Abstract
The assembly of the musculoskeletal system in Drosophila relies on the integration of chemical and mechanical signaling between the developing muscles with ectodermal cells specialized as “tendon cells.” Mechanical tension generated at the junction of flight muscles and tendon cells of the notum epithelium is required for muscle morphogenesis, and is balanced by the epithelium in order to not deform. We report that Drosophila Rho kinase (DRok) is necessary in tendon cells to assemble stable myotendinous junctions (MTJ), which are required for muscle morphogenesis and survival. In addition, DRok is required in tendon cells to maintain epithelial shape and cell orientation in the notum, independently of chascon (chas). Loss of DRok function in tendon cells results in mis-orientation of tendon cell extensions and abnormal accumulation of Thrombospondin and βPS-integrin, which may cause abnormal myotendinous junction formation and muscle morphogenesis. This role does not depend exclusively on nonmuscular Myosin-II activation (Myo-II), indicating that other DRok targets are key in this process. We propose that DRok function in tendon cells is key to promote the establishment of MTJ attachment and to balance mechanical tension generated at the MTJ by muscle compaction.
Keywords: myotendinous junction, Rho-kinase, epithelial morphogenesis
THE development of muscle–tendon interaction is a great example of how chemical and mechanical signaling between interacting tissues integrates to regulate cell differentiation and morphogenesis (Schweitzer et al. 2010; Xu et al. 2012; Weitkunat et al. 2014). In Drosophila, a great amount of work has been devoted to understanding the mechanisms by which embryonic muscles target and attach to specific epidermal muscle attachment sites (MAS) (Frommer et al. 1996; Subramanian et al. 2007; Wayburn and Volk 2009; Gilsohn and Volk 2010a, 2010b; Ordan et al. 2015; Ordan and Volk 2015), how MAS are specified by patterning signals (Volk and VijayRaghavan 1994; Usui et al. 2004), and the role of attachment in MAS differentiation as tendon cells (Nabel-Rosen et al. 1999, 2002; Volohonsky et al. 2007). Moreover, studies of the interaction between the indirect flight muscle (IFM) and the notum epithelium have contributed to unveiling the role of mechanical signaling in muscle and epithelial morphogenesis (Olguin et al. 2011; Weitkunat et al. 2014). Nevertheless, the mechanisms by which tendon cells sense chemical and mechanical cues at the developing junction to generate stable muscle–tendon connections and balance mechanical stress is still poorly understood.
Differentiating embryonic and adult tendon cells provide positional cues that direct the targeting of myotube filopodia toward them. Targeting of embryonic muscle toward tendon cells relies on the Slit-Robo system, the leucine-rich tendon specific protein (Lrt), and the matrix proteins Thrombospondin (Tsp) and Slowdown (Chanana et al. 2007; Subramanian et al. 2007; Wayburn and Volk 2009; Gilsohn and Volk 2010a, 2010b). Attachment initiates with the secretion of extracellular matrix components, including Tsp and laminin, and accumulation of integrins at myotube and tendon membranes (Subramanian et al. 2007; Maartens and Brown 2015). Subsequently, a few hours later, myofibrillogenesis begins, driving myotube compaction, which in turn pulls tendon cells inside the thoracic cavity (Metcalfe 1970; Olguin et al. 2011; Weitkunat et al. 2014). In response to mechanical tension, tendon cells then extend thin processes enriched in microtubules, F-actin, and Myosin-II (Myo-II) along their apical–basal axis (Reedy and Beall 1993; Olguin et al. 2011; Weitkunat et al. 2014), similar to embryonic tendon cells (Subramanian et al. 2003; Alves-Silva et al. 2008). Inhibiting tension buildup either by laser ablation of tendon processes or by genetically compromising muscle–tendon attachment formation, results in strong myofibrillogenesis defects, indicating that mechanical stimuli are required for muscle morphogenesis (Weitkunat et al. 2014). In addition, disturbing the structure of the actin meshwork of tendon cells, either by loss of function of the actin crosslinker jbug/Filamin or chas (a Jbug/Filamin partner), or interfering with nonmuscle Myo-II, results in cell and tissue deformation (Olguin et al. 2011). Thus, to establish muscle–tendon interactions and in order not to deform, tendon cells respond to pulling forces by adjusting their adhesive and mechanical properties through strengthening of the myotendinous junction and reorganization of the cytoskeleton.
Rho-kinase is a serine/threonine kinase that regulates cell adhesion, axon growth, planar cell polarity, and cytokinesis, among other cellular processes (Winter et al. 2001; Riento and Ridley 2003). In both vertebrates and flies, Rho-kinase regulates acto-myosin contractility. Once activated by Rho-GTPase, it phosphorylates the myosin regulatory light chain (MRLC) and the myosin-binding subunit of myosin phosphatase, leading to the activation of nonmuscle Myo-II and the formation of stress fibers and focal adhesion in cultured cells (Amano et al. 1996; Kimura et al. 1996; Kawano et al. 1999). Rho-kinase activation mediated by RhoA is regulated by integrin-based cell-matrix adhesion and requires traction forces mediated by cytoskeletal tension (Rossman et al. 2005; Schiller 2006; Bhadriraju et al. 2007). Since Rho-kinase signaling is situated at the interface between mechanical and chemical signaling, we analyze here its role in the development of muscle–tendon interactions and notum epithelium morphogenesis. Flies lacking Drosophila Rho kinase (DRok) in the notum epithelium display deformation of the notum epithelium and defects in the targeting of tendon cell processes to the IFMs, which leads to defective myotendinous junction, abnormal muscle morphogenesis, and detachment. In contrast to its role in regulating trichome number (Winter et al. 2001), DRok function in myotendinous junction formation does not rely exclusively on nonmuscle Myo-II, suggesting that other yet unknown DRok targets are crucial for this function.
Our work shows that DRok is required in tendon cells to establish stable connection with the IFMs and to respond to the pulling forces generated by muscle compaction in order to maintain epithelial integrity and polarity.
Materials and Methods
Fly strains and genetics
Overexpression studies were performed using the Gal4/UAS system (Brand and Perrimon 1993). We used the following UAS lines: UAS-DrokiR (3793 and 104675), UAS-chasiR (31766), UAS-MysiR (29619), UAS-SqhiR (7917) (Vienna Drosophila RNAi Center), UAS-CD8-ChRFP (Bloomington Drosophila Stock Center; BDSC), UAS-sqh[E20E21] (Winter et al. 2001), and UAS-DFosN Ala (Ciapponi et al. 2001). We also used the following Gal4 lines: Pnr-Gal4 and Sr-Gal4 (BDSC). Mitotic chas1 clones were generated using the FLP/FRT (Xu and Rubin 1993) marked with yellow. For chas rescue with DrokiR, and for Drok2 (BDSC) clonal analysis we used the mosaic analysis with a repressible cell marker (MARCM) system (Lee and Luo 1999). MHC-TauGFP was used to visualize muscle fibers (Chen and Olson 2001). All phenotypes were analyzed at 25° unless stated otherwise.
Immunohistochemistry and imaging
Primary antibodies were β-integrin (1/20, Hybridoma bank, DSHB), Tsp (1/100, courtesy of T. Volk, Department of Molecular Genetics, Weizmann Institute of Science, Rehovot, Israel), anti-phospho-sqh (1/500, courtesy of Robert E. Ward IV, Department of Molecular Biosciences, University of Kansas), and anti-GFP (1/1000, Molecular Probes, Eugene, OR). Secondary antibodies were from Jackson Immunological Laboratories (1/200), F-actin was stained with Rhodamine-phalloidin (1/500, Molecular Probes), and nuclei were stained with DAPI (1/1000, Molecular Probes). Dissected muscle preparations were obtained from staged pupae. Pupae of the desired age were removed from the pupal case, pinned down on Sylgard plates, and dissected in cold PBS. The fixation was carried out with 4% paraformaldehyde in PBS (PFA) for 40 min at room temperature. Following washes in PBS containing 0.3% Triton X-100, the tissue was incubated with antibodies diluted in 0.3% Triton X-100 and 10% bovine serum albumin as blocking reagent. Stained samples were mounted in Vectashield (Vector Laboratories, Burlingame, CA), confocal images were captured using a Carl Zeiss LSM 700 confocal microscope (Thornwood, NY). Agarose sectioning of pupae using a vibratome was performed according to (Weitkunat and Schnorrer 2014). For scanning electron microscopy adult flies were fixed in ethanol 95%, dried to critical point, sputter coated, and imaged with a Hitachi S-2600H scanning electron microscope. Pictures of adults were taken in an Infinity 1 digital camera and processed using Adobe Photoshop CS5 Extended.
Live imaging of pupal notum
White pupae of the indicated genotype were collected, aged at 25°, and mounted as described (Bellaiche et al. 2001). Images were acquired using a Leica TCS LSI confocal microscope. Fifteen confocal planes (0.2 μm depth) of nota expressing UAS-CD8-RFP under the control of srGal4 and MHC-TauGFP were taken at 20 min intervals for 5 hr. Filopodial angles of tendon cells and myotubes with respect to the lateral posterior end of the sr domain were measured in three independent movies by using ImageJ (National Institutes of Health), analyzed with Prism 4 software, shown as Rose diagrams (Rose 2.1.0, http://mypage.iu.edu/%7Etthomps/programs/home.htm), and analyzed with Student’s t-test. P values were calculated using Student’s t-test. A P value < 0.01 was considered to show a significant difference. For the filopodia angle profile, Welch’s correction was used assuming similar means, but differences in SD.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results and Discussion
DRok is required for notum epithelium morphogenesis and polarity
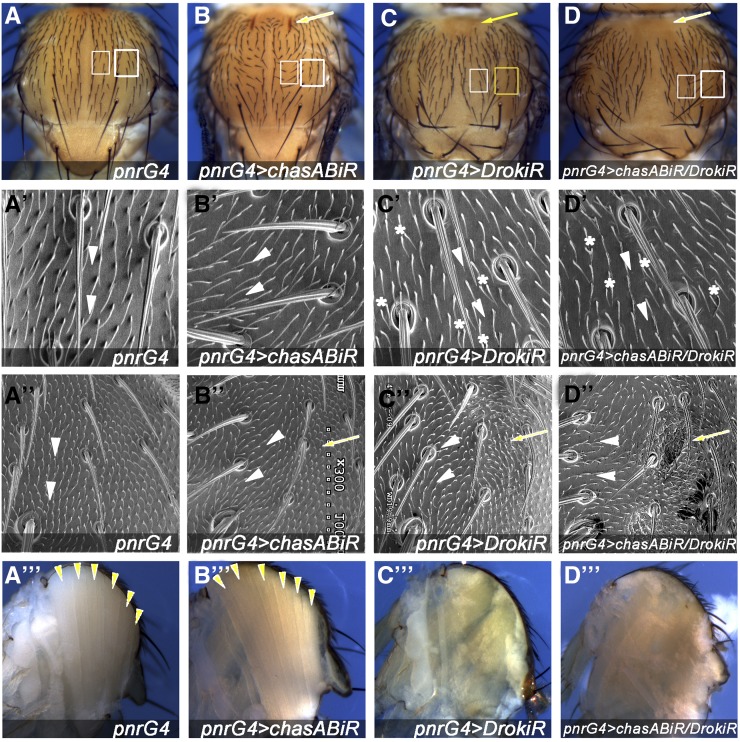
We have shown previously that nonmuscle Myo-II and chas are required in tendon cells to maintain notum epithelium shape and polarity in response to the pulling forces generated by dorsal longitudinal muscles (DLMs) compaction (Olguin et al. 2011). Since DRok is the main regulator of Myo-II activity through phosphorylation of MRLC, and its activity is regulated by mechanical tension and integrin signaling in other models (Rossman et al. 2005; Schiller 2006; Bhadriraju et al. 2007), we tested whether it plays a role in the mechanoresponse of tendon cells to mechanical forces generated by interactions with IFMs. To interfere with Drok expression we used two nonoverlapping double-stranded RNAis (dsRNAis), DrokiR and DrokiR2 (Supplemental Material, Figure S1). The expression of either dsRNAi targeting Drok under the control of the pnrGal4 driver results in mild notum closure defects at the midline (Figure 1, cf. C and 1A, and Figure S1) and in multiple hairs per cell, as has been described in the wing epithelium lacking fz or Drok (Figure 1C’) (Winter et al. 2001). Since both dsRNAis display similar phenotypes, we used DrokiR for subsequent experiments (Figure S1). DrokiR-expressing cells located at the most lateral region of the pnr expression domain display bristles and trichomes pointing toward the midline, similar to chas knockdown phenotypes (Figure 1, cf. C” and B”, arrowheads) (Olguin et al. 2011). At this lateral domain (Figure 1, A–C, indicated with yellow squares), animals expressing either chasiR or fziR in combination with DrokiR displayed enhanced trichome orientation phenotypes (Figure 1D” and Figure S2). In addition, scanning electron microscope (SEM) images reveal small invaginations of the epithelium toward the thoracic cavity (Figure 1D” and Figure S2), suggesting that Drok is required in tendon cells to maintain epithelial integrity and cellular orientation through the balance of the pulling forces generated by muscle compaction. Epithelial cell deformation is consistent with the deformation of Drok2 oocytes due to detachment of the apical membrane of follicular cells during oogenesis. This effect has been attributed to disorganization of cortical actin networks due to diminished levels of moesin and Myo-II (Verdier et al. 2006). DRok might play a similar role organizing the apical actin network through regulation of these proteins.
Figure 1.
Drok is required for notum morphogenesis. (A–D) Adult notum of flies expressing the indicated dsRNAi under the control of pnrGal4. Arrowheads indicate indentations of the notum epithelium. (A–D) White square marks central notum region analyzed in (A’–D’); yellow square indicates lateral regions analyzed in (A”–D”). (A’–D”) SEM images of adult flies showing orientation of trichomes (arrowheads), invaginations of the notum epithelium (arrows) and multiple hairs per cell (asterisks). (A”’–D”’) Sagittal sections of adult thorax, showing dorsal longitudinal muscles (DLMs) (arrowheads). Note that dorsal longitudinal IFMs appears to be reduced or absent (C”’) and (D”’).
Unexpectedly, Drok knockdown rescues both the indentation and the orientation defects associated with chas knockdown in the anterior and central region of the pnr domain, respectively (Figure 1, D and D’). Since the expression of a dominant negative of Zipper, the Drosophila Myo-II heavy chain, in a chas background enhances chas phenotypes (Olguin et al. 2011), we presume that other targets of DRok are involved in this phenotype.
Drok notum closure phenotypes are reminiscent of defective heminota migration toward the midline and/or fusion, a process that is finished by 6.5 hr after puparium formation (hAPF), previous to the early stages of muscle–tendon attachment (Zeitlinger and Bohmann 1999; Martin-Blanco et al. 2000). Heminota fusion defects associated with loss of Jnk result in bristle orientation toward the lateral side (with respect to the midline), similar to DrokiR expression, although in a much stronger manner (Martin-Blanco et al. 2000). Based on these data, we reasoned that defective heminota migration could indirectly revert chas cell orientation phenotypes. Knockdown of the Jnk pathway in combination with chas knockdown only partially suppresses cell orientation defects associated with chas loss of function, indicating that polarity defects associated with heminota migration or fusion are not sufficient to revert the chas phenotype (not shown). To avoid heminota migration defects, we expressed DrokiR in chas1 mitotic clones using the MARCM system (Lee and Luo 2001). Cells expressing DrokiR did not affect heminotum migration, and MARCM clones located at lateral and central positions of the notum reverted their chas1 orientation phenotypes (Figure S3). Taken together, these data show that Drok knockdown rescues chas associated cell orientation defects, independently of its role in dorsal thorax closure.
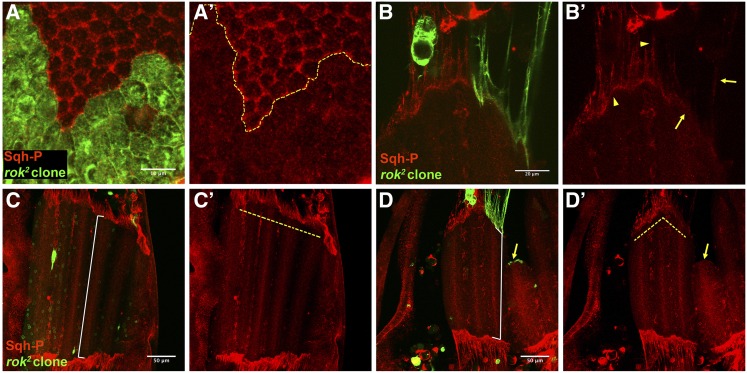
DRok is required in tendon cells for muscle survival independently of Myo-II
Since Myo-II knockdown and chas cell orientation phenotypes depend on the interaction of the notum epithelium with the underlying IFMs (Olguin et al. 2011), we reasoned that Drok could be required in tendon cells for muscle development or myotendinous junction formation in addition to its role in epithelial morphogenesis. Therefore, diminished DRok activity in tendons should reduce or abrogate pulling forces generated by muscle compaction. Sagittal sections of adults expressing DrokiR under pnrGal4 appears to lack dorsal longitudinal IFMs (Figure 1C”’), suggesting that Drok is required in tendon cells for muscle development or maintenance. Since MRLC, encoded by the spaghetti squash (sqh) gene, is the main effector of DRok, we asked whether its phosphorylation is altered in tendon cells lacking Drok. Drok2 mutant cells displayed reduced levels of phosphorylated Sqh (Sqh-P) from the apical level through tendon cells processes (Figure 2, A and B’) (Zhang and Ward 2011). At the myotendinous junction the reduction of Sqh-P staining appears weaker than at the other levels, which can be due to expression of Drok at the muscle cells. Interestingly, myotubes attached to mutant tendons are shorter displaying irregular anterior edge compared with its contralateral counterparts (Figure 2, cf. C and D, and C’ and D’). Moreover, other myotubes detach from the epithelium, supporting our previous observations in adults using DrokiR (Figure 2, D and D’). To answer whether inhibition of Myo-II activity results in muscle degeneration, we expressed a dsRNAi targeting (sqh), at different larval developmental stages using pnrGal4 and tubGal80ts to bypass its cytokinesis role (Murthy and Wadsworth 2005). Expression of sqhiR starting at second instar larval stage resulted in dorsal thorax closure defects and muscle degeneration, suggesting that the role of Drok in muscle development may depend on Myo-II activity, although this effect could be an indirect consequence of abnormal thorax closure (Figure S4). In addition, the activation of sqhiR expression at early third instar larval stage results in anterior invaginations of the notum epithelium and in cell orientation defects without affecting muscle integrity, confirming Myo-II role tendon cell adaptation to mechanical stress (Figure S4). Strikingly, the expression of a constitutively active form of MRLC (SqhE20E21) in the epithelium did not rescue the muscle degeneration phenotype associated with Drok loss of function (Figure 3, cf. I and H), but it did partially rescue notum dorsal closure and multiple hairs per cell phenotypes (Figure 3, A–F), as was shown previously in the wing epithelium (Winter et al. 2001), supporting the idea that other DRok targets are required in tendon cells for muscle survival.
