 is in the conserved arginine finger that is important for ATPase function in AAA+ proteins (Besche et al. 2004; Iyer et al. 2004; Snider and Houry 2008; Cha et al. 2010). The distinct phenotypes of lon2-9 suggest that the arginine finger is not essential for all LON2 functions. Perhaps the lon2-9 protein retains partial function because the AAA+ domain promotes normal peroxisome morphology but has a smaller role in other LON2 functions. This result agrees with previous findings that the AAA+ domain is needed for a function related to the microscopic phenotypes, whereas the protease domain is important for peroxisomal protein degradation (Goto-Yamada et al. 2014). The subset of defects displayed by lon2-9 further supports the notion that LON2 has multiple functions.
Distinct cofactor requirements for IBA-to-IAA conversion and fatty acid β-oxidation
PMDH1 and PMDH2 recycle NAD+ in the peroxisome (Pracharoenwattana et al. 2007). Interestingly, pmdh1 mutants and the pmdh1-1 pmdh2-1 double mutant were resistant to 2,4-DB but sensitive to IBA (Figure 5D). This result suggests that IBA-to-IAA conversion may be less dependent on NAD+ than fatty acid or 2,4-DB β-oxidation. In fatty acid β-oxidation, the MFP2 dehydrogenase step uses NAD+ (Figure 1). Our mfp2 mutants and previously characterized mfp2 mutants do not show strong IBA resistance (Figure 7E; Rylott et al. 2006). Instead, the dehydrogenase step of IBA β-oxidation might be catalyzed by IBR1, which contains a Rossmann fold that might bind NAD+, NADP+, or FAD (Zolman et al. 2008). The specific cofactor requirements of IBR1 have not been reported.
Conclusions
In summary, this microscopy-based screen recovered a suite of mutants providing insight into peroxisomal pathways. Our screen underscored the connection between peroxisome position (near oil bodies) and function (fatty acid β-oxidation) during seedling establishment. We detected matrix protein import defects in a subset of fatty acid β-oxidation mutants. Because many of the mutants delayed but did not eliminate lipid mobilization, many would not have been recovered had we screened earlier or later in development. Indeed, a previous screen for aberrant peroxisome morphology recovered drp3a (Mano et al. 2004), lon2 (Goto-Yamada et al. 2014), and pxn (Mano et al. 2011) alleles, which we also found, but did not report the metabolic mutants that made up the bulk of our isolates.
The depth of screening allowed recovery of multiple mutations in 10 of the 15 genes uncovered (Table 1), including mutants defective in the major seedling isoforms of most enzymes implicated in seedling fatty acid mobilization (Figure 1), mutants in a gene not previously implicated in oil body mobilization (PME31), and mutants not previously recovered in forward-genetic screens (acx2). We recovered not only null alleles but also partial loss-of-function alleles (ped1-4, icl-3, icl-4, and lon2-9) that confer phenotypes distinct from the corresponding null alleles and may aid in future structure-function analyses. This mutant collection will provide useful tools to address nuanced questions about peroxisomal processes in the future.
Acknowledgments
We thank Steven Smith (University of Western Australia) for pmdh1-1 pmdh1-2 seeds and the PMDH2 antibody, Ian Graham (University of York) for icl-1 seeds, Pierce Young for the lon2-9 genotyping marker, and Kim Gonzalez, Yun-Ting Kao, Roxanna Llinas, Andrew Woodward, Zachary Wright, Pierce Young, and an anonymous reviewer for critical comments on the manuscript. This research was supported by the National Science Foundation (MCB-1516966), the National Institutes of Health (NIH) (R01-GM079177), and the Robert A. Welch Foundation (C-1309). Confocal microscopy was performed on equipment obtained through a Shared Instrumentation grant from the NIH (S10-RR026399-01). Whole-genome sequencing at the Genome Technology Access Center at Washington University in St. Louis was supported by the NIH National Center for Research Resources (UL1-RR024992).
Footnotes
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.193169/-/DC1.
