Abstract
Objective
To assess whether the degree of steatosis as determined by Controlled Attenuation Parameter (CAP) measurements correlates with that observed on liver biopsies in a single-center pediatric and young adult cohort.
Study design
This was a cross-sectional study in patients undergoing liver biopsy as part of standard clinical care between January 25, 2012, and April 1, 2015 at Boston Children’s Hospital. Eligible patients, with a variety of liver diseases, had CAP measurements within 1 year of biopsy. CAP values were compared across histologic steatosis grades using ANOVA.
Results
Sixty-nine patients (mean age, 16.0 ± 2.9 years; 62% male) were studied. CAP measurements were obtained at a median of 1.3 months (IQR, 0.5–3.2) after biopsy. Of the 69 subjects, 23 had steatosis on biopsy. Mean CAP value (dB/m) for subjects with no steatosis was 198 ± 37 vs 290 ± 47 for subjects with steatosis (P < .0001). There were statistically significant differences between CAP values in individuals with no steatosis vs mild/moderate steatosis (P < .0001), no steatosis vs marked steatosis (P < .0001), and mild/moderate vs marked steatosis (P = .004)
Conclusion
This study demonstrated a difference in CAP between no steatosis and steatosis, and between grades of steatosis. CAP may be a useful non-invasive tool to detect hepatic steatosis in children.
Keywords: Non-alcoholic fatty liver disease, NAFLD, liver disease, child, fatty liver, elastography
Introduction
Hepatic steatosis results from the accumulation of excess lipids in hepatocytes and is considered abnormal when the hepatic fat content exceeds 5% of liver weight. Non-alcoholic fatty liver disease (NAFLD) is currently the most common form of chronic liver disease in both children and adults.1 Based on an autopsy study, one in 10 children or adolescents in the United States has NAFLD.2 A recent systematic review and meta-analysis of children ages 1–19 years reported a pooled mean prevalence of NAFLD of 7.6% among children treated at general clinics, and 34% among children treated at obesity clinics.3 Aside from obesity and metabolic syndrome, other diseases and medications can result in hepatic fat accumulation.4
The most commonly used noninvasive tests to diagnose NAFLD include liver enzymes and ultrasound. However, these lack sensitivity and the ability to consistently detect and quantify hepatic fibrosis. In the SAFETY study, the sensitivity of ALT for the detection of NAFLD, hepatitis B, and hepatitis C ranged from 32–48% when using hospital based cut-offs.5 Ultrasound is machine and operator dependent, is affected by extrahepatic adipose tissue, has low positive predictive value, and has low sensitivity when steatosis is less than 30%.6,7 Liver biopsy remains the standard for assessment of the degree of steatosis but is limited by invasiveness, cost, and the potential for sampling error, and rarely can result in complications.8 Other methods include quantification of fat by computed tomography (CT) and magnetic resonance imaging (MRI), but these are expensive and either involve radiation or require special technology, so are not routinely used at this time in clinical practice.9
A novel non-invasive method utilizing ultrasound, called Controlled Attenuation Parameter (CAP), has been developed to assess hepatic steatosis. Since fat affects ultrasound propagation, CAP is based on the radiofrequency ultrasound signal acquired by the transient elastography device (Fibroscan® Echosens, Paris, France). CAP is an estimate of the ultrasonic attenuation coefficient at 3.5 MHz. It is evaluated in the same region of interest as that used for liver stiffness measurement and uses the same radio-frequency data. CAP can only be acquired if the TE liver stiffness measurement is valid (since it depends on an ultrasonic attenuation value). 10 These measures are reproducible, as well as operator and machine independent.11
Given the ability of CAP to detect and grade hepatic steatosis in adults, the aim of this study was to assess whether the degree of steatosis as determined by liver biopsy correlates with CAP measurements in a pediatric and young adult cohort with liver disease.
Methods
This is a cross sectional study of children and young adults who underwent liver biopsy and CAP measurement at Boston Children’s Hospital between January 2012 and April 2015. Patients with incomplete critical biochemical data or unavailable/uninterpretable biopsy specimens were not included. Patients were also excluded if they were not candidates for CAP because of ascites, pregnancy, or an implantable cardiac device (as suggested by the manufacturer). Clinical and biochemical data were the closest values within 6 months before or after the date of CAP measurement. Data collected included AST, ALT, GGTP, and albumin. Anthropometric data includes height and weight.
