Abstract
BACKGROUND
Our objective was to determine the hospital resources at high volume cancer resection centers required for low volume, high quality care.
METHODS
Patients who underwent esophageal, pancreatic, and rectal resection for malignancy were identified using HCUP SID (Florida and California) between 2007 and 2011. Annual case volume, by procedure, was used to identify high and low volume centers. Hospital data were obtained from the AHA Annual Survey Database. Procedure risk-adjusted mortality was calculated for each hospital using multilevel, mixed-effects models.
RESULTS
24,784 patients from 302 hospitals met inclusion criteria. Of these, 13 hospitals were classified as having a high volume oncologic resection ecosystem by being a high volume hospital ≥ 2 studied procedures. A total 11 of 31 studied hospital factors were strongly associated with hospitals that performed a high volume of cancer resections and used to develop the High Volume Ecosystem for Oncologic Resections (HIVE-OR) score. At low volume centers, increasing HIVE-OR score resulted in decreased mortality for rectal cancer resection (p=0.038). HIVE-OR was not related to risk-adjusted mortality for esophagectomy (p=0.421) or pancreatectomy (p=0.413) at low volume centers.
CONCLUSIONS
Our study demonstrates in some settings, low volume, high quality cancer surgical care can be explained by a having high volume ecosystem.
INTRODUCTION
The relationship between increasing volume and improved outcomes in surgery is a well-studied phenomenon. First described in the late 1970s1, the volume and outcome association has since been demonstrated in cardiac surgery, cancer resection, vascular surgery, and colorectal surgery, amongst others.2–6 The impact of increasing volume on improved postoperative outcomes is especially well-validated for patients undergoing major oncologic resection.7, 8
Two causal models have traditionally explained the relationship between increasing surgical volume and improved outcomes. The first postulates that high volume centers leverage the concept of a learning curve, both at the level of the provider and the system. For the provider, proficiency improves with repetition. For the system, team-based familiarity improves outcomes. The second model cites a referral system that has a tendency to send patients to places already providing high quality care, hence increasing their volume.9 More recently, a third explanation offered is that system characteristics within institutions, including technology, staffing, and expertise in other surgical procedures, may equip them with tools to optimize perioperative care for both malignant and non-malignant diseases.10–12 While many of these characteristics would be present in high volume hospitals, they could also be used by low volume hospitals to provide high quality care.
Although there is little debate that increasing volume can result in improved outcomes, a better understanding of the hospital ecosystem that supports low volume, high quality cancer care may offer new insights into improving surgical outcomes and bolster patient access. Therefore, the overarching goal of this study was to understand the role of a high volume cancer resection ecosystem on surgical quality. To achieve this, we first characterized the hospital resources that define a high volume ecosystem for cancer resections. Next, we identified low volume centers with a high volume ecosystem. Finally, we measured if a high volume ecosystem could explain low volume, high quality care for patients undergoing three representative cancer operations.
METHODS
Data Sources
Data from the Healthcare Cost and Utilization Project State Inpatient Database (HCUP SID) were linked to the American Hospital Association (AHA) Annual Survey Database. Given the use of de-identified, publicly available data, this study was deemed exempt from needing institutional review board approval. HCUP SID is a state-specific, patient-level dataset that was developed by the Agency of Health Research and Quality (AHRQ) to inform healthcare decision-making. HCUP SID is an administrative dataset that includes over 100 clinical and nonclinical variables obtained from discharge records for all payers.
The AHA Annual Survey database is a hospital-level data source with information from over 6,000 hospitals across the United States. The AHA Annual Survey is given to hospital administrators and includes questions that characterize an institution’s structure, service lines, utilization, budget, and staffing. Answers are then compiled into a single database with over 1,000 fields.
Patient Inclusion Criteria
All patients ≥18 years old that underwent esophageal, pancreatic, or rectal resection for a diagnosis of cancer between 2007 and 2011 in the states of Florida or California were included. International Classification of Diseases, 9th Edition, Clinical Modification (ICD-9-CM) diagnosis and procedure codes were used to define the population of interest. Specific ICD-9-CM case-finding codes are shown in Supplementary Table 1.
For each procedure, hospitals were excluded if they performed <1 case per year. Patients without an associated hospital identifier were also excluded. Procedures selected for study were all gastrointestinal cancer resections included in the “Take the Volume Pledge” campaign, each with strong evidence supporting the volume-outcome relationship.13
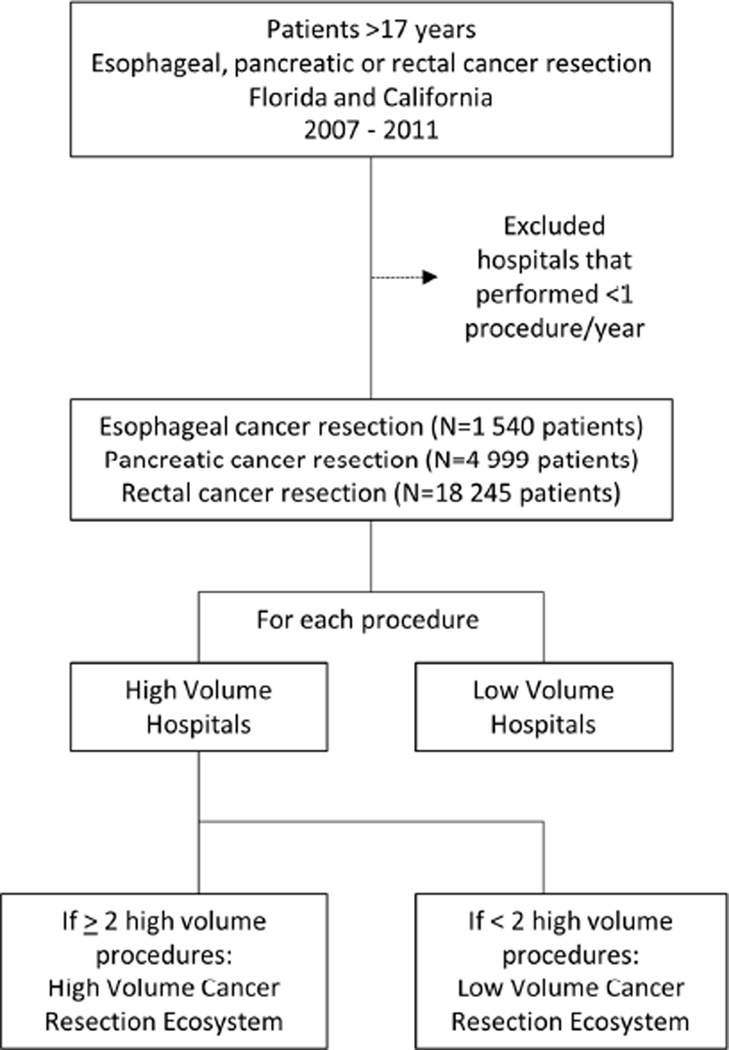
Overall study design is shown in Figure 1.
Figure 1.
Overview of study design.
Volume Thresholds
Hospitals were classified as high or low volume for each procedure. Volume assignments for each procedure were based on published numbers outlined in the “Take the Volume Pledge” campaign.13 For esophageal cancer resection, high volume was 20. For pancreatic cancer resection high volume was 20. For rectal cancer resection, high volume was 15.
Hospital Variables
Hospital-specific variables from the 2011 AHA Annual Survey database were used to characterize the resources available across centers and grouped into the following categories: infrastructure, size, staffing, perioperative services, and support intensity. Characteristics and groupings were created using a modified nominal group technique with participating members selected by the study authors.14 Missing values from the AHA Annual Survey were omitted from analyses. Sensitivity analyses using random and mean imputation demonstrated no difference in results.
Analytic Approach
Patient-level descriptive statistics
Patient demographic and clinical factors are reported using either arithmetic means with standard deviations for continuous variables with symmetric distributions or medians with interquartile range for continuous variables with skewed distributions. Proportions were computed to report categorical variables.
Hospital-specific, risk-adjusted mortality rates
The primary outcome of study was hospital risk-adjusted mortality, by procedure. Risk adjustment models were constructed using multi-level, mixed effects logistic regression to account for patients clustered within hospitals. Forward, stepwise selection with logistic regression was used to determine covariates for multi-level models, without consideration of interactions. Interactions were assessed during model fitting. Separate risk-adjustment models were built for each procedure using a 70% training set to fit models (minimization of Akaike Information Criterion) and a 30% validation set to measure performance (in and out of sample c-statistic). Final fitted model fixed and random effects for each procedure are shown in Supplementary Table 2. Model-derived observed and expected outcomes were calculated for each patient (with reliability adjustment) and used to calculate risk-adjusted mortality for each hospital.
Hospital-level descriptive statistics
Analyses of group differences in hospital characteristics were performed using X2 tests (categorical variables), with the hospital as the unit of measurement. All continuous measures of hospital characteristics were split into quartiles for treatment as categorical variables. Unadjusted hospital-level analyses were conducted using simple logistic regression and results reported as odds ratios (with 95% confidence intervals).
All statistical analyses were performed using STATA version 13 (StataCorp LP, College Station, Texas) and R version 3.1.2 (R Foundation for Statistical Computing).
RESULTS
The overall analytic sample included 24,784 patients at 302 hospitals between 2007 and 2011. Baseline characteristics of the study population are shown in Table 1. A total of 1,540 (6.2%) included patients underwent esophageal cancer resection, 4,999 (20.2%) pancreatic cancer resection, and 18,245 (73.6%) rectal cancer resection. For esophageal cancer resection, pancreatic cancer resection, and rectal cancer resection, 391 (25.4%), 2,615 (52.3%), and 9,940 (54.5%) received care at high volume centers, respectively.
Table 1.
Baseline characteristics of overall study population (N=24,784).
| Demographic Characteristics | N (%) |
|---|---|
| Mean Age (years, SD) | 64.2 (12.8) |
| Gender (% female) | 10,404 (42.0%) |
| Race | |
| Caucasian | 16,825 (67.9%) |
| African-American | 1,393 (5.6%) |
| Hispanic | 3,339 (13.5%) |
| Asian or Pacific Islander | 1,808 (7.3%) |
| Native American | 839 (3.4%) |
| Other | 580 (2.3%) |
| Insurance type (n, %) | |
| Medicare | 11,763 (47.5%) |
| Medicaid | 1,923 (7.8%) |
| Private | 9,965 (40.2%) |
| Other | 1,133 (4.6%) |
| Comorbidities | |
| Alcohol abuse | 616 (2.5%) |
| Deficiency anemia | 4,980 (20.1%) |
| Rheumatoid arthritis | 331 (1.3%) |
| Chronic blood loss anemia | 628 (2.5%) |
| Congestive heart failure | 997 (4.0%) |
| Chronic pulmonary disease | 3,254 (13.1%) |
| Coagulopathy | 967 (3.9%) |
| Depression | 1,356 (5.5%) |
| Diabetes Mellitus | 4,713 (19.0%) |
| Diabetes with complications | 677 (2.7%) |
| Hypertension | 12,690 (51.2%) |
| Hypothyroidism | 1,960 (7.9%) |
| Liver disease | 651 (2.6%) |
| Lymphoma | 103 (0.4%) |
| Fluid and electrolyte disorders | 5,003 (20.2%) |
| Neurological disorders | 656 (2.7%) |
| Obesity | 2,235 (9.0%) |
| Paralysis | 171 (0.7%) |
| Peripheral vascular disease | 925 (3.7%) |
| Psychoses | 540 (2.2%) |
| Pulmonary circulation disorders | 368 (1.5%) |
| Renal failure | 1,124 (4.5%) |
| Valvular disease | 919 (3.7%) |
| Weight loss | 1,943 (7.8%) |
| CCI (mean, SD) | 3.8 (2.1) |
| Procedures | |
| Esophagus Cancer Resection | 1,540 (6.2%) |
| Pancreatic Cancer Resection | 4,999 (20.2%) |
| Rectal Resection | 18,245 (73.6%) |
SD: standard deviation; CCI: Charlson Comorbidity Index (Deyo modification)
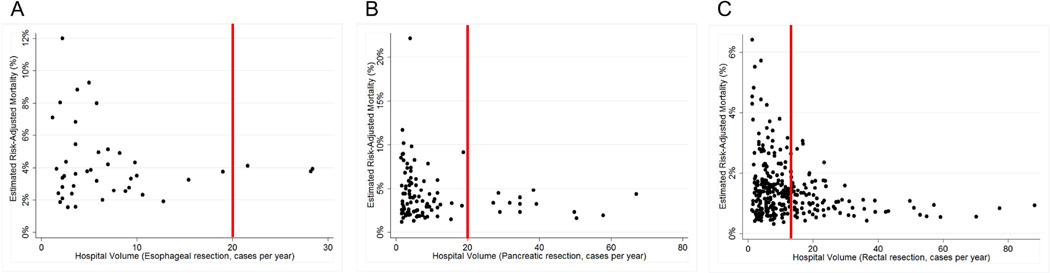
Hospital risk-adjusted mortality for each procedure, by procedural volume is shown in Figure 2. For esophageal cancer resection, risk-adjusted mortality ranged from 1.5% – 12.0%. For pancreatic cancer resection, risk-adjusted mortality ranged from 1.2% – 22.1%. For rectal resection, risk-adjusted mortality ranged from 0.3% – 6.4%.
Figure 2.
Hospital risk-adjusted mortality for each procedure, by volume. A) Esophagus cancer resection (N=1,540). B) Pancreatic cancer resection (N=4,999). C) Rectal cancer resection (N=18,245). Vertical line in each panel demarcates the high volume threshold.
At the hospital-level, 3 of 43 were high-volume hospitals for esophageal cancer resection, 13 of 97 were high volume hospitals for pancreatic cancer resection, and 70 of 301 were high volume hospitals for rectal cancer resection. Only 3 hospitals (1.0%) were high volume centers for all procedures studied. Thirteen hospitals (4.3%) were high volume for at least 2 procedures and 70 hospitals (23.2%) were high volume for at least 1 procedure.
Hospitals that were high volume providers for at least 2 procedures were classified as having a high volume cancer resection ecosystem (N=13). Hospital characteristics for these hospitals were compared to all other hospitals (Table 2). When comparing the relative association between hospital characteristics and classification as having a high volume cancer resection ecosystem, level 1 trauma center and transplant services were key infrastructural elements. Admission volume, surgical volume, residents, and nurses were important size and staff elements. Geriatric services, speech and language therapy, inpatient palliative care, and inpatient rehabilitation were important perioperative elements. Finally, high oncology support was an important support element (Table 3).
Table 2.
Comparison of hospital characteristics between institutions with high and low volume cancer resection healthcare ecosystems (N=302).
| Hospital Characteristics | Hospitals with High Volume Cancer Resection Ecosystem (N=13) |
Hospitals with Low Volume Cancer Resection Ecosystem (N=289) |
p value |
|---|---|---|---|
| Infrastructure | |||
| Joint Commission Accreditation | 13 (100.0) | 271 (93.8) | 0.353 |
| COC Accreditation | 9 (69.2) | 149 (51.6) | 0.212 |
| COTH Accreditation | 13 (100.0) | 14 (4.8) | <0.001 |
| Level 1 Trauma Center | 9 (69.2) | 24 (8.3) | <0.001 |
| Transplant Services | 12 (92.3) | 14 (4.8) | <0.001 |
| Size | |||
| Number of Annual Admissions | 0.001 | ||
| < 8,830 | 0 (0.0) | 76 (26.3) | |
| 8,830–13,250 | 3 (23.1) | 72 (24.9) | |
| 13,251–19,500 | 1 (7.7) | 75 (26.0) | |
| >19,500 | 9 (69.2) | 66 (22.8) | |
| Surgical Volume | <0.001 | ||
| <2,050 | 0 (0.0) | 76 (26.3) | |
| 2,050–3,080 | (0.0) | 75 (26.0) | |
| 3,081–5,000 | 1 (7.7) | 75 (26.0) | |
| >5,000 | 12 (92.3) | 63 (21.8) | |
| ICU Beds/Total Hospital Beds* | 0.032 | ||
| <50.6 | 2 (15.4) | 60 (20.7) | |
| 50.6–75.0 | 2 (15.4) | 60 (20.7) | |
| 76.0–104.0 | 2 (15.4) | 61 (21.1) | |
| >104.0 | 7 (53.8) | 54 (18.7) | |
| Unknown | 0 (0.0) | 54 (18.7) | |
| Staffing | |||
| Physicians/Bed ratio | 0.041 | ||
| <0.0025 | 4 (30.8) | 88 (30.5) | |
| 0.0025–0.0245 | 3 (23.1) | 56 (19.4) | |
| 0.0246–0.1100 | 2 (15.4) | 74 (25.6) | |
| >0.1100 | 4 (30.8) | 71 (24.6) | |
| Residents/Bed ratio | <0.001 | ||
| <0.004 | 3 (23.1) | 110 (38.1) | |
| 0.004–0.032 | 0 (0.0) | 38 (13.2) | |
| 0.033–0.0672 | 0 (0.0) | 76 (26.3) | |
| >0.0672 | 10 (76.9) | 65 (22.5) | |
| Nurses/Bed ratio | <0.001 | ||
| <1.045 | 1 (7.7) | 75 (26.0) | |
| 1.045–1.341 | 0 (0.0) | 75 (26.0) | |
| 1.342–1.745 | 2 (15.4) | 74 (25.6) | |
| >1.745 | 10 (76.9) | 65 (22.5) | |
| Perioperative Services | |||
| Inpatient Rehabilitation | 9 (69.2) | 70 (29.8) | 0.003 |
| Skilled Nursing | 2 (15.4) | 53 (22.6) | 0.545 |
| Geriatric Services† | 13 (100.0) | 109 (46.4) | <0.001 |
| Hospice Care† | 5 (38.5) | 53 (22.6) | 0.187 |
| Speech and Language | |||
| Therapy† | 13 (100.0) | 175 (74.5) | 0.036 |
| Nutrition Services† | 12 (92.3) | 197 (83.8) | 0.414 |
| Interventional Catheterization | |||
| Lab† | 12 (92.3) | 163 (69.4) | 0.077 |
| Inpatient Palliative Care† | 7 (53.9) | 41 (17.5) | 0.001 |
| Wound Management Services† | 13 (100.0) | 193 (82.1) | 0.094 |
| Support Intensity | |||
| High Gastrointestinal Support | 13 (100.0) | 261 (90.3) | 0.239 |
| High Radiology Support | 9 (69.2) | 113 (39.1) | 0.030 |
| High Oncology Support | 11 (84.6) | 136 (47.1) | 0.008 |
per 1000 hospital beds.
based on 235 low volume and 13 high volume hospitals.
COC: Commission on Cancer; COTH: Council of Teaching Hospitals
Table 3.
Unadjusted associations between hospital characteristics and a hospital having a high volume cancer resection ecosystem (N=302).
| Hospital Characteristics | Odds Ratio | p value | Lower | Upper |
|---|---|---|---|---|
| Joint Commission Accreditation | * | |||
| COTH Accreditation | * | |||
| Geriatric Services | * | |||
| Speech and Language Therapy | * | |||
| Wound Management Services | * | |||
| High Gastrointestinal Support | * | |||
| Overall Surgical Volume (Highest quartile) |
43.05 | <0.001 | 5.49 | 337.42 |
| Level 1 Trauma Center | 24.84 | <0.001 | 7.12 | 86.69 |
| Transplant Services | 19.46 | <0.001 | 7.00 | 54.12 |
| Resident-to-bed (Highest quartile) | 11.49 | <0.001 | 3.07 | 42.98 |
| Nurse-to-bed (Highest quartile) | 11.49 | <0.001 | 3.07 | 42.98 |
| Admissions (Highest quartile) | 7.60 | <0.001 | 2.27 | 25.48 |
| High Oncology Support | 6.19 | 0.019 | 1.35 | 28.41 |
| Inpatient Palliative Care | 5.52 | <0.001 | 1.76 | 17.28 |
| Inpatient Rehabilitation | 5.30 | 0.007 | 1.58 | 17.79 |
| Interventional Catheterization Lab | 5.30 | 0.112 | 0.68 | 41.54 |
| ICU Beds (Highest quartile) | 5.08 | 0.005 | 1.64 | 15.71 |
| High Radiology Support | 3.50 | 0.041 | 1.05 | 11.65 |
| Nutrition Services | 2.31 | 0.427 | 0.29 | 18.33 |
| Hospice Care | 2.15 | 0.196 | 0.67 | 6.84 |
| COC Accreditation | 2.11 | 0.221 | 0.64 | 7.02 |
| Physician-to-bed (Highest quartile) | 1.36 | 0.614 | 0.41 | 4.57 |
| Skilled Nursing Care | 0.62 | 0.548 | 0.13 | 2.90 |
All centers classified as a high volume cancer resection ecosystem have this resource.