Figure 2.
Drok loss of function in tendon cells results in diminished Sqh phosphorylation and disturbs muscle morphogenesis. (A, A’) Drok2 mutant clone of notum epithelial cells (27 hAPF) marked with CD8-GFP displays diminished levels of monophosphorylated Sqh (Sqh-P) at the apical side. (B, B’) Drok2 mutant tendon cell processes and myotendinous junction display diminished levels of Sqh-P (arrows), cf. wild type tendon extension and myotendinous junction (arrowheads). (C–D’) Confocal projections of DLMs at 27 hAPF of an animal carrying a Drok2 clone at the right side (D, D’). DLMs attached to wild type tendon cells display a regular anterior edge (dashed yellow line) (C’), while DLMs attached to a group of Drok2 tendon cell processes display irregular anterior edge (dashed yellow line) (D’) and display detachment of the epithelia (arrow). Note that myotubes attached to Drok2 tendon processes are shorter than the contra lateral muscles, cf. white guide of (C) and (D).
Figure 3.
Drok requirement for muscle survival does not depend exclusively on Myo-II. (A–C) Expression of SqhE20E21 driven by pnrG4 partially rescues dorsal thorax closure, cf. (B) and (C) (20% penetrance n = 20) (arrows); and multiple hairs per cell, indicated with yellow asterisks, cf. (D) with (E), P = 0.0022 (F), associated with DrokiR expression. Regions analyzed in (D) and (E) correspond to the region indicated with a square in (A). (G–I) Sagittal sections of thorax stained with phalloidin and DAPI. Expression of SqhE20E21 using srG4 driver does not rescue muscle degeneration associated to expression of DrokiR (arrowhead). (J–L) Detail showing myofibrils. Note that remaining muscle fibers are normal, cf. (K) and (L) with (J). (G–I) Bar, 200 μm. (J–L) Bar, 2 μm. Asterisks in (F) indicate significance P = 0.0022.
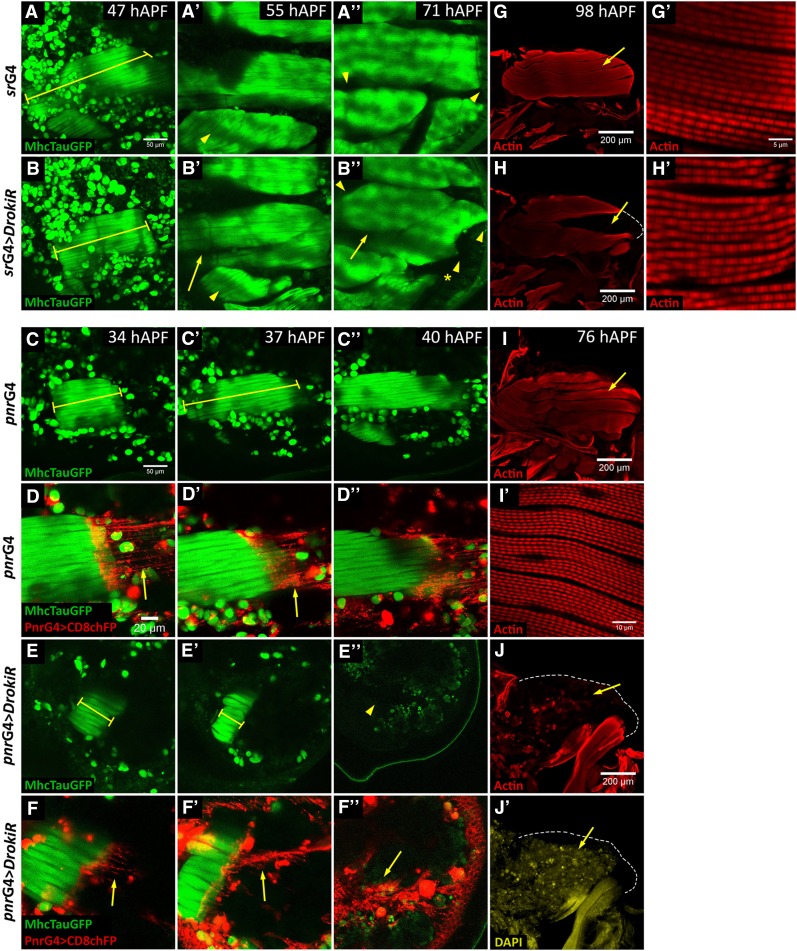
To investigate at which stage of development IFMs degenerate in Drok knockdown conditions, we monitored muscle development in animals expressing tauGFP under the control of the muscle myosin heavy chain promoter (MHC-tauGFP) (Chen and Olson 2001) (Figure 4). Animals expressing DrokiR under srGal4 (srG4 > DrokiR) displayed shortened muscles compared to control animals expressing only the srGal4 driver at 47 hAPF (Figure 4, cf. A and B). At 55 hAPF a gap develops between DLMs dorsal and ventral fibers, which display reduced size and irregular anterior and posterior edges, suggesting irregular attachments to the epithelium (Figure 4B’’). Sagittal sections of 98 hAPF nota reveal a gap between dorsal and ventral myotubes, indicating the lack of DLMs fibers (Figure 4, cf. H and G). Finally, inspection of remaining DLMs in srGal4 > DrokiR animals reveals mildly irregular myofibrils (Figure 4H’). To confirm our results we expressed DrokiR driven by pnrGal4 (pnrGal4 > DrokiR), since it resulted in stronger phenotypes than with srGal4. Like srGal4 > DrokiR, these animals displayed enhanced shortening of DLMs (Figure 4, cf. C and C’ with E and E’), which is already significant at 24 hAPF, although with similar width (Figure S5). In contrast to srGal4 > DrokiR, the overshortening of DLMs increases through development and correlates with irregular attachment to tendon cell processes (Figure 4, D’ and F’). Finally, pnrG4 > DrokiR animals display completely degenerated muscles, which is confirmed by sagittal sections of 76 hAPF nota (Figure 4, J and J’). We did not find differences in the number of nuclei between IFMs attached to either wild-type or DrokiR-expressing tendon cells at 24 hAPF using both drivers (Figure S5). Thus, we conclude that muscle morphological defects and degeneration occurs after myoblast fusion and muscle–tendon connection.
Figure 4.
Drok is required in tendon cells for muscle morphogenesis. (A–F”) Time lapse of DLMs development of flies expressing Tau-GFP under the control of MHC. Genotypes are as indicated to the left. (A, B) Note that developing DLMs remain short in flies expressing DrokiR under the control of srGal4 at 31hAPF, cf. (A) with (B) (rulers). (B’) At 55 hAPF a gap between dorsal and ventral myotubes is evident (arrow), cf. (B). Note that dorso-ventral muscle is smaller than the control, cf. (B) with (B’). (B”) At 71 hAPF lack DLMs are smaller than the control displaying abnormal shape at the anterior and posterior edges (arrowheads), cf. (A”). Note that anterior dorsoventral muscle is missing (B”) (asterisk) cf. (B). (C, E’) Overshortening of DLMs of flies expressing DrokiR under the control of pnrGal4. Note that overshortening increases from 34 to 37 hAPF (E, E’), cf. (C, C’). (E”) At 40 hAPF DLMs are absent, cf. (C”). (D, D”, F, F”) Details of (C, C”, E, E”) showing a confocal projection of the most dorsal tendon processes marked with CD8RFP. Note that tendon processes are irregularly attached to DLMs (arrows). (G–J’) Sagittal sections of 98 hAPF thorax stained with phalloidin (G–J) and DAPI (J’). (H) Gap generated between dorsal and ventral group of DLMs, cf. (G). (H’) Remaining myofibers appear mildly disorganized. (J) Absence of DLMs at 76 hAPF in animals expressing DrokiR.
Based on these results we propose that a muscle overcompaction and deformation phenotypes, along with the loss of myotube ability to extend back after compaction (Figure 4) are due to irregular attachment and unbalanced pulling forces by tendon cells. These phenotypes are consistent with the morphology of muscles observed in Drok2 mosaic animals, where myotubes attached to mutant tendon cells are shorter than myotubes attached to wild type cells, and even detach from mutant tendon cells (Figure 2). Since pulling forces would not be distributed homogeneously along the anterior and posterior edges of myotubes at the myotendinous junction, deformation of muscle fibers may result. This idea is in agreement with observations from Frank Schnorrer’s group, who has shown elegantly that pulling forces generated by muscle compaction at myotendinous junction are required for correct myofibrillogenesis and muscle morphogenesis (Weitkunat et al. 2014).
DRok is required in tendon cells for myotendinous junction formation
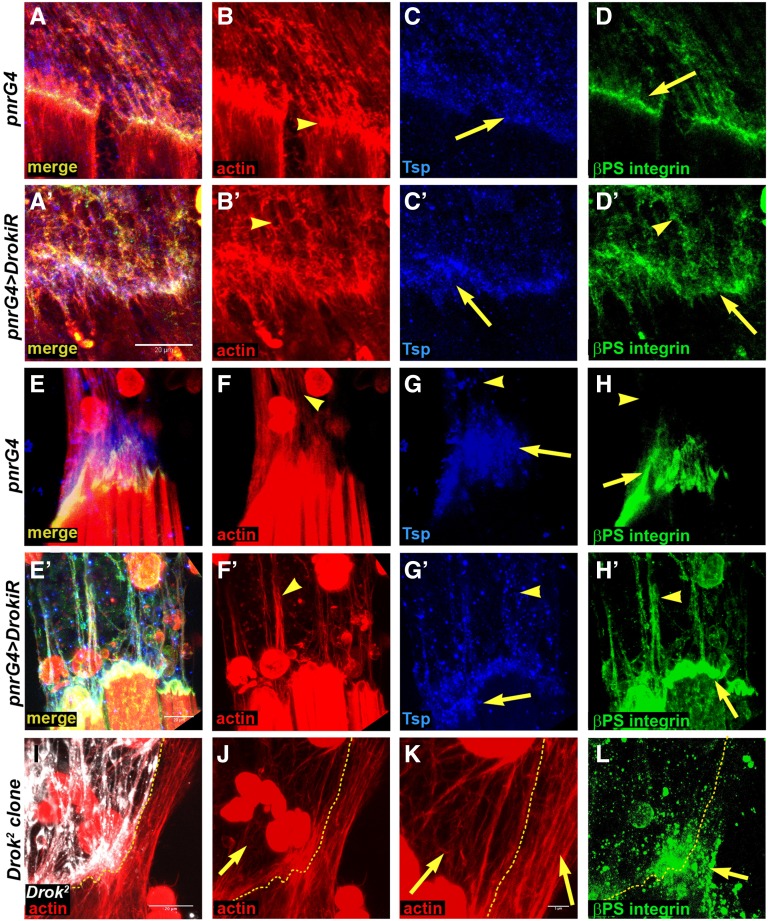
It has been shown previously that, during embryogenesis, the correct assembly of the myotendinous junction depends on the coordinated accumulation of αPS2-βPS integrin heterodimers at the muscle leading edge, with the deposition of the tendon-derived extracellular matrix (ECM) protein Tsp (Subramanian et al. 2007; Gilsohn and Volk 2010b). Tsp mediates the binding of αPS2-βPS heterodimers to the ECM during myotendinous junction formation, and its premature deposition results in accumulation of αPS2-βPS and irregular architecture of the myotendinous junction (Gilsohn and Volk 2010b). We observed that Drok loss of function resulted in normal accumulation of Tsp and irregular accumulation of βPS integrin at the attachment region at 24 hAPF (Figure 5, cf. D with D’). At 32 hAPF Tsp and βPS integrin accumulates along the tendon processes (Figure 5H’), with Tsp displaying a spotty pattern (Figure 5, G and G’). Strikingly, F-actin staining of notum epithelia expressing DrokiR, revealed disorganized tendon extensions that appeared to interact with each other or group into bundles (Figure 5, F and F’). Similarly, phalloidin staining of Drok2 tendon cell processes reveals disorganized filaments compared to wild-type cells, which display more parallel arrangement of tendon processes (Figure 5K, cf. left and right side). On the other hand, integrin staining accumulates mildly in a spotty fashion on Drok2 clone territory, although not as clearly as in the DrokiR condition (Figure 5L). This phenotype is reminiscent of animals with diminished expression of kon-tiki (kon) in embryonic myotubes (Schnorrer et al. 2007). Kon is a single pass transmembrane protein of the neurexin family that is required in ventrolateral embryonic myotubes to recognize its tendon targets and generate stable junctions (Schnorrer et al. 2007). How can loss of function of either DRok at tendons, or Kon at myotubes, generate such similar phenotypes? During development of the adult myotendinous junction, Kon appears to be required for the accumulation of βPS integrin at the tips of myotubes (Weitkunat et al. 2014). Therefore its loss of function results in defective recognition between tendon and myotubes. Accordingly, abnormal deposition of Tsp by tendon cells lacking Drok might lead to defective recognition by myotube αPS2-βPS integrin receptors and abnormal myotendinous junction formation.