Communicating editor: S. Poethig
Literature Cited
- Adham A. R., Zolman B. K., Millius A., Bartel B., 2005. Mutations in Arabidopsis acyl-CoA oxidase genes reveal distinct and overlapping roles in β-oxidation. Plant J. 41: 859–874. [DOI] [PubMed] [Google Scholar]
- Agrimi G., Russo A., Pierri C. L., Palmieri F., 2012. The peroxisomal NAD+ carrier of Arabidopsis thaliana transports coenzyme A and its derivatives. J. Bioenerg. Biomembr. 44: 333–340. [DOI] [PubMed] [Google Scholar]
- Antonenkov V. D., Hiltunen J. K., 2012. Transfer of metabolites across the peroxisomal membrane. Biochim. Biophys. Acta 1822: 1374–1386. [DOI] [PubMed] [Google Scholar]
- Arent S., Christensen C. E., Pye V. E., Norgaard A., Henriksen A., 2010. The multifunctional protein in peroxisomal beta-oxidation: structure and substrate specificity of the Arabidopsis thaliana protein MFP2. J. Biol. Chem. 285: 24066–24077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung K., Hu J., 2009. The Arabidopsis peroxisome division mutant pdd2 is defective in the Dynamin-Related Protein3A (DRP3A) gene. Plant Signal. Behav. 4: 542–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel B., Farmer L. M., Rinaldi M. A., Young P. G., Danan C. H., et al. , 2014. Mutation of the Arabidopsis LON2 peroxisomal protease enhances pexophagy. Autophagy 10: 518–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge R. K., Aarsland A., Kryvi H., Bremer J., Aarsaether N., 1989. Alkylthioacetic acid (3-thia fatty acids)–a new group of non-beta-oxidizable, peroxisome-inducing fatty acid analogues. I. A study on the structural requirements for proliferation of peroxisomes and mitochondria in rat liver. Biochim. Biophys. Acta 1004: 345–356. [DOI] [PubMed] [Google Scholar]
- Bernhardt K., Wilkinson S., Weber A. P., Linka N., 2012. A peroxisomal carrier delivers NAD(+) and contributes to optimal fatty acid degradation during storage oil mobilization. Plant J. 69: 1–13. [DOI] [PubMed] [Google Scholar]
- Besche H., Tamura N., Tamura T., Zwickl P., 2004. Mutational analysis of conserved AAA+ residues in the archaeal Lon protease from Thermoplasma acidophilum. FEBS Lett. 574: 161–166. [DOI] [PubMed] [Google Scholar]
- Burkhart S. E., Lingard M. J., Bartel B., 2013. Genetic dissection of peroxisome-associated matrix protein degradation in Arabidopsis thaliana. Genetics 193: 125–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhart S. E., Kao Y. T., Bartel B., 2014. Peroxisomal ubiquitin-protein ligases Peroxin2 and Peroxin10 have distinct but synergistic roles in matrix protein import and Peroxin5 retrotranslocation in Arabidopsis. Plant Physiol. 166: 1329–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo M. C., Leon J., 2008. Expression of the beta-oxidation gene 3-ketoacyl-CoA thiolase 2 (KAT2) is required for the timely onset of natural and dark-induced leaf senescence in Arabidopsis. J. Exp. Bot. 59: 2171–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza J. L., Grisafi P. L., Fink G. R., 1995. A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev. 9: 2131–2142. [DOI] [PubMed] [Google Scholar]
- Cha S. S., An Y. J., Lee C. R., Lee H. S., Kim Y. G., et al. , 2010. Crystal structure of Lon protease: molecular architecture of gated entry to a sequestered degradation chamber. EMBO J. 29: 3520–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman K. D., Dyer J. M., Mullen R. T., 2012. Biogenesis and functions of lipid droplets in plants: thematic review series: lipid droplet synthesis and metabolism: from yeast to man. J. Lipid Res. 53: 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani P., Platts A., Wang le L., Coon M., Nguyen T., et al. , 2012. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 6: 80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornah J. E., Germain V., Ward J. L., Beale M. H., Smith S. M., 2004. Lipid utilization, gluconeogenesis, and seedling growth in Arabidopsis mutants lacking the glyoxylate cycle enzyme malate synthase. J. Biol. Chem. 279: 42916–42923. [DOI] [PubMed] [Google Scholar]
