Abstract
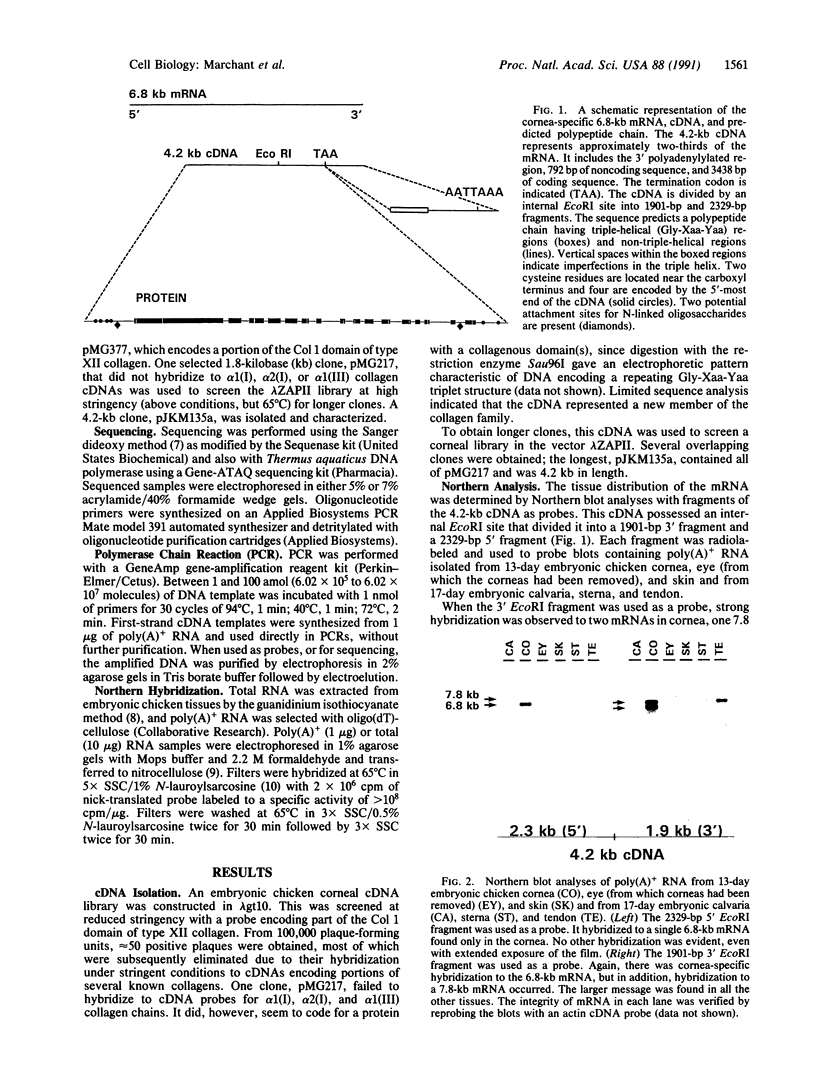
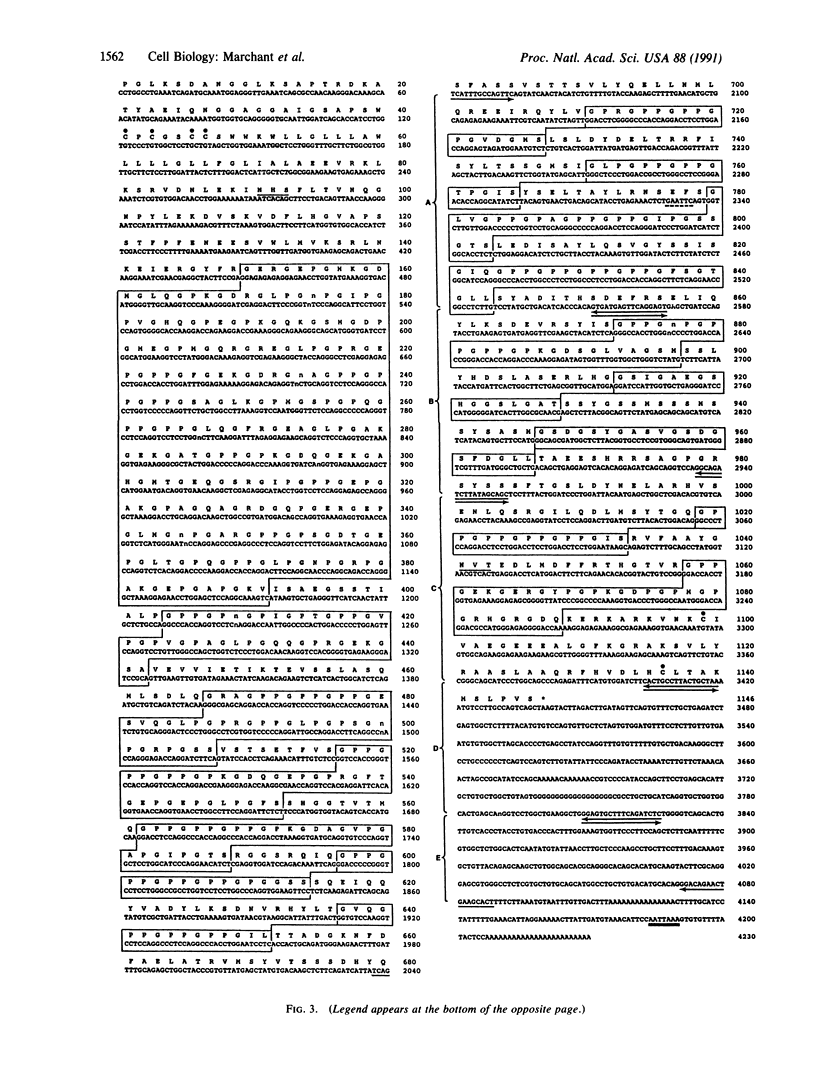
In the development of chicken corneal stroma, two or more collagens often interact, either as constituents of a single heterotypic fibril or as components of the fibril surface. The latter, fibril-associated collagens, may facilitate interactions between fibrils and the surrounding extracellular matrix or between fibrils themselves. In an effort to isolate putative nonfibrillar collagens that may have such a function, we screened a 13-day embryonic cornea cDNA library under reduced stringency conditions, using a cDNA probe for a collagenous domain of type XII collagen. We isolated a 4.2-kilobase (kb) cDNA that predicts a "collagenous" protein that has three unusual, if not unique, features. (i) The putative polypeptide encoded by this cDNA has a structural arrangement in which numerous stretches of Gly-Xaa-Yaa triplets, typical of collagens, are interrupted by non-Gly-Xaa-Yaa regions. One of the potential triple-helical domains is 246 amino acids long, but most are much smaller, consisting of 15-36 amino acids. Many are very rich in the helix-stabilizing imino acid proline. (ii) Northern blot analyses demonstrated strong cDNA hybridization to a 6.8-kb mRNA whose expression is restricted to the cornea. No hybridization was observed to mRNAs from the nine other tissues used in these analyses, even with extended exposure of the film. (iii) The cDNA contains a short (less than or equal to 425-base-pair) sequence in the 3' untranslated region of the 6.8-kb mRNA that hybridizes to a 7.8-kb mRNA that has a wide tissue distribution.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Q. A., Gibney E., Fitch J. M., Linsenmayer C., Schmid T. M., Linsenmayer T. F. Long-range movement and fibril association of type X collagen within embryonic cartilage matrix. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8046–8050. doi: 10.1073/pnas.87.20.8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cox G. N., Fields C., Kramer J. M., Rosenzweig B., Hirsh D. Sequence comparisons of developmentally regulated collagen genes of Caenorhabditis elegans. Gene. 1989;76(2):331–344. doi: 10.1016/0378-1119(89)90173-x. [DOI] [PubMed] [Google Scholar]
- Fitch J. M., Mentzer A., Mayne R., Linsenmayer T. F. Acquisition of type IX collagen by the developing avian primary corneal stroma and vitreous. Dev Biol. 1988 Aug;128(2):396–405. doi: 10.1016/0012-1606(88)90301-6. [DOI] [PubMed] [Google Scholar]
- Gordon M. K., Gerecke D. R., Dublet B., van der Rest M., Olsen B. R. Type XII collagen. A large multidomain molecule with partial homology to type IX collagen. J Biol Chem. 1989 Nov 25;264(33):19772–19778. [PubMed] [Google Scholar]