Figure 5.
Drok is required for myotendinous junction formation. Confocal projections of myotendinous junctions at 24 hAPF (A–D’) and 32 hAPF (E–H’) stained with phalloidin/F-actin (B, B’, F, F’, J, K), Tsp (C, C’, G, G’) and βPS-integrin (D, D’, H, H’, L). Genotypes as indicated on left. (B, B’, F, F’, J, K) Tendon cells extensions arrange irregularly: cf. (B) and (B’), (F) and (F’), and Drok2 clones [I, J, K (detail)] (arrows). Tsp and βPS-integrin accumulate irregularly in tendon cell extensions (arrowheads) and myotube edge (arrows), cf. (C’) and (C); (D’) and (D); (G’) and (G); (H’) and (H); and mitotic clones (L). Note that at 24 hAPF, at the myotendinous junction, the abnormal accumulation of Tsp and integrin is not as aberrant as at 32 hAPF. Bar, 20 μm for all images and 5 μm in (K).
DRok regulates tendon extension orientation and shape
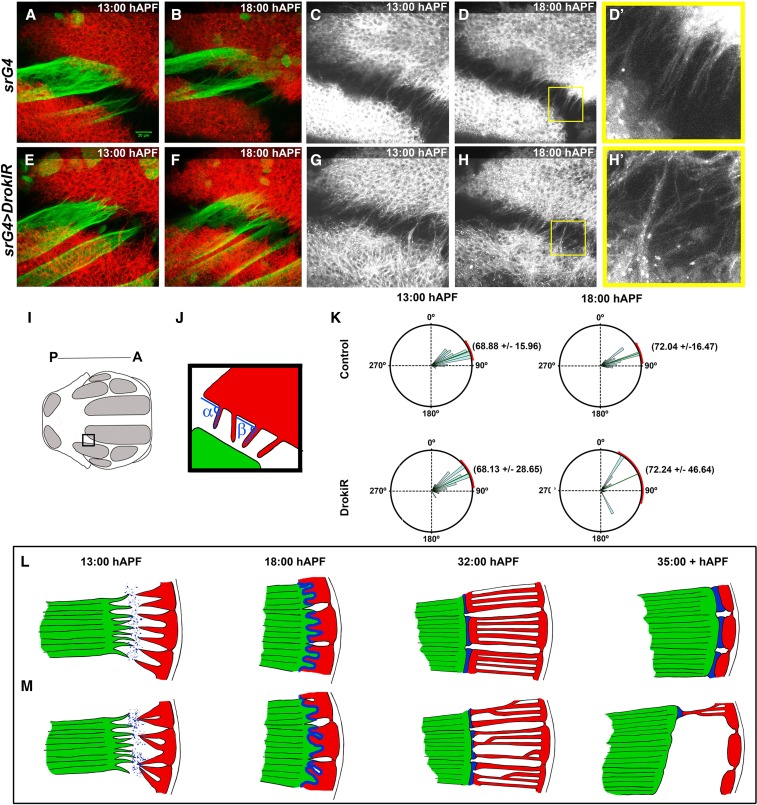
Since Kon and Drok display similar phenotypes during the myotube compaction stage, we reasoned that junction and muscle phenotypes are a consequence of defects that occur at earlier stages of myotendinous junction establishment. We monitored attachment initiation using time lapse confocal microscopy of flies expressing MHC-tau-GFP and CD8-RFP driven by srGal4, to examine myotube and tendon processes, respectively (Figure 6, Figure S6, File S1, and File S2). Tendon extensions of cells expressing DrokiR appeared disorganized and to cluster from 13 to 18 hAPF (Figure 6, D and D’, H and H’). Measurements of the angles formed between tendon processes and the lateral–posterior edge of the stripe domain (Figure 6, I and J) showed that DrokiR SD of angle measurements doubled the wild type value at 13 hAPF (P < 0.0001) and tripled it by 18 hAPF (P < 0.0001) (Figure 6K). Interestingly, the SD increased significantly during that time in DrokiR expressing animals (P = 0.0042) in contrast to wild type, which maintained a similar distribution and average angle values. Distribution of angle measurements of myotube extension of DrokiR was wider than controls at 13 hAPF (P < 0.0001), but did not show significant differences at 18 hAPF (Figure S6). Taken together, these results indicate that DRok is required in tendon cells for orientation of tendon extension during attachment initiation.
Figure 6.
Drok regulates tendon processes orientation and morphology. (A–H) Time points of time-lapse confocal microscopy movies taken from 13 to 18 hAPF of flies expressing MHC-TauGFP and CD8-RFP driven by srGal4. (D’ and H’) Details of (D and H) with a magnification of 3X. (I) Thorax region imaged, posterior is to the left and anterior to the right. (J) Method to measure the angles formed between tendon processes and the lateral–posterior edge of the sr domain. (K) Quantification of tendon extensions angles (P < 0.0001). (L, M) Scheme of myotendinous junction formation under normal (L) and Drok loss of function (M) conditions. Tendon cells (red) with Drok loss of function display irregular morphology and orientation during attachment initiation, abnormal secretion of matrix proteins (blue), failure to attach uniformly to myotubes (green), and detachment during muscle compaction.
Conclusions
The RhoA-ROCK pathway plays a key role in coordinating mechanical and chemical signaling in a variety of processes, including human mesenchymal stem cell differentiation into tendon/ligament-like lineages (Riento and Ridley 2003; Xu et al. 2012). We propose that coordination between “outside-in and inside-out mechanical signaling” called mechano-reciprocity, might play a fundamental role in tendon cells for myotendinous junction formation. In our model, Drok is pivotal for mechano-reciprocity at the myotendinous junction (Figure 6, L and M). Tendon cells with diminished levels of Drok would not be able to respond to mechanical signaling from ECM (outside-in) leading to production of unstable cellular extensions with a wider range of orientations and as a consequence, abnormal secretion (inside-out) of ECM proteins that are required for myotube filopodia attachment, such as Tsp (13–18 hAPF, Figure 6, cf. M and L). Irregular and unstable muscle tendon attachments (Figure 6M, 32 hAPF) result in muscle morphogenesis defects, detachment, and death (Figure 6M, 35+ hAPF).
In summary we have shown that Drok is not only required for epithelial morphogenesis and polarity, but importantly also for myotendinous junction formation and subsequently for muscle development. Our genetic and cellular analyses suggest that DRok is required at different cellular levels, and its role in myotendinous junction formation does not depend exclusively on Myo-II activation, indicating that other DRok targets are key in this process.
Acknowledgments
We thank Alvaro Glavic, Jimena Sierralta, Kenneth Prehoda, Daniel Kiehart, Robert E. Ward IV, and Talila Volk, Berkeley Drosophila Genome Project, Vienna Drosophila RNAi Stock Center, and the Bloomington Center for flies and reagents; Angélica Figueroa, Fernando Vergara, Paola Sepúlveda, Inés Negrete, and Noemi Candia for technical support; and Ursula Weber for advice and discussion. This work was supported by National Institutes of Health grant GM-62917 to M.M., FONDECYT no. 112053 and Anillo ACT-1401 grants, and Program U-Apoya (University of Chile) to P.O. C.M. is a recipient of a fellowship for graduate students from Consejo Nacional de Ciencia y Technológia.
Footnotes
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.189548/-/DC1.
Communicating editor: I. K. Hariharan
Literature Cited
- Alves-Silva J., Hahn I., Huber O., Mende M., Reissaus A., et al. , 2008. Prominent actin fiber arrays in Drosophila tendon cells represent architectural elements different from stress fibers. Mol. Biol. Cell 19: 4287–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M., Ito M., Kimura K., Fukata Y., Chihara K., et al. , 1996. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271: 20246–20249. [DOI] [PubMed] [Google Scholar]
- Bellaiche Y., Gho M., Kaltschmidt J. A., Brand A. H., Schweisguth F., 2001. Frizzled regulates localization of cell-fate determinants and mitotic spindle rotation during asymmetric cell division. Nat. Cell Biol. 3: 50–57. [DOI] [PubMed] [Google Scholar]
- Bhadriraju K., Yang M., Alom Ruiz S., Pirone D., Tan J., et al. , 2007. Activation of ROCK by RhoA is regulated by cell adhesion, shape, and cytoskeletal tension. Exp. Cell Res. 313: 3616–3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N., 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- Chanana B., Graf R., Koledachkina T., Pflanz R., Vorbruggen G., 2007. AlphaPS2 integrin-mediated muscle attachment in Drosophila requires the ECM protein Thrombospondin. Mech. Dev. 124: 463–475. [DOI] [PubMed] [Google Scholar]
- Chen E. H., Olson E. N., 2001. Antisocial, an intracellular adaptor protein, is required for myoblast fusion in Drosophila. Dev. Cell 1: 705–715. [DOI] [PubMed] [Google Scholar]
- Ciapponi L., Jackson D. B., Mlodzik M., Bohmann D., 2001. Drosophila Fos mediates ERK and JNK signals via distinct phosphorylation sites. Genes Dev. 15: 1540–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommer G., Vorbruggen G., Pasca G., Jackle H., Volk T., 1996. Epidermal egr-like zinc finger protein of Drosophila participates in myotube guidance. EMBO J. 15: 1642–1649. [PMC free article] [PubMed] [Google Scholar]
- Gilsohn E., Volk T., 2010a Fine tuning cellular recognition: the function of the leucine rich repeat (LRR) trans-membrane protein, LRT, in muscle targeting to tendon cells. Cell Adhes. Migr. 4: 368–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsohn E., Volk T., 2010b Slowdown promotes muscle integrity by modulating integrin-mediated adhesion at the myotendinous junction. Development 137: 785–794. [DOI] [PubMed] [Google Scholar]
- Kawano Y., Fukata Y., Oshiro N., Amano M., Nakamura T., et al. , 1999. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J. Cell Biol. 147: 1023–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K., Ito M., Amano M., Chihara K., Fukata Y., et al. , 1996. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273: 245–248. [DOI] [PubMed] [Google Scholar]
- Lee T., Luo L., 1999. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22: 451–461. [DOI] [PubMed] [Google Scholar]