- Cui S., Hayashi Y., Otomo M., Mano S., Oikawa K., et al. , 2016. Sucrose production mediated by lipid metabolism suppresses physical interaction of peroxisomes and oil bodies during germination of Arabidopsis thaliana. J. Biol. Chem. DOI: 10.1074/jbc.M116.748814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Craemer D., Zweens M. J., Lyonnet S., Wanders R. J., Poll-The B. T., et al. , 1991. Very large peroxisomes in distinct peroxisomal disorders (rhizomelic chondrodysplasia punctata and acyl-CoA oxidase deficiency): novel data. Virchows Arch. A Pathol. Anat. Histopathol. 419: 523–525. [DOI] [PubMed] [Google Scholar]
- De Marcos Lousa C., van Roermund C. W., Postis V. L., Dietrich D., Kerr I. D., et al. , 2013. Intrinsic acyl-CoA thioesterase activity of a peroxisomal ATP binding cassette transporter is required for transport and metabolism of fatty acids. Proc. Natl. Acad. Sci. USA 110: 1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedeurwaerder S., Menu-Bouaouiche L., Mareck A., Lerouge P., Guerineau F., 2009. Activity of an atypical Arabidopsis thaliana pectin methylesterase. Planta 229: 311–321. [DOI] [PubMed] [Google Scholar]
- Delker C., Zolman B. K., Miersch O., Wasternack C., 2007. Jasmonate biosynthesis in Arabidopsis thaliana requires peroxisomal beta-oxidation enzymes–additional proof by properties of pex6 and aim1. Phytochemistry 68: 1642–1650. [DOI] [PubMed] [Google Scholar]
- Dietrich D., Schmuths H., De Marcos Lousa C., Baldwin J. M., Baldwin S. A., et al. , 2009. Mutations in the Arabidopsis peroxisomal ABC transporter COMATOSE allow differentiation between multiple functions in planta: insights from an allelic series. Mol. Biol. Cell 20: 530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond P. J., 2006. Sugar-dependent1 encodes a patatin domain triacylglycerol lipase that initiates storage oil breakdown in germinating Arabidopsis seeds. Plant Cell 18: 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond P. J., 2007. Monodehyroascorbate reductase 4 is required for seed storage oil hydrolysis and postgerminative growth in Arabidopsis. Plant Cell 19: 1376–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond P. J., Germain V., Lange P. R., Bryce J. H., Smith S. M., et al. , 2000a Postgerminative growth and lipid catabolism in oilseeds lacking the glyoxylate cycle. Proc. Natl. Acad. Sci. USA 97: 5669–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond P. J., Hooks M., Graham I. A., 2000b The Arabidopsis acyl-CoA oxidase gene family. Biochem. Soc. Trans. 28: 95–99. [PubMed] [Google Scholar]
- Enders T. A., Strader L. C., 2015. Auxin activity: past, present, and future. Am. J. Bot. 102: 180–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C. Y., Pan J., Chu R., Lee D., Kluckman K. D., et al. , 1996. Hepatocellular and hepatic peroxisomal alterations in mice with a disrupted peroxisomal fatty acyl-coenzyme A oxidase gene. J. Biol. Chem. 271: 24698–24710. [DOI] [PubMed] [Google Scholar]
- Farmer L. M., Rinaldi M. A., Young P. G., Danan C. H., Burkhart S. E., et al. , 2013. Disrupting autophagy restores peroxisome function to an Arabidopsis lon2 mutant and reveals a role for the LON2 protease in peroxisomal matrix protein degradation. Plant Cell 25: 4085–4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Footitt S., Slocombe S. P., Larner V., Kurup S., Wu Y., et al. , 2002. Control of germination and lipid mobilization by COMATOSE, the Arabidopsis homologue of human ALDP. EMBO J. 21: 2912–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Footitt S., Cornah J. E., Pracharoenwattana I., Bryce J. H., Smith S. M., 2007. The Arabidopsis 3-ketoacyl-CoA thiolase-2 (kat2–1) mutant exhibits increased flowering but reduced reproductive success. J. Exp. Bot. 58: 2959–2968. [DOI] [PubMed] [Google Scholar]