This study was approved by the Boston Children’s Hospital Institutional Review Board. Written informed consent was obtained from parents, guardians, and subjects 18 years of age and older.
Liver Histology
All subjects underwent liver biopsy for clinical indications. Histologic specimens were retrospectively reviewed by one of two pathologists who were blinded to clinical diagnosis, initial pathology assessment, and CAP measurement. A biopsy specimen was considered adequate for evaluation if 1.5 cm long and it contained a minimum of 6 portal tracts. Steatosis was categorized as: None (S0, <5%); Mild (S1, 5–30%); Moderate (S2, 31–60%), and Marked (S3, >60%).
CAP Measurement
Hepatic steatosis was measured by CAP using signals acquired by FibroScan® (Echosens, Paris, France). CAP measures ultrasonic attenuation in the liver at 3.5 MHz at depth between 25 and 65 mm. CAP was performed by trained study investigators who were certified by the manufacturer and blinded to the liver biopsy results. All female subjects aged >12 years were required to have a negative urine pregnancy test before undergoing CAP. All subjects had a thoracic perimeter >75 cm and CAP measurements were ascertained using the “M” or “XL” probe according to the manufacturer’s specifications. CAP measurements are not available using the “S” probe, so children with thoracic perimeter < 75 cm were excluded. The reported CAP measurement (dB/m) was the median of 10 measurements.
Statistical Analysis
Categorical data are reported as frequency (%) and continuous data as mean ± SD when normally distributed and median (interquartile range; IQR) otherwise. CAP measurements were approximately normally distributed, but due to the small sample size were analyzed both non-parametrically and parametrically. Results were consistent, and only parametric results are shown. Subjects with mild (n=7) and moderate (n=4) steatosis were combined to increase power. Two-group comparisons were made with Student’s t-test, and three-group comparisons with analysis of variance (ANOVA). Pairwise comparisons were made using Fisher’s protected least significant difference. Receiver operator characteristic (ROC) analysis was used to determine an optimal cut point for predicting steatosis from CAP measurements. Due to the small number of subjects with steatosis, no attempt was made to determine CAP cut points for distinguishing mild, moderate and marked steatosis. All tests were two-sided, and P<0.05 was established a priori as statistically significant. Analysis was performed with SAS® version 9.3 (Cary, NC).
Results
A total of 145 subjects were enrolled, of whom 76 were excluded from analysis due to invalid measurements (N = 5; 3 patients with a fatty abdominal wall, 1 with high IQR, and 1 unknown), scan with S probe (N = 67), or lack of liver steatosis assessment on biopsy (N = 4). A total of 69 patients were analyzed (64 M probe and 5 XL probe).
The mean age of subjects was 16.0 ± 2.9 years, 84% were younger than 18 years and 62% were male. The median BMI was 22.6 kg/m2 (IQR 19.6 – 29.2). Subjects had a variety of liver disease diagnoses. These and other subject characteristics are shown in Table 1. Subjects with steatosis were similar in age and sex to those without steatosis, but had greater BMI and greater prevalence of NAFLD (Table 2).
Table 1.
Subject characteristics (n=69)
| Characteristic | N (%) |
|---|---|
| Age, years | |
| Mean±SD | 16.0±2.9 |
| 0–6 | 0 (0.0) |
| 7–11 | 5 (7.3) |
| 12–17 | 53 (76.8) |
| 18–24 | 11 (15.9) |
| Female sex | 26 (37.7) |
| Race/ethnicity (n=4 unknown) | |
| White | 38 (58.5) |
| Asian | 9 (13.8) |
| Black | 9 (13.8) |
| Hispanic | 8 (12.3) |
| Other | 1 ( 1.5) |
| Liver disease diagnosis | |
| Autoimmune hepatitis (includes drug-induced + PSC overlap) | 18 (26.1) |
| NAFLD (n=10 NASH) | 14 (20.3) |
| Viral hepatitis | 13 (18.8) |
| Wilson’s disease/Alpha 1 antitrypsin deficiency | 3 ( 4.5) |
| Cellular rejection | 1 ( 1.4) |
| Cholestasis (includes PFIC) | 1 ( 1.4) |
| Other1 | 19 (27.5) |
| Anthropometry | |
| BMI (kg/m2) | 22.6 (19.6, 29.2) |
| BMI percentile (among 63 subjects <20y) | 74.9 (37.8, 97.4) |
| BMI z-score (among 63 subjects <20y) | 0.67 (−0.31, 1.95) |
| Blood tests nearest to time of CAP | |
| ALT, U/L (n=62) | 85 (43, 180) |
| AST, U/L (n=56) | 47 (34, 111) |
| GGTP, U/L (n=49) | 83 (37, 154) |
| Steatosis | 23 (33.3) |
| Mild | 7 (30.4) |
| Moderate | 4 (17.4) |
| Marked | 12 (52.2) |
7 with PSC, 5 with chronic hepatitis of unknown etiology, 2 with lysosomal acid lipase deficiency, 1 routine liver biopsy at the time of bariatric surgery, 1 with diabetes associated glycogenic hepatopathy, 1 with veno-occlusive disease, 1 with nodular regenerative hyperplasia, and 1 with severe combined immunodeficiency s/p stem cell transplant
Table 2.
Comparison of subjects with and without steatosis(n=69)
| Characteristic | No steatosis (n=46) | Steatosis (n=23) | P |
|---|---|---|---|
| Age, years | 15.9 ± 2.9 | 16.3 ± 3.0 | 0.55 |
| Female sex | 18 (39%) | 8 (35%) | 0.73 |
| Anthropometry | |||
| BMI (kg/m2) | 20.9 (18.5, 23.3) | 28.7 (25.4, 36.4) | <0.0001 |
| BMI percentile (among 63 subjects <20y) | 60.8 (20.9, 86.8) | 96.4 (84.4, 98.7) | <0.0001 |
| BMI z-score (among 63 subjects <20y) | 0.28 (−0.81, 1.12) | 1.79 (1.01, 2.22) | <0.0001 |
| Liver disease diagnosis | <0.0001 | ||
| Autoimmune hepatitis (includes drug-induced + PSC overlap) | 18 (39) | 0 (0) | |
| NAFLD (n=10 NASH) | 0 (0) | 14 (61) | |
| Viral hepatitis | 9 (20) | 4 (17) | |
| Wilson’s disease/Alpha 1 antitrypsin deficiency | 2 (4) | 1 (4) | |
| Cellular rejection | 1 (2) | 0 (0) | |
| Cholestasis (includes PFIC) | 0 (0) | 1 (4) | |
| Other1 | 16 (35) | 3 (13) | |
| Blood tests nearest to time of CAP | |||
| ALT, U/L (n=62) | 63 (41, 151) | 129 (86, 262) | 0.07 |
| AST, U/L (n=56) | 41 (33, 80) | 66 (43, 129) | 0.13 |
| GGTP, U/L (n=49) | 84 (27, 161) | 83 (56, 118) | 0.57 |
7 with PSC, 5 with chronic hepatitis of unknown etiology, 2 with lysosomalacid lipase deficiency, 1 routine liver biopsy at the time of bariatric surgery, 1 with diabetes associated glycogenic hepatopathy, 1 with veno-occlusive disease, 1 with nodular regenerative hyperplasia, and 1 with severe combined immunodeficiency s/p stem cell transplant
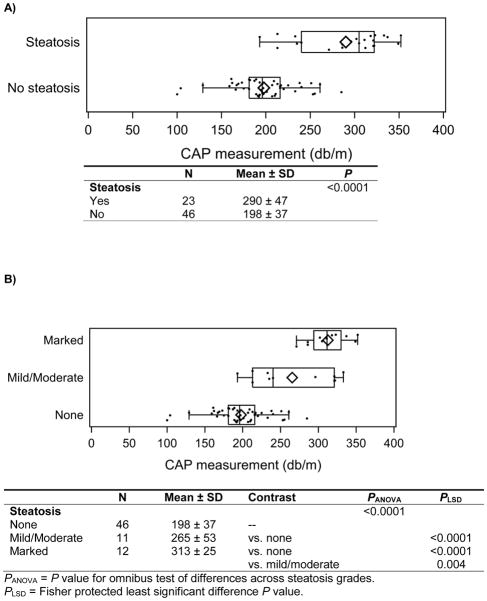
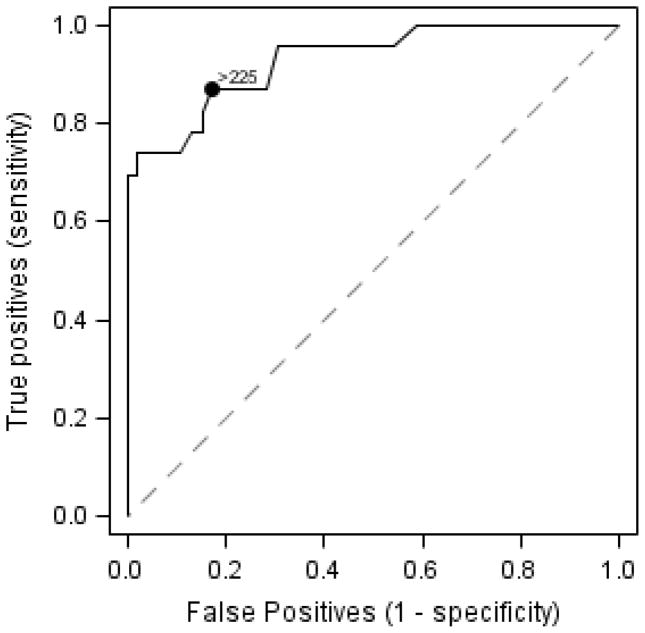
The median time between liver biopsy and CAP measurement was 1.3 months (IQR 0.5 – 3.2). The mean CAP measurement for patients without steatosis was 198 ± 37 versus 290 ± 47 dB/m for patients with steatosis, P<0.0001 (Figure 1a). CAP also distinguished severity of steatosis. Mean CAP measurement for patients with no steatosis was 198 ± 37 dB/m compared to 265 ± 53 dB/m for patients with mild/moderate steatosis (P<0.0001) and 313 ± 25 dB/m for patients with marked steatosis (P<0.0001). In addition, mean CAP in patients with mild/moderate steatosis was statistically different from that in patients with marked steatosis (P=0.004) (Figure 1b). The effect of being overweight or obese (BMI 85th percentile if age <20y; BMI 25kg/m2 otherwise) on CAP measurement was investigated by including an interaction for the effect of being overweight or obese (yes/no) with steatosis (yes/no) in an analysis of variance. The interaction effect was not statistically significant (P=0.81). CAP measurements were statistically higher in patients with steatosis than those without steatosis, regardless of whether patients were overweight or obese (P=0.0008) or normal weight (P=0.003; Table 1, online). The sample size was too small to investigate whether or not being overweight or obese modifies the effect of CAP across steatosis severity levels (mild, moderate, marked). An optimal cut point of 225 dB/m for predicting steatosis was identified, with 0.87 sensitivity, 0.83 specificity, positive predictive value 0.71, negative predictive value 0.93, and area under the curve 0.93 (95% CI 0.87–0.99) (Figure 2).
Figure 1.
A) CAP measurement by presence of biopsy-confirmed steatosis (n=69). The box-whisker plots describe the distribution of CAP measurements, with the left and right edges of each box corresponding to the lower and upper quartiles, the vertical line and diamond within representing the median and mean respectively, and the whiskers extending up to 1.5 times the interquartile range (i.e. box width). B) Distribution of CAP measurement by degree of biopsy-confirmed steatosis (n=69). The box-whisker plots describe the distribution of CAP measurements, with the left and right edges of each box corresponding to the lower and upper quartiles, the vertical line and diamond within representing the median and mean respectively, and the whiskers extending up to 1.5 times the interquartile range (i.e. box width).
Figure 2.
ROC for presence of steatosis. A cut point of 225 dB/m for predicting steatosis was determined with 0.87 sensitivity, 0.83 specificity, positive predictive value 0.71, negative predictive value 0.93, and area under the curve 0.93 (95% CI 0.87–0.99)
Discussion
We compared CAP measurements to the degree of steatosis in children. CAP was able to distinguish absence from presence of steatosis, as well as differentiate between some grades of steatosis.
These findings are consistent with those from adult studies, in which CAP measurements for S0 steatosis are reported as 184–222 dB/m.12–14 Sasso, et al. found a median CAP for S0 to be 205 dB/m; an optimal cut point of 238 dB/m to detect 11% steatosis was suggested.11 In a study of 264 healthy subjects the mean CAP value was 224.8 ± 38.7 dB/m.15 In this study of pediatric patients we found the optimal CAP threshold to detect steatosis to be >225 dB/m.
In a cohort of adults with chronic liver disease, CAP values correlated well with steatosis and distinguished mild from marked steatosis .13 In a later study of patients with hepatitis C, CAP was able to differentiate between consecutive steatosis grades.16
Currently, liver biopsy is considered the reference standard for assessing hepatic steatosis. However, there are associated risks and limitations.8 Numerous non-invasive options to assess hepatic steatosis have been studied including ultrasound, MRI, MR spectroscopy, and CT. Ultrasound has been found to have a positive predictive value between 47–62% for hepatic steatosis. However, ultrasound does not directly measure fat and the results are subjective and non-quantitative.17 In some institutions MRI is used in standard clinical practice to measure liver fat fraction, however MRI is expensive and there are confounding factors such as noise bias, eddy currents, and T1 relaxation, so that MRI may sometimes be inaccurate or unreproducible. MR spectroscopy is more robust but not universally available, and MRI studies often require sedation in children. CT scan requires delivery of ionizing radiation.9
CAP has the advantages of being non-invasive, inexpensive, painless, as well as machine and operator independent. The results obtained by using CAP are also reproducible and can assess steatosis in patients with a variety of liver diseases. Additionally, since it utilizes vibration controlled transient elastography, liver stiffness is measured at the same acquisition. CAP has been found to be feasible in a pediatric population.18
Thus far, ideal cut points to differentiate grades of steatosis have varied by study. In a study on CAP in patients with hepatitis C, optimal cut points between steatosis grades were lower than the original report which included patients with a variety of liver diseases. Additionally, even with cut points reported, the difference between S2 and S3 was only 11 dB/m.11,16 Our study was conducted in a heterogeneous group of pediatric patients with liver disease. In this group we were able to distinguish between some, but not all, steatosis categories. Even adult studies on a heterogeneous group of patients with liver disease do not report consistent thresholds between grades of steatosis. Kumar listed different optimal cut points based on the type of liver disease, however the sample size for chronic hepatitis B, chronic hepatitis C, and NAFLD was only 146, 108, and 63 patients respectively.13 CAP has been found to correlate with BMI and waist circumference.19 Further studies on larger groups of patients are needed to further clarify cut points between steatosis grades.
The primary limitation of this study is the small number of subjects with hepatic steatosis, resulting in the inability to differentiate the moderate and severe categories of steatosis. Additionally, this cohort includes patients with a variety of liver disease, so it remains unclear whether these results can be extrapolated to individual diseases. Pathology specimens are subjective and semi-quantitative, however in this study pathology slides were assessed systematically and the estimate of steatosis was based on the entire biopsy rather than a single section. Since steatosis can change quickly, another limitation is the time between biopsy and CAP; however the interval between biopsy and CAP was relatively short in our cohort, with a median of 1.3 months. Larger studies in children are needed to confirm accurate CAP cut points.
This preliminary study in children shows CAP is a noninvasive tool that may be useful in the detection of steatosis. A CAP threshold of 225 dB/m may be optimal to detect steatosis in a pediatric population, which is comparable to that proposed from adult studies. Further studies will help develop appropriate cut points between grades of steatosis in children.
Supplementary Material
Abbreviations
- CAP
Controlled Attenuation Parameter
- NAFLD
non-alcoholic fatty liver disease
- TE
transient elastography
- MRI
Magnetic resonance imaging
- CT
computed tomography
- ROC
Receiver operator characteristic
Footnotes
Conflict of Interest: Echosens (Paris, France) provided the FibroScan machine, technical support, and training of investigators for the purpose of this and other studies. Echosens did not have a role in study design, collection/analysis/interpretation of data, writing of the manuscript, or the decision to submit the manuscript for publication. The other authors declare no other conflicts of interest pertinent to this study.
Bibliography
- 1.Wieckowska A, Feldstein AE. Nonalcoholic fatty liver disease in the pediatric population: a review. Curr Opin Pediatr. 2005 Oct;17:636–41. doi: 10.1097/01.mop.0000172816.79637.c5. [DOI] [PubMed] [Google Scholar]
- 2.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006 Oct;118:1388–93. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 3.Anderson EL, Howe LD, Jones HE, Higgins JPT, Lawlor DA, Fraser A. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS One. 2015 Jan;10:e0140908. doi: 10.1371/journal.pone.0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kneeman JM, Misdraji J, Corey KE. Secondary causes of nonalcoholic fatty liver disease. Therap Adv Gastroenterol. 2012 May;5:199–207. doi: 10.1177/1756283X11430859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwimmer JB, Dunn W, Norman GJ, Pardee PE, Middleton MS, Kerkar N, et al. SAFETY Study: Alanine Aminotransferase Cutoff Values Are Set Too High for Reliable Detection of Pediatric Chronic Liver Disease. Gastroenterology. 2010 Apr;138:1357–64. e2. doi: 10.1053/j.gastro.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. 2011 Jan;21:87–97. doi: 10.1007/s00330-010-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mottin CC, Moretto M, Padoin AV, Swarowsky AM, Toneto MG, Glock L, et al. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obes Surg. 2004 May;14:635–7. doi: 10.1381/096089204323093408. [DOI] [PubMed] [Google Scholar]
- 8.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005 Jun;128:1898–906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 9.Awai HI, Newton KP, Sirlin CB, Behling C, Schwimmer JB. Evidence and recommendations for imaging liver fat in children, based on systematic review. Clin Gastroenterol Hepatol. 2014 May;12:765–73. doi: 10.1016/j.cgh.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandrin L, Fourquet B, Hasquenoph J-M, Yon S, Fournier C, Mal F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003 Dec;29:1705–13. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, et al. Controlled attenuation parameter (CAP): a novel VCTETM guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010 Nov;36:1825–35. doi: 10.1016/j.ultrasmedbio.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Chan W-K, Nik Mustapha NR, Mahadeva S. Controlled attenuation parameter for the detection and quantification of hepatic steatosis in nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2014 Jul;29:1470–6. doi: 10.1111/jgh.12557. [DOI] [PubMed] [Google Scholar]
- 13.Kumar M, Rastogi A, Singh T, Behari C, Gupta E, Garg H, et al. Controlled attenuation parameter for non-invasive assessment of hepatic steatosis: does etiology affect performance? J Gastroenterol Hepatol. 2013 Jul;28:1194–201. doi: 10.1111/jgh.12134. [DOI] [PubMed] [Google Scholar]
- 14.Yilmaz Y, Yesil A, Gerin F, Ergelen R, Akin H, Celikel ÇA, et al. Detection of hepatic steatosis using the controlled attenuation parameter: a comparative study with liver biopsy. Scand J Gastroenterol. 2014 May;49:611–6. doi: 10.3109/00365521.2014.881548. [DOI] [PubMed] [Google Scholar]
- 15.Chon YE, Jung KS, Kim KJ, Joo DJ, Kim BK, Park JY, et al. Normal controlled attenuation parameter values: a prospective study of healthy subjects undergoing health checkups and liver donors in Korea. Dig Dis Sci. 2015 Jan;60:234–42. doi: 10.1007/s10620-014-3293-1. [DOI] [PubMed] [Google Scholar]
- 16.Sasso M, Tengher-Barna I, Ziol M, Miette V, Fournier C, Sandrin L, et al. Novel controlled attenuation parameter for noninvasive assessment of steatosis using Fibroscan(®): validation in chronic hepatitis C. J Viral Hepat. 2012 Apr;19:244–53. doi: 10.1111/j.1365-2893.2011.01534.x. [DOI] [PubMed] [Google Scholar]
- 17.Shannon A, Alkhouri N, Carter-Kent C, Monti L, Devito R, Lopez R, et al. Ultrasonographic quantitative estimation of hepatic steatosis in children With NAFLD. J Pediatr Gastroenterol Nutr. 2011 Aug;53:190–5. doi: 10.1097/MPG.0b013e31821b4b61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho Y, Tokuhara D, Morikawa H, Kuwae Y, Hayashi E, Hirose M, et al. Transient Elastography-Based Liver Profiles in a Hospital-Based Pediatric Population in Japan. PLoS One. 2015 Jan;10:e0137239. doi: 10.1371/journal.pone.0137239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Lédinghen V, Vergniol J, Capdepont M, Chermak F, Hiriart J-B, Cassinotto C, et al. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014 May;60:1026–31. doi: 10.1016/j.jhep.2013.12.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.