- Gordon M. K., Gerecke D. R., Olsen B. R. Type XII collagen: distinct extracellular matrix component discovered by cDNA cloning. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6040–6044. doi: 10.1073/pnas.84.17.6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay E. D., Linsenmayer T. F., Trelstad R. L., von der Mark K. Origin and distribution of collagens in the developing avian cornea. Curr Top Eye Res. 1979;1:1–35. [PubMed] [Google Scholar]
- Kodama T., Freeman M., Rohrer L., Zabrecky J., Matsudaira P., Krieger M. Type I macrophage scavenger receptor contains alpha-helical and collagen-like coiled coils. Nature. 1990 Feb 8;343(6258):531–535. doi: 10.1038/343531a0. [DOI] [PubMed] [Google Scholar]
- Linsenmayer T. F., Gibney E., Schmid T. M. Intracellular avian type X collagen in situ and determination of its thermal stability using a conformation-dependent monoclonal antibody. Exp Cell Res. 1986 Sep;166(1):15–22. doi: 10.1016/0014-4827(86)90504-5. [DOI] [PubMed] [Google Scholar]
- Overbeek P. A., Merlino G. T., Peters N. K., Cohn V. H., Moore G. P., Kleinsmith L. J. Characterization of five members of the actin gene family in the sea urchin. Biochim Biophys Acta. 1981 Dec 28;656(2):195–205. doi: 10.1016/0005-2787(81)90087-3. [DOI] [PubMed] [Google Scholar]
- Pikkarainen J. The molecular structures of vertebrate skin collagens. A comparative study. Acta Physiol Scand Suppl. 1968;309:1–72. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Schmid T. M., Linsenmayer T. F. A short chain (pro)collagen from aged endochondral chondrocytes. Biochemical characterization. J Biol Chem. 1983 Aug 10;258(15):9504–9509. [PubMed] [Google Scholar]
- Schmid T. M., Linsenmayer T. F. Immunoelectron microscopy of type X collagen: supramolecular forms within embryonic chick cartilage. Dev Biol. 1990 Mar;138(1):53–62. doi: 10.1016/0012-1606(90)90176-j. [DOI] [PubMed] [Google Scholar]
- Sugrue S. P., Gordon M. K., Seyer J., Dublet B., van der Rest M., Olsen B. R. Immunoidentification of type XII collagen in embryonic tissues. J Cell Biol. 1989 Aug;109(2):939–945. doi: 10.1083/jcb.109.2.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda K. K., Nishimura I., Sugrue S. P., Ninomiya Y., Olsen B. R. Embryonic chicken cornea and cartilage synthesize type IX collagen molecules with different amino-terminal domains. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7496–7500. doi: 10.1073/pnas.85.20.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan L., Mendler M., Huber S., Bruckner P., Winterhalter K. H., Irwin M. I., Mayne R. D-periodic distribution of collagen type IX along cartilage fibrils. J Cell Biol. 1988 Mar;106(3):991–997. doi: 10.1083/jcb.106.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]