- Froyland L., Helland K., Totland G. K., Kryvi H., Berge R. K., 1996. A hypolipidemic peroxisome proliferating fatty acid induces polydispersity of rat liver mitochondria. Biol. Cell 87: 105–112. [PubMed] [Google Scholar]
- Fujimoto M., Arimura S., Mano S., Kondo M., Saito C., et al. , 2009. Arabidopsis dynamin-related proteins DRP3A and DRP3B are functionally redundant in mitochondrial fission, but have distinct roles in peroxisomal fission. Plant J. 58: 388–400. [DOI] [PubMed] [Google Scholar]
- Fulda M., Shockey J., Werber M., Wolter F. P., Heinz E., 2002. Two long-chain acyl-CoA synthetases from Arabidopsis thaliana involved in peroxisomal fatty acid β-oxidation. Plant J. 32: 93–103. [DOI] [PubMed] [Google Scholar]
- Fulda M., Schnurr J., Abbadi A., Heinz E., Browse J., 2004. Peroxisomal acyl-CoA synthetase activity is essential for seedling development in Arabidopsis thaliana. Plant Cell 16: 394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabaldon T., 2010. Peroxisome diversity and evolution. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365: 765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain V., Rylott E. L., Larson T. R., Sherson S. M., Bechntold N., et al. , 2001. Requirement for 3-ketoacyl-CoA thiolase-2 in peroxisome development, fatty acid β-oxidation and breakdown of triacylglycerol in lipid bodies of Arabidopsis seedlings. Plant J. 28: 1–12. [DOI] [PubMed] [Google Scholar]
- Goldfischer S., Collins J., Rapin I., Neumann P., Neglia W., et al. , 1986. Pseudo-Zellweger syndrome: deficiencies in several peroxisomal oxidative activities. J. Pediatr. 108: 25–32. [DOI] [PubMed] [Google Scholar]
- Goto S., Mano S., Nakamori C., Nishimura M., 2011. Arabidopsis aberrant peroxisome morphology9 is a peroxin that recruits the PEX1–PEX6 complex to peroxisomes. Plant Cell 23: 1573–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto-Yamada S., Mano S., Nakamori C., Kondo M., Yamawaki R., et al. , 2014. Chaperone and protease functions of LON protease 2 modulate the peroxisomal transition and degradation with autophagy. Plant Cell Physiol. 55: 482–496. [DOI] [PubMed] [Google Scholar]
- Gould S. J., Keller G. A., Hosken N., Wilkinson J., Subramani S., 1989. A conserved tripeptide sorts proteins to peroxisomes. J. Cell Biol. 108: 1657–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham I. A., 2008. Seed storage oil mobilization. Annu. Rev. Plant Biol. 59: 115–142. [DOI] [PubMed] [Google Scholar]
- Graham I. A., Li Y., Larson T. R., 2002. Acyl-CoA measurements in plants suggest a role in regulating various cellular processes. Biochem. Soc. Trans. 30: 1095–1099. [DOI] [PubMed] [Google Scholar]
- Hamada T., Igarashi H., Yao M., Hashimoto T., Shimmen T., et al. , 2006. Purification and characterization of plant dynamin from tobacco BY-2 cells. Plant Cell Physiol. 47: 1175–1181. [DOI] [PubMed] [Google Scholar]
- Haughn G. W., Somerville C., 1986. Sulfonylurea-resistant mutants of Arabidopsis thaliana. Mol. Gen. Genet. 204: 430–434. [Google Scholar]
- Hayashi H., Nito K., Takei-Hoshi R., Yagi M., Kondo M., et al. , 2002. Ped3p is a peroxisomal ATP-binding cassette transporter that might supply substrates for fatty acid β-oxidation. Plant Cell Physiol. 43: 1–11. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Toriyama K., Kondo M., Nishimura M., 1998. 2,4-dichlorophenoxybutyric acid-resistant mutants of Arabidopsis have defects in glyoxysomal fatty acid β-oxidation. Plant Cell 10: 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm M., Luck C., Prestele J., Hierl G., Huesgen P. F., et al. , 2007. Dual specificities of the glyoxysomal/peroxisomal processing protease Deg15 in higher plants. Proc. Natl. Acad. Sci. USA 104: 11501–11506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J. K., Moser H., Kishimoto Y., Hamilton J. A., 1995. Interactions of a very long chain fatty acid with model membranes and serum albumin. Implications for the pathogenesis of adrenoleukodystrophy. J. Clin. Invest. 96: 1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks M. A., Kellas F., Graham I. A., 1999. Long-chain acyl-CoA oxidases of Arabidopsis. Plant J. 20: 1–13. [DOI] [PubMed] [Google Scholar]
- Hu J., Baker A., Bartel B., Linka N., Mullen R. T., et al. , 2012. Plant peroxisomes: biogenesis and function. Plant Cell 24: 2279–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer L. M., Leipe D. D., Koonin E. V., Aravind L., 2004. Evolutionary history and higher order classification of AAA+ ATPases. J. Struct. Biol. 146: 11–31. [DOI] [PubMed] [Google Scholar]
- Kelly A. A., Quettier A. L., Shaw E., Eastmond P. J., 2011. Seed storage oil mobilization is important but not essential for germination or seedling establishment in Arabidopsis. Plant Physiol. 157: 866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan B. R., Adham A. R., Zolman B. K., 2012. Peroxisomal Acyl-CoA oxidase 4 activity differs between Arabidopsis accessions. Plant Mol. Biol. 78: 45–58. [DOI] [PubMed] [Google Scholar]
- Knazek R. A., Rizzo W. B., Schulman J. D., Dave J. R., 1983. Membrane microviscosity is increased in the erythrocytes of patients with adrenoleukodystrophy and adrenomyeloneuropathy. J. Clin. Invest. 72: 245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryvi H., Aarsland A., Berge R. K., 1990. Morphologic effects of sulfur-substituted fatty acids on rat hepatocytes with special reference to proliferation of peroxisomes and mitochondria. J. Struct. Biol. 103: 257–265. [DOI] [PubMed] [Google Scholar]
- Kunce C. M., Trelease R. N., Doman D. C., 1984. Ontogeny of glyoxysomes in maturing and germinated cotton seeds-a morphometric analysis. Planta 161: 156–164. [DOI] [PubMed] [Google Scholar]
- Lachtermacher M. B., Seuanez H. N., Moser A. B., Moser H. W., Smith K. D., 2000. Determination of 30 X-linked adrenoleukodystrophy mutations, including 15 not previously described. Hum. Mutat. 15: 348–353. [DOI] [PubMed] [Google Scholar]
- Lai Z., Wang F., Zheng Z., Fan B., Chen Z., 2011. A critical role of autophagy in plant resistance to necrotrophic fungal pathogens. Plant J. 66: 953–968. [DOI] [PubMed] [Google Scholar]
- Lange P., Eastmond P., Madagan K., Graham I., 2004. An Arabidopsis mutant disrupted in valine catabolism is also compromised in peroxisomal fatty acid beta-oxidation. FEBS Lett. 571: 147–153. [DOI] [PubMed] [Google Scholar]
- Li F., Vierstra R. D., 2012. Autophagy: a multifaceted intracellular system for bulk and selective recycling. Trends Plant Sci. 17: 526–537. [DOI] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., et al. , 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingard M. J., Bartel B., 2009. Arabidopsis LON2 is necessary for peroxisomal function and sustained matrix protein import. Plant Physiol. 151: 1354–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingard M. J., Monroe-Augustus M., Bartel B., 2009. Peroxisome-associated matrix protein degradation in Arabidopsis. Proc. Natl. Acad. Sci. USA 106: 4561–4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas K. A., Filley J. R., Erb J. M., Graybill E. R., Hawes J. W., 2007. Peroxisomal metabolism of propionic acid and isobutyric acid in plants. J. Biol. Chem. 282: 24980–24989. [DOI] [PubMed] [Google Scholar]
- Maeshima M., Yokoi H., Asahi T., 1988. Evidence for no proteolytic processing during transport of isocitrate lyase into glyoxysomes in castor bean endosperm. Plant Cell Physiol. 29: 381–384. [Google Scholar]
- Mano S., Nakamori C., Hayashi M., Kato A., Kondo M., et al. , 2002. Distribution and characterization of peroxisomes in Arabidopsis by visualization with GFP: dynamic morphology and actin-dependent movement. Plant Cell Physiol. 43: 331–341. [DOI] [PubMed] [Google Scholar]
- Mano S., Nakamori C., Kondo M., Hayashi M., Nishimura M., 2004. An Arabidopsis dynamin-related protein, DRP3A, controls both peroxisomal and mitochondrial division. Plant J. 38: 487–498. [DOI] [PubMed] [Google Scholar]
- Mano S., Nakamori C., Nito K., Kondo M., Nishimura M., 2006. The Arabidopsis pex12 and pex13 mutants are defective in both PTS1- and PTS2-dependent protein transport to peroxisomes. Plant J. 47: 604–618. [DOI] [PubMed] [Google Scholar]
- Mano S., Nakamori C., Fukao Y., Araki M., Matsuda A., et al. , 2011. A defect of peroxisomal membrane protein 38 causes enlargement of peroxisomes. Plant Cell Physiol. 52: 2157–2172. [DOI] [PubMed] [Google Scholar]
- McLachlan D. H., Lan J., Geilfus C. M., Dodd A. N., Larson T., et al. , 2016. The breakdown of stored triacylglycerols is required during light-induced stomatal opening. Curr. Biol. 26: 707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe-Augustus M., Ramon N. M., Ratzel S. E., Lingard M. J., Christensen S. E., et al. , 2011. Matrix proteins are inefficiently imported into Arabidopsis peroxisomes lacking the receptor-docking peroxin PEX14. Plant Mol. Biol. 77: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanly J., 1997. Auxin metabolism. Physiol. Plant. 100: 431–442. [Google Scholar]
- Olsen L. J., Ettinger W. F., Damsz B., Matsudaira K., Webb M. A., et al. , 1993. Targeting of glyoxysomal proteins to peroxisomes in leaves and roots of a higher plant. Plant Cell 5: 941–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinfield-Wells H., Rylott E. L., Gilday A. D., Graham S., Job K., et al. , 2005. Sucrose rescues seedling establishment but not germination of Arabidopsis mutants disrupted in peroxisomal fatty acid catabolism. Plant J. 43: 861–872. [DOI] [PubMed] [Google Scholar]
- Poll-The B. T., Roels F., Ogier H., Scotto J., Vamecq J., et al. , 1988. A new peroxisomal disorder with enlarged peroxisomes and a specific deficiency of acyl-CoA oxidase (pseudo-neonatal adrenoleukodystrophy). Am. J. Hum. Genet. 42: 422–434. [PMC free article] [PubMed] [Google Scholar]
- Pracharoenwattana I., Cornah J. E., Smith S. M., 2007. Arabidopsis peroxisomal malate dehydrogenase functions in beta-oxidation but not in the glyoxylate cycle. Plant J. 50: 381–390. [DOI] [PubMed] [Google Scholar]
- Ramón N. M., Bartel B., 2010. Interdependence of the peroxisome-targeting receptors in Arabidopsis thaliana: PEX7 facilitates PEX5 accumulation and import of PTS1 cargo into peroxisomes. Mol. Biol. Cell 21: 1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond T. A., Bleecker A. B., 1999. A defect in β-oxidation causes abnormal inflorescence development in Arabidopsis. Plant Cell 11: 1911–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L., Larner V., Kurup S., Bougourd S., Holdsworth M., 2000. The Arabidopsis comatose locus regulates germination potential. Development 127: 3759–3767. [DOI] [PubMed] [Google Scholar]
- Rylott E. L., Rogers C. A., Gilday A. D., Edgell T., Larson T. R., et al. , 2003. Arabidopsis mutants in short- and medium-chain acyl-CoA oxidase activities accumulate acyl-CoAs and reveal that fatty acid β-oxidation is essential for embryo development. J. Biol. Chem. 278: 21370–21377. [DOI] [PubMed] [Google Scholar]