- Lee T., Luo L., 2001. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 24: 251–254. [DOI] [PubMed] [Google Scholar]
- Maartens A. P., Brown N. H., 2015. The many faces of cell adhesion during Drosophila muscle development. Dev. Biol. 401: 62–74. [DOI] [PubMed] [Google Scholar]
- Martin-Blanco E., Pastor-Pareja J. C., Garcia-Bellido A., 2000. JNK and decapentaplegic signaling control adhesiveness and cytoskeleton dynamics during thorax closure in Drosophila. Proc. Natl. Acad. Sci. USA 97: 7888–7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe J. A., 1970. Developmental genetics of thoracic abnormalities of dumpy mutants of Drosophila melanogaster. Genetics 65: 627–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy K., Wadsworth P., 2005. Myosin-II-dependent localization and dynamics of F-actin during cytokinesis. Curr. Biol. 15: 724–731. [DOI] [PubMed] [Google Scholar]
- Nabel-Rosen H., Dorevitch N., Reuveny A., Volk T., 1999. The balance between two isoforms of the Drosophila RNA-binding protein how controls tendon cell differentiation. Mol. Cell 4: 573–584. [DOI] [PubMed] [Google Scholar]
- Nabel-Rosen H., Volohonsky G., Reuveny A., Zaidel-Bar R., Volk T., 2002. Two isoforms of the Drosophila RNA binding protein, how, act in opposing directions to regulate tendon cell differentiation. Dev. Cell 2: 183–193. [DOI] [PubMed] [Google Scholar]
- Olguin P., Glavic A., Mlodzik M., 2011. Intertissue mechanical stress affects frizzled-mediated planar cell polarity in the Drosophila notum epidermis. Curr. Biol. 21: 236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordan E., Volk T., 2015. A non-signaling role of Robo2 in tendons is essential for Slit processing and muscle patterning. Development 142: 3512–3518. [DOI] [PubMed] [Google Scholar]
- Ordan E., Brankatschk M., Dickson B., Schnorrer F., Volk T., 2015. Slit cleavage is essential for producing an active, stable, non-diffusible short-range signal that guides muscle migration. Development 142: 1431–1436. [DOI] [PubMed] [Google Scholar]
- Reedy M. C., Beall C., 1993. Ultrastructure of developing flight muscle in Drosophila. II. Formation of the myotendon junction. Dev. Biol. 160: 466–479. [DOI] [PubMed] [Google Scholar]
- Riento K., Ridley A. J., 2003. Rocks: multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 4: 446–456. [DOI] [PubMed] [Google Scholar]
- Rossman K. L., Der C. J., Sondek J., 2005. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 6: 167–180. [DOI] [PubMed] [Google Scholar]
- Schiller M. R., 2006. Coupling receptor tyrosine kinases to Rho GTPases–GEFs what’s the link. Cell. Signal. 18: 1834–1843. [DOI] [PubMed] [Google Scholar]
- Schnorrer F., Kalchhauser I., Dickson B. J., 2007. The transmembrane protein Kon-tiki couples to Dgrip to mediate myotube targeting in Drosophila. Dev. Cell 12: 751–766. [DOI] [PubMed] [Google Scholar]
- Schweitzer R., Zelzer E., Volk T., 2010. Connecting muscles to tendons: tendons and musculoskeletal development in flies and vertebrates. Development 137: 2807–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A., Prokop A., Yamamoto M., Sugimura K., Uemura T., et al. , 2003. Shortstop recruits EB1/APC1 and promotes microtubule assembly at the muscle-tendon junction. Curr. Biol. 13: 1086–1095. [DOI] [PubMed] [Google Scholar]
- Subramanian A., Wayburn B., Bunch T., Volk T., 2007. Thrombospondin-mediated adhesion is essential for the formation of the myotendinous junction in Drosophila. Development 134: 1269–1278. [DOI] [PubMed] [Google Scholar]
- Usui K., Pistillo D., Simpson P., 2004. Mutual exclusion of sensory bristles and tendons on the notum of dipteran flies. Curr. Biol. 14: 1047–1055. [DOI] [PubMed] [Google Scholar]
- Verdier V., Johndrow J. E., Betson M., Chen G. C., Hughes D. A., et al. , 2006. Drosophila Rho-kinase (DRok) is required for tissue morphogenesis in diverse compartments of the egg chamber during oogenesis. Dev. Biol. 297: 417–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk T., VijayRaghavan K., 1994. A central role for epidermal segment border cells in the induction of muscle patterning in the Drosophila embryo. Development 120: 59–70. [DOI] [PubMed] [Google Scholar]
- Volohonsky G., Edenfeld G., Klambt C., Volk T., 2007. Muscle-dependent maturation of tendon cells is induced by post-transcriptional regulation of stripeA. Development 134: 347–356. [DOI] [PubMed] [Google Scholar]
- Wayburn B., Volk T., 2009. LRT, a tendon-specific leucine-rich repeat protein, promotes muscle-tendon targeting through its interaction with Robo. Development 136: 3607–3615. [DOI] [PubMed] [Google Scholar]
- Weitkunat M., Schnorrer F., 2014. A guide to study Drosophila muscle biology. Methods 68: 2–14. [DOI] [PubMed] [Google Scholar]
- Weitkunat M., Kaya-Copur A., Grill S. W., Schnorrer F., 2014. Tension and force-resistant attachment are essential for myofibrillogenesis in Drosophila flight muscle. Curr. Biol. 24: 705–716. [DOI] [PubMed] [Google Scholar]
- Winter C. G., Wang B., Ballew A., Royou A., Karess R., et al. , 2001. Drosophila rho-associated kinase (Drok) links frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell 105: 81–91. [DOI] [PubMed] [Google Scholar]
- Xu B., Song G., Ju Y., Li X., Song Y., et al. , 2012. RhoA/ROCK, cytoskeletal dynamics, and focal adhesion kinase are required for mechanical stretch-induced tenogenic differentiation of human mesenchymal stem cells. J. Cell. Physiol. 227: 2722–2729. [DOI] [PubMed] [Google Scholar]
- Xu T., Rubin G. M., 1993. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117: 1223–1237. [DOI] [PubMed] [Google Scholar]
- Zeitlinger J., Bohmann D., 1999. Thorax closure in Drosophila: involvement of Fos and the JNK pathway. Development 126: 3947–3956. [DOI] [PubMed] [Google Scholar]
- Zhang L., Ward R. E. t., 2011. Distinct tissue distributions and subcellular localizations of differently phosphorylated forms of the myosin regulatory light chain in Drosophila. Gene Expr. Patterns 11: 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.