COTH: Council of Teaching Hospitals; ICU: Intensive Care Unit; COC: Commission on Cancer
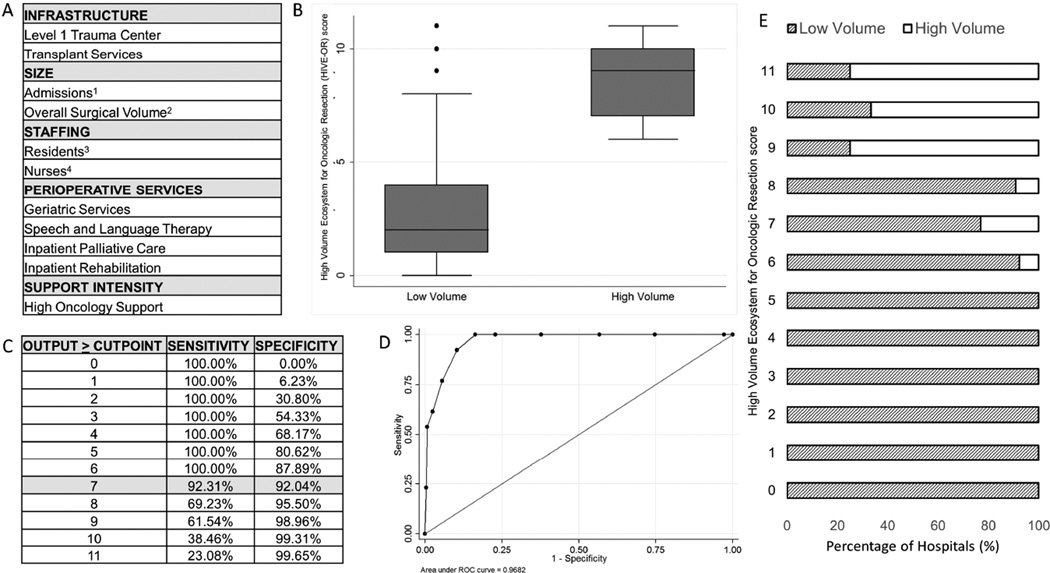
To better quantify the characteristics of a high volume cancer resection ecosystem, a High Volume Ecosystem for Oncologic Resections (HIVE-OR) score was developed. The process used to create the HIVE-OR score is shown in Figure 3. Two approaches were used to make the HIVE-OR score: (1) an additive score and (2) regression-based coefficient approach. In the additive score, the presence of each hospital characteristic was summated with the highest HIVE-OR score equal to 11. In the regression-based approach, a logistic regression was fit with each factor identified and post-estimation results of the fitted regression were used to develop the HIVE-OR score. The area under the curve for each approach was statistically the same (0.97 vs. 0.98). Given the performance similarities between approaches, the summated HIVE-OR score was selected due to its ease of use and simple interpretation.
Figure 3.
Development of the High Volume Ecosystem for Oncologic Resection (HIVE-OR) score. A) Components used to calculate the HIVE-OR score. Hospitals given 1 point for the presence of each characteristic to create a maximum score of 11. B) Box-plot HIVE-OR score distribution between high and low volume centers. C) Sensitivity and specificity for correctly classifying a hospital as having a high volume healthcare ecosystem at each HIVE-OR score. D) Receiver operating curve using the additive HIVE-OR score for classifying hospitals as having a high volume healthcare ecosystem. E) Number of high and low volume hospitals at each value of the HIVE-OR score. 1Highest quartile (>19,500 total annual admissions); 2Highest quartile (>5,000 total annual cases; 3Highest quartile (>0.07 total residents per hospital bed); 4Highest quartile (>1.7 total nurses per hospital bed).
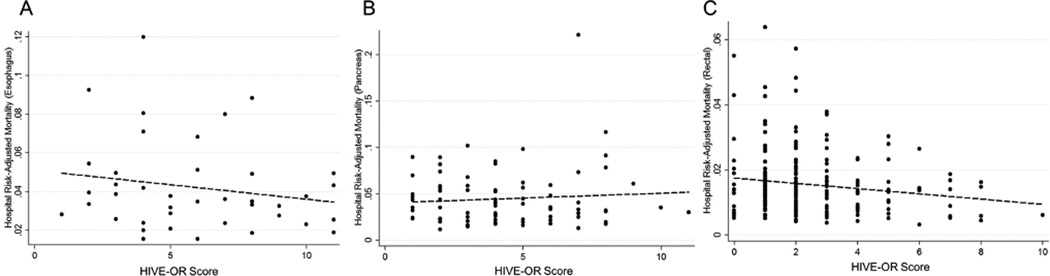
The HIVE-OR score for each low volume center was compared to its risk-adjusted mortality for each procedure (Figure 4). For both esophageal cancer resection and pancreatic resection, there was not a linear relationship between a hospital’s HIVE-OR score and risk-adjusted mortality (p>0.05). For patients undergoing rectal resection, however, increasing hospital HIVE-OR score was associated with improved risk-adjusted mortality (coefficient: −0.001, p=0.021).
Figure 4.
Measuring the relationship between the HIVE-OR score and risk-adjusted mortality at low volume centers. A) HIVE-OR score and risk-adjusted mortality for esophagus cancer resection (N=40). B) HIVE-OR score and risk-adjusted mortality for pancreatic cancer resection (N=84). C) HIVE-OR score and risk-adjusted mortality for rectal cancer resection (N=231). Dotted line in each panel fitted linear regression.
At a HIVE-OR score of ≥7, the sensitivity and specificity of identifying a hospital with a high volume ecosystem was 92.3% and 92.0%, respectively. Of the 40 hospitals performing a low volume of esophageal resections, 16 hospitals had a HIVE-OR score ≥ 7 and a mean risk-adjusted mortality rate of 3.9% (vs. 4.4% at hospitals with a HIVE-OR score <7, p>0.05). Of the 84 hospitals performing a low volume of pancreatic resections, 18 hospitals had a HIVE-OR score ≥ 7 and a risk-adjusted mortality rate of 4.1% (vs. 5.7%, p=0.050). Of the 231 hospitals performing a low volume of rectal resections, 11 hospitals had a HIVE-OR score ≥ 7 and a risk-adjusted mortality rate of 1.1% (vs. 1.6%, p=0.045).
DISCUSSION
In this study, we identified 11 hospital factors that define high volume cancer resection ecosystems. We also demonstrated that high volume ecosystems can be present at low volume hospitals. For rectal cancer resection in particular, a high volume ecosystem may explain low volume, high quality care.
Increasingly, the provision of high quality care in high volume settings is being ascribed to more than a “practice makes perfect” mechanism.15 Previous work by Funk and colleagues demonstrates how system factors can bolster outcomes for esophagectomy in low volume hospitals. Specifically, they identified lung transplantation services, bariatric surgical services, complex medical oncology services, position emission tomography scanning, and nurse to bed ratio as important predictors of decreased mortality in low volume hospitals. Although the direct mechanism for how these factors improved outcomes could not be determined, the authors proposed they may have more globally represented markers for an environment that led to optimized perioperative care.10 Notably, transplant services, nurse to bed ratio, and oncology support each were elements captured within the HIVE-OR described in this study.
Of the 11 hospital factors used to calculate the HIVE-OR and describe a high volume ecosystem, several overlap with prior studies measuring the association between hospital characteristics and mortality. Solid organ transplant programs may explain decreased mortality following colon resection.16 Failure to rescue rates are decreased in teaching hospitals and hospitals with increased nurse-to-patient ratios.17 In pancreatic cancer, the presence of a surgical residency is associated with improved postoperative mortality.18 Notably, these factors were not just surrogates for identifying large, busy hospitals. For example, in rectal resection, 215 low volume hospitals had outcomes on par or better than high volume hospitals. Of those, only 31 had annual admissions in the top quartile of hospitals and 29 had surgical volumes in the top quartile. Other components of the hospital ecosystem were therefore responsible for increasing the HIVE-OR score.
Nevertheless, the prevalence and utilization of many of these elements can be challenging to reliably measure and causal associations are difficult to determine. Therefore, volume continues to be used as a surrogate for quality.19 However, the difficulty with volume alone as a quality measurement is that it is not a simple, actionable target. This is reflected by the paucity of health care policies that exist to limit the number of hospitals and surgeons performing major surgery in low volume settings. The most well-known initiative for volume limitation was put forth by the Leapfrog coalition in the early 2000s, which targeted 5 surgical procedures.20 These volume thresholds, and the associated regionalization of care, are often difficult to implement as a result of several barriers including patient preference, potential to create new health access disparities, and financial considerations.21–23
In 2015, three major health systems developed the “Take the Volume Pledge” campaign as an attempt to overcome these barriers and limit the number of patients exposed to low volume care. This pledge set specific hospital-level volume thresholds for several procedures including esophageal resection (20/year), pancreatic resection (20/year), and rectal resection (15/year). In health networks that take the pledge, hospitals that do not achieve these volume marks no longer would be allowed to perform that particular operation.13 The number of hospitals that met the criteria for being defined as high volume by the “Take the Volume Pledge” was notably small, particularly for esophageal resection. Further study is warranted to understand the potential impact the strict limits created by this pledge would have if it was widely implemented.
Efforts like this largely do not acknowledge centers that are able to provide high quality care in a low volume setting. This study demonstrates a substantial number of low volume centers are providing high quality care for patients undergoing major cancer resection. In addition, there is a gap in understanding how low volume hospitals are able to produce acceptable outcomes following major surgery. In this study, we demonstrate that hospital characteristics associated with high volume hospitals may explain how low volume hospitals achieve high quality for some procedures. Interestingly, having a high volume ecosystem is a highly specific, though not sensitive predictor of quality suggesting additional features of low volume centers are contributing to surgical outcomes. Features not available in the data used for this study including provider-specific volume, information technology infrastructure, adherence to process measures, and more granular patient-level data such as tumor staging, laboratory information, and imaging results could help further our understanding of how to achieve high quality in low volume settings.
Nevertheless, the results presented in this study suggest three potential areas where volume threshold policies could be modified. The first is in identifying low volume, high quality care and supporting those centers. This would increase the number of providers available and may buffer lost access to care for patients that currently receive care at low volume hospitals. Second, our data support a role for bolstering the hospital ecosystem within low volume hospitals to improve outcomes. Third, existing policies could lower the volume limits and include requirements for perioperative support systems. This would be a strategy similar to that first used by the Leapfrog safety initiative which created hospital standards required to care for Leapfrog employees. In addition to volume limits, these included the presence of computerized physician order entry and intensive care units staffed by full-time intensivists.24
An important question is why the relationship was strongest between HIVE-OR score and rectal resection. Although our data cannot answer that conclusively, one possibility is that outcomes following rectal resection are less dependent on volume than the other procedures we studied. Both Schrag et al. and Simunovic, Marko, et al. have previously demonstrated that inpatient mortality in high and low volume centers is similar in rectal cancer surgery.25, 26 As a result, in low volume settings, quality of care could more strongly be influenced by other factors – such as the hospital resources identified in this study. It is worthwhile nothing that while there was not a linear relationship between HIVE-OR score and the other procedures we studied, HIVE-OR score was able to identify low volume, high quality care with a high positive predictive value.
There are several limitations that warrant further discussion. The data used in this study are administrative and subject to the known limitations of using billing information to study health outcomes.27, 28 In particular, this guided our decision to focus on mortality as the primary outcome since it can be reliably assessed. Secondary outcomes such as the quality of the cancer operation, patient satisfaction, and readmissions, all of which may benefit from high volume care, could not be studied. In addition, provider-specific volume could not be assessed due to limitations of the dataset.
Another limitation is the generalizability of the study results to a national population since we focused on two states. Both Florida and California provide diverse patient populations and a broad range of hospitals to study, however the further validation of the HIVE-OR score is needed before it can reliably be used for policy decision-making. Finally, this study cannot establish a causal relationship between the HIVE-OR score, hospital characteristics, and risk-adjusted outcomes. Given the retrospective nature of the study design, these results are meant to be hypothesis-generating with the goal of creating prospective studies to look at how implementing a high volume ecosystem in low volume settings could impact outcomes.
CONCLUSION
This study supports that hospitals performing a high volume of cancer resections are characterized by several infrastructure, size, staffing, resource, and support factors. The differences in availability of these factors in high compared to low volume hospitals was leveraged to quantify the high volume ecosystem with the HIVE-OR score.
In rectal cancer resection, as low volume hospitals more closely resembled high volume hospitals, risk-adjusted mortality improved. In esophageal cancer and pancreatic cancer resection, this relationship was not apparent. These data support that a high volume ecosystem can improve surgical quality in some low volume settings.
Supplementary Material
Acknowledgments
This work supported by NIH T32 GM08750-16.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented March 10, 2016 at the 2016 Annual Meeting of the Central Surgical Association in Montreal, Quebec.
Contributor Information
Anai N. Kothari, Email: ankothari@lumc.edu.
Barbara A. Blanco, Email: aldiblanco@gmail.com.
Ann E. Evans, Email: anevans@lumc.edu.
Victor A. Chang, Email: vchang@luc.edu.
Gerard J. Abood, Email: gabood@lumc.edu.
Raffaella Settimi, Email: rsettimi@cdm.depaul.edu.
Daniela S. Raicu, Email: draicu@cdm.depaul.edu.
Paul C. Kuo, Email: paul.kuo@luhs.org.
REFERENCES
- 1.Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? the empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364–1369. doi: 10.1056/NEJM197912203012503. [DOI] [PubMed] [Google Scholar]
- 2.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the united states. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 3.Karthikesalingam A, Hinchliffe RJ, Loftus IM, Thompson MM, Holt PJ. Volume-outcome relationships in vascular surgery: The current status. J Endovasc Ther. 2010;17:356–365. doi: 10.1583/10-3035.1. [DOI] [PubMed] [Google Scholar]
- 4.Faiz O, Brown T, Bottle A, Burns EM, Darzi AW, Aylin P. Impact of hospital institutional volume on postoperative mortality after major emergency colorectal surgery in english national health service trusts, 2001 to 2005. Dis Colon Rectum. 2010;53:393–401. doi: 10.1007/DCR.0b013e3181cc6fd2. [DOI] [PubMed] [Google Scholar]
- 5.Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: A national study. Archives of Surgery. 2003;138:721–725. doi: 10.1001/archsurg.138.7.721. [DOI] [PubMed] [Google Scholar]
- 6.Katz JN, Barrett J, Mahomed NN, Baron JA, Wright RJ, Losina E. Association between hospital and surgeon procedure volume and the outcomes of total knee replacement. J Bone Joint Surg Am. 2004;86-A:1909–1916. doi: 10.2106/00004623-200409000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Wouters MW, Gooiker GA, van Sandick JW, Tollenaar RA. The volume-outcome relation in the surgical treatment of esophageal cancer. Cancer. 2012;118:1754–1763. doi: 10.1002/cncr.26383. [DOI] [PubMed] [Google Scholar]
- 8.Wong SL, Revels SL, Yin H, Stewart AK, McVeigh A, Banerjee M, et al. Variation in hospital mortality rates with inpatient cancer surgery. Ann Surg. 2015;261:632–636. doi: 10.1097/SLA.0000000000000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luft HS, Hunt SS, Maerki SC. The volume-outcome relationship: Practice-makes-perfect or selective-referral patterns? Health Serv Res. 1987;22:157–182. [PMC free article] [PubMed] [Google Scholar]
- 10.Funk LM, Gawande AA, Semel ME, Lipsitz SR, Berry WR, Zinner MJ, et al. Esophagectomy outcomes at low-volume hospitals: The association between systems characteristics and mortality. Ann Surg. 2011;253:912–917. doi: 10.1097/SLA.0b013e318213862f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakeam E, Hevelone ND, Maine R, Swain J, Lipsitz SA, Finlayson SR, et al. Failure to rescue in safety-net hospitals: Availability of hospital resources and differences in performance. JAMA surgery. 2014;149:229–235. doi: 10.1001/jamasurg.2013.3566. [DOI] [PubMed] [Google Scholar]
- 12.McCrum ML, Lipsitz SR, Berry WR, Jha AK, Gawande AA. Beyond volume: Does hospital complexity matter?: An analysis of inpatient surgical mortality in the united states. Med Care. 2014;52:235–242. doi: 10.1097/MLR.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 13.Urbach DR. Pledging to eliminate low-volume surgery. N Engl J Med. 2015;373:1388–1390. doi: 10.1056/NEJMp1508472. [DOI] [PubMed] [Google Scholar]
- 14.Bartunek JM, Murninghan JK. The nominal group technique: Expanding the basic procedure and underlying assumptions. Group & organization management. 1984;9:417–432. [Google Scholar]
- 15.Mack M. Balancing optimal outcomes with access to care: It can be done! JACC: Cardiovascular Interventions. 2015;8:1952–1953. doi: 10.1016/j.jcin.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 16.Billingsley KG, Morris AM, Dominitz JA, Matthews B, Dobie S, Barlow W, et al. Surgeon and hospital characteristics as predictors of major adverse outcomes following colon cancer surgery: Understanding the volume-outcome relationship. Archives of Surgery. 2007;142:23–31. doi: 10.1001/archsurg.142.1.23. [DOI] [PubMed] [Google Scholar]
- 17.Johnston MJ, Arora S, King D, Bouras G, Almoudaris AM, Davis R, et al. A systematic review to identify the factors that affect failure to rescue and escalation of care in surgery. Surgery. 2015;157:752–763. doi: 10.1016/j.surg.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Joseph B, Morton JM, Hernandez-Boussard T, Rubinfeld I, Faraj C, Velanovich V. Relationship between hospital volume, system clinical resources, and mortality in pancreatic resection. J Am Coll Surg. 2009;208:520–527. doi: 10.1016/j.jamcollsurg.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Jha AK. Back to the future: Volume as a quality metric. JAMA. 2015;314:214–215. doi: 10.1001/jama.2015.7580. [DOI] [PubMed] [Google Scholar]
- 20.Birkmeyer JD, Finlayson EV, Birkmeyer CM. Volume standards for high-risk surgical procedures: Potential benefits of the leapfrog initiative. Surgery. 2001;130:415–422. doi: 10.1067/msy.2001.117139. [DOI] [PubMed] [Google Scholar]
- 21.Finlayson SR, Birkmeyer JD, Tosteson AN, Nease RF., Jr Patient preferences for location of care: Implications for regionalization. Med Care. 1999:204–209. doi: 10.1097/00005650-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Livingston EH, Burchell I. Reduced access to care resulting from centers of excellence initiatives in bariatric surgery. Archives of Surgery. 2010;145:993–997. doi: 10.1001/archsurg.2010.218. [DOI] [PubMed] [Google Scholar]
- 23.Chhabra KR, Dimick JB. Hospital networks and value-based payment: Fertile ground for regionalizing high-risk surgery. JAMA. 2015;314:1335–1336. doi: 10.1001/jama.2015.9803. [DOI] [PubMed] [Google Scholar]
- 24.Birkmeyer JD, Finlayson EV, Birkmeyer CM. Volume standards for high-risk surgical procedures: Potential benefits of the leapfrog initiative. Surgery. 2001;130:415–422. doi: 10.1067/msy.2001.117139. [DOI] [PubMed] [Google Scholar]
- 25.Schrag D, Panageas KS, Riedel E, Cramer LD, Guillem JG, Bach PB, et al. Hospital and surgeon procedure volume as predictors of outcome following rectal cancer resection. Ann Surg. 2002;236:583–592. doi: 10.1097/00000658-200211000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simunovic M, To T, Baxter N, Balshem A, Ross E, Cohen Z, et al. Hospital procedure volume and teaching status do not influence treatment and outcome measures of rectal cancer surgery in a large general population. Journal of Gastrointestinal Surgery. 2000;4:324–330. doi: 10.1016/s1091-255x(00)80083-9. [DOI] [PubMed] [Google Scholar]
- 27.Grimes DA. Epidemiologic research with administrative databases: Red herrings, false alarms and pseudo-epidemics. Hum Reprod. 2015;30:1749–1752. doi: 10.1093/humrep/dev151. [DOI] [PubMed] [Google Scholar]
- 28.Haut ER, Pronovost PJ, Schneider EB. Limitations of administrative databases. JAMA. 2012;307:2589–2590. doi: 10.1001/jama.2012.6626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.