- Rylott E. L., Eastmond P. J., Gilday A. D., Slocombe S. P., Larson T. R., et al. , 2006. The Arabidopsis thaliana multifunctional protein gene (MFP2) of peroxisomal beta-oxidation is essential for seedling establishment. Plant J. 45: 930–941. [DOI] [PubMed] [Google Scholar]
- Schilmiller A. L., Koo A. J., Howe G. A., 2007. Functional diversification of acyl-coenzyme A oxidases in jasmonic acid biosynthesis and action. Plant Physiol. 143: 812–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani N., Watkins P. A., Valle D., 1995. PXA1, a possible Saccharomyces cerevisiae ortholog of the human adrenoleukodystrophy gene. Proc. Natl. Acad. Sci. USA 92: 6012–6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani N., Sapag A., Valle D., 1996. Characterization and analysis of conserved motifs in a peroxisomal ATP-binding cassette transporter. J. Biol. Chem. 271: 8725–8730. [DOI] [PubMed] [Google Scholar]
- Smith J. J., Brown T. W., Eitzen G. A., Rachubinski R. A., 2000. Regulation of peroxisome size and number by fatty acid beta-oxidation in the yeast Yarrowia lipolytica. J. Biol. Chem. 275: 20168–20178. [DOI] [PubMed] [Google Scholar]
- Snider J., Houry W. A., 2008. AAA+ proteins: diversity in function, similarity in structure. Biochem. Soc. Trans. 36: 72–77. [DOI] [PubMed] [Google Scholar]
- Stasinopoulos T. C., Hangarter R. P., 1990. Preventing photochemistry in culture media by long-pass light filters alters growth of cultured tissues. Plant Physiol. 93: 1365–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzi A., Browse J., 2000. The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc. Natl. Acad. Sci. USA 97: 10625–10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader L. C., Bartel B., 2011. Transport and metabolism of the endogenous auxin precursor indole-3-butyric acid. Mol. Plant 4: 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader L. C., Wheeler D. L., Christensen S. E., Berens J. C., Cohen J. D., et al. , 2011. Multiple facets of Arabidopsis seedling development require indole-3-butyric acid-derived auxin. Plant Cell 23: 984–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinkels B. W., Gould S. J., Bodnar A. G., Rachubinski R. A., Subramani S., 1991. A novel, cleavable peroxisomal targeting signal at the amino-terminus of the rat 3-ketoacyl-CoA thiolase. EMBO 10: 3255–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thazar-Poulot N., Miquel M., Fobis-Loisy I., Gaude T., 2015. Peroxisome extensions deliver the Arabidopsis SDP1 lipase to oil bodies. Proc. Natl. Acad. Sci. USA 112: 4158–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. M., Strader L. C., 2015. Next-generation sequencing as a tool to quickly identify causative EMS-generated mutations. Plant Signal. Behav. 10: e1000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. M., Beisner E. R., Liu J., Venkova S. V., Strader L. C., 2014. Abscisic acid regulates root elongation through the activities of auxin and ethylene in Arabidopsis thaliana. G3 (Bethesda) 4: 1259–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roermund C. W., Tabak H. F., van Den Berg M., Wanders R. J., Hettema E. H., 2000. Pex11p plays a primary role in medium-chain fatty acid oxidation, a process that affects peroxisome number and size in Saccharomyces cerevisiae. J. Cell Biol. 150: 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roermund C. W., Schroers M. G., Wiese J., Facchinelli F., Kurz S., et al. , 2016. The peroxisomal NAD carrier from Arabidopsis imports NAD in exchange with AMP. Plant Physiol. 171: 2127–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain R. L., Wightman F., 1954. The growth-regulating activity of certain ω-substituted alkyl carboxylic acids in relation to their β-oxidation within the plant. Proc. R. Soc. Lond. B Biol. Sci. 142: 525–536. [DOI] [PubMed] [Google Scholar]
- Waterham H. R., Ferdinandusse S., Wanders R. J., 2016. Human disorders of peroxisome metabolism and biogenesis. Biochim. Biophys. Acta. 1863: 922–933. [DOI] [PubMed] [Google Scholar]
- Whitcomb R. W., Linehan W. M., Knazek R. A., 1988. Effects of long-chain, saturated fatty acids on membrane microviscosity and adrenocorticotropin responsiveness of human adrenocortical cells in vitro. J. Clin. Invest. 81: 185–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers M., Kohler W., Brennemann W., Boese V., Sokolowski P., et al. , 1999. X-linked adrenomyeloneuropathy associated with 14 novel ALD-gene mutations: no correlation between type of mutation and age of onset. Hum. Genet. 105: 116–119. [DOI] [PubMed] [Google Scholar]
- Wiszniewski A. A., Bussell J. D., Long R. L., Smith S. M., 2014. Knockout of the two evolutionarily conserved peroxisomal 3-ketoacyl-CoA thiolases in Arabidopsis recapitulates the abnormal inflorescence meristem 1 phenotype. J. Exp. Bot. 65: 6723–6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward A. W., Bartel B., 2005. Auxin: regulation, action, and interaction. Ann. Bot. (Lond.) 95: 707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward A. W., Fleming W. A., Burkhart S. E., Ratzel S. E., Bjornson M., et al. , 2014. A viable Arabidopsis pex13 missense allele confers severe peroxisomal defects and decreases PEX5 association with peroxisomes. Plant Mol. Biol. 86: 201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P. G., Bartel B., 2016. Pexophagy and peroxisomal protein turnover in plants. Biochim. Biophys. Acta 1863: 999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Hu J., 2009. Two small protein families, dynamin-related protein3 and fission1, are required for peroxisome fission in Arabidopsis. Plant J. 57: 146–159. [DOI] [PubMed] [Google Scholar]
- Zhang X., Hu J., 2010. The Arabidopsis chloroplast division protein dynamin- related protein5b also mediates peroxisome division. Plant Cell 22: 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. C., Hu J. P., 2008. Fission1A and fission1B proteins mediate the fission of peroxisomes and mitochondria in Arabidopsis. Mol. Plant 1: 1036–1047. [DOI] [PubMed] [Google Scholar]
- Zolman B. K., Bartel B., 2004. An Arabidopsis indole-3-butyric acid-response mutant defective in peroxin6, an apparent ATPase implicated in peroxisomal function. Proc. Natl. Acad. Sci. USA 101: 1786–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman B. K., Yoder A., Bartel B., 2000. Genetic analysis of indole-3-butyric acid responses in Arabidopsis thaliana reveals four mutant classes. Genetics 156: 1323–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman B. K., Monroe-Augustus M., Thompson B., Hawes J. W., Krukenberg K. A., et al. , 2001a chy1, an Arabidopsis mutant with impaired β-oxidation, is defective in a peroxisomal β-hydroxyisobutyryl-CoA hydrolase. J. Biol. Chem. 276: 31037–31046. [DOI] [PubMed] [Google Scholar]
- Zolman B. K., Silva I. D., Bartel B., 2001b The Arabidopsis pxa1 mutant is defective in an ATP-binding cassette transporter-like protein required for peroxisomal fatty acid beta-oxidation. Plant Physiol. 127: 1266–1278. [PMC free article] [PubMed] [Google Scholar]
- Zolman B. K., Nyberg M., Bartel B., 2007. IBR3, a novel peroxisomal acyl-CoA dehydrogenase-like protein required for indole-3-butyric acid response. Plant Mol. Biol. 64: 59–72. [DOI] [PubMed] [Google Scholar]
- Zolman B. K., Martinez N., Millius A., Adham A. R., Bartel B., 2008. Identification and characterization of Arabidopsis indole-3-butyric acid response mutants defective in novel peroxisomal enzymes. Genetics 180: 237–251. [DOI] [PMC free article] [PubMed] [Google Scholar]