Abstract
Introduction
Osteopontin (OPN) mediates metastasis and invasion of hepatocellular carcinoma (HCC). Epigallocatechin-3-gallate (EGCG), found in green tea, suppresses HCC tumor growth in vitro. We sought to investigate the role of EGCG in modulating OPN in cell lines of metastatic HCC.
Methods
Experimental HCC cell lines included HepG2 and MHCC-97H HCC cells, which express high levels of OPN, and the Hep3B cells, which express lesser levels of OPN. Cells were treated with EGCG (0.02–20 μg/mL) before measurement of OPN with enzyme-linked immunosorbent assay and reverse transcriptase-polymerase chain reaction. Scratch assay measured cell migration. Binding of the OPN promoter to RNA pol II was evaluated by the use of Chromatin-IP assay after EGCG treatment. Transcriptional regulation of OPN was investigated with luciferase reporter plasmids containing various deletion fragments of the human OPN promoter. Measurement of the half-life of OPN mRNA was conducted using actinomycin D.
Results
Treatment of MHCC-97H and HepG2 cells with 2 μg/mL and 20 μg/mL EGCG caused a ~6-fold and ~90-fold decrease in secreted protein levels of OPN (All P < .001). OPN mRNA was decreased with EGCG concentrations of 0.2–20 μg/ml (All P < .001). The 3-(4, 5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (ie, MTT) assay revealed that differences in OPN expression were not due to viability of the HCC cell lines. Promoter assay and chromatin immunoprecipitation analysis revealed no effect of EGCG on the transcriptional regulation of OPN. Posttranscriptionally, EGCG decreased the half-life of OPN mRNA from 16.8 hours (95% confidence interval 9.0–125.1) to 2.5 hours (95% confidence interval 2.1–3.2) (P < .001). Migration was decreased in EGCG treated cells at 24 hours (8.0 ± 2.4% vs 21.2 ± 10.8%, P < .01) and at 48 hours (13.2 ± 3.6% vs 53.5 ± 19.8%, P < .001).
Conclusion
We provide evidence that EGCG decreases OPN mRNA and secreted OPN protein levels by decreasing the half-life of OPN mRNA in MHCC-97H cells. The translatability of EGCG for patients with HCC is promising, because EGCG is an inexpensive, easily accessible chemical with an extensive history of safety.
Hepatocellular carcinoma (HCC) is the fifth most-common cancer and the third leading cause of cancer-related death worldwide.1 HCC is an aggressive tumor with a poor prognosis and a high mortality rate attributed largely to metastasis and recurrence even after curative therapy. Risk factors include infection with viral hepatitis, hepatic cirrhosis, obesity, and consumption of dietary hepatocarcinogens.1,2 Recent work elucidated the importance of the tumor microenvironment in the growth and metastasis of HCC.3
Osteopontin (OPN), a secreted phosphoprotein, is an important factor regulating metastasis and invasion in multiple types of cancer, including HCC.3 In the extracellular environment, OPN affects several signaling pathways by binding integrin receptor family units as well as CD44, a ubiquitously expressed surface glycoprotein that mediates cell–cell and cell–matrix interactions.3 A diverse array of signaling pathways regulate OPN expression, including activator protein 1, Myc, viral-Src, Runt-related transcription factor/core binding factor, transforming-growth factor β/bone morphogenic protein/smad/homeobox, and Wnt/ß–catenin/adenomatous polyposis coli tumor suppressor/glycogen synthase kinase 3β/transcription factor 4.
The expression of OPN is enhanced or induced during tumor progression, which mediates cancer processes, including extracellular matrix degradation, tumor cell migration, evasion of host immunity, neovascularization, and inhibition of apoptosis. In addition, induction of OPN expression in Hep3B cells, which express lesser constitutive levels of OPN, results in increased migration and invasion.4
The expression of OPN serves as a prognostic marker and potential therapeutic target in HCC. Increased tissue levels of OPN in HCC correlate with vascular and bile duct invasion, Edmondson grade, and intrahepatic spread, as well as lesser time to tumor recurrence and less overall survival compared with patients with a lesser expression of OPN.5,6 Resection of HCC results in a decrease in OPN expression to low levels >2 months after operation, and, in recurrent HCC, OPN levels again increase.5,6 Substantial in vitro and animal data suggest blocking OPN in HCC results in decreased invasion and metastatic potential; however, there have been few human trials to date.3
In this study we investigated the effect of epigallocatechin-3-gallate (EGCG) on OPN expression in HCC cell lines. EGCG is the most abundant of the catechin antioxidants found in green tea. In epidemiologic studies, consumption of green tea is associated with a decreased recurrence of breast cancer, and, in in vitro models of HCC, EGCG suppresses tumor growth and induces apoptosis.7–9 In addition, EGCG decreases OPN expression in models of breast cancer.10 The aim of our study was to explore whether EGCG modulates OPN expression in models of HCC, and thus elucidate potential anti-cancer mechanisms of the antioxidant.
METHODS
Cell culture
HepG2, Hep3B, and MHCC 97-H cell lines were seeded at approximately 1.0 × 106 cells/plate and grown in standard Eagles Minimum Essential Medium (Corning Cellgro, Mediatech, Inc, Manassas, VA) with 10% fetal bovine serum, glutamine, and penicillin streptomycin at 37°C and 5% CO2. In the following experiments, cells were treated with EGCG at concentrations 20 ng/mL, 200 ng/mL, 2 μg/mL, and 20 μg/mL for 48 hours unless otherwise specified. The only experiment performed on Hep3B cells was enzyme-linked immunosorbent assay (ELISA) for OPN.
MTT assay
Cells were plated at 5,000 cells/well in a 96-well plate. After overnight incubation, cells were treated with 2 μg/mL EGCG. MTT (3-[4,5-dimethylthiazolyl-2]-2,5-diphenyltetrazolium bromide) was added, and cells were incubated for 3–4 hours according to kit protocol (ATCC, Manassas, VA). Detergent reagent was added, and cells were incubated in the dark for 4 hours. Absorbance was measured using the EMAX Precision Microplate Reader (Molecular Devices, Sunnyvale, CA), and the absorbance value of each group was normalized to nontreated controls.
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
RNA was isolated from cell culture using TRIzol RNA Purification following manufacturer guidelines (Life Technologies, Carlsbad, CA). qRT-PCR was performed with the 2-step reaction protocol using iQ SYBR Green Detection Kit (Bio-Rad Laboratories, Hercules, CA). First-strand cDNA were synthesized from 1 μg of total RNA using the iScript Select cDNA Synthesis Kit (Bio-Rad laboratories). The reverse transcription protocol was as follows: 25°C for 5 minutes, 42°C for 30 minutes, and 85°C for 5 minutes. qRT-PCR parameters were: 95°C for 3 minutes; 95°C for 60 seconds, 55°C for 45 seconds (40 cycles); 55°C for 1 minute. The cycle threshold (CT) is defined as the number of cycles required for the target fluorescent signal to exceed the background fluorescence threshold. All CT values were normalized to either input CT values to calculate a percent input or normalized to IgG CT values to calculate fold increase. ΔΔCT values were calculated with β-actin normalization. The sequence of the OPN PCR primer was as follows and resulted in an 86-bp fragment: 5′-tgaatggtgcatacaaggccataa-3′, 5′-ttcataactgtccttcccacggct-3′.
ELISA
Cell supernatant was collected and centrifuged at 3,000g for 5 minutes at 4°C. Supernatants were analyzed using ELISAs of OPN according to manufacturer protocol (R&D Systems, Minneapolis, MN). Kit standards were used to quantify secreted levels of OPN.
Construction of promoter plasmids
Full-length promoter and 5′ deletion fragments were generated by PCR. All fragments were cloned into pGL3 Luciferase Reporter vectors (Promega, Madison, WI). The lengths of the OPN promoter fragments were OPN −80 (nt −80 to nt +86), OPN −108 (nt −108 to nt +86), OPN −135 (nt −135 to nt +86), OPN −174 (nt −174 to nt +86), OPN −190 (nt −190 to nt +86), OPN −400 (nt −400 to nt +86), and OPN –Full (−2,098 to +86). All constructs were confirmed previously by DNA sequencing.11
Dual luciferase reporter assay
Four micrograms of luciferase-reporter constructs or empty PGL3 vector were cotransfected with 0.4 μg of Renilla vector into HepG2 and MHCC 97-H cell lines using Lipofectamine 2000 (Invitrogen, Life Technologies) according to the manufacturer's instruction. After treatment with 2 μg/mL EGCG for 48 hours, cells were lysed, and the promoter activity was measured as a ratio of Firefly:Renilla luciferase using a Dual-Luciferase assay system (Promega), with the Modulus Luminometer (Promega).
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed after treatment of HCC cell lines with EGCG in culture before DNA cross-linking and lysis. Chromatin was fixed, sonicated, and immunoprecipitated using the EZ-ChIP assay kit (Millipore, Billerica, MA) following guidelines by the manufacturer. The DNA was sonicated using previously determined settings to 500 bp fragments. Purified chromatin was immunoprecipitated using mouse anti-RNA polymerase II Ab (1 μg), and normal mouse IgG (5 μg). After DNA purification, qRT-PCR was used to determine the presence of RNA polymerase II bound to the transforming-growth factor β1 TATA box. The qRT-PCR DNA products were then visualized on a 1% agarose gel. Primer for the OPN TATA box promoter/gene qRT-PCR for ChIP assays are as follows and resulted in a 440 bp fragment: 5′-TCA CAATTGCTTCAAAGAGA-3′, 5′-TGAGGCCTCTG TTCTGTTCC-3′.
RNA half-life RT-PCR
MHCC-97H cells were treated with EGCG 2 μg/mL for 24 hours before incubation with actinomycin D 10 μg/mL (Sigma-Aldrich, Inc, Saint Louis, MO). RNA was collected using TRIzol RNA Purification (Life Technologies) at times 0 hours, 30 minutes, and 1, 2, 4, 8, 16, and 32 hours of actinomycin D treatment. qRT-PCR of OPN was completed as described previously. The proportion of OPN RNA at a given time was standardized to the amount of OPN RNA at time 0. Least-square analysis identified the best-fit lines, and the decay rate constant obtained from the slope of the semi-logarithmic plot of [RNA] over time was used to calculate the RNA half-life.12
Statistical analysis
All experiments were performed in triplicate. Quantitative values are expressed as mean ± SEM. Comparisons of independent samples were compared using Student t test, regression modeling, and ANOVA.
Scratch wound assay
HepG2 and MHCC-97H cells were seeded at 100,000 cells per well of a 24-well plate and grown to confluence in serum-free medium. Cells were pretreated with EGCG 2 μg/mL for 24 hours. A pipette tip was used to make a wound that was followed by treatment with EGCG 2 μg/mL for 48 hours. Images were taken at 24 and 48 hours. ImageJ (National Institutes of Health) was used to calculate the percent of wound closure.
RESULTS
Expression of OPN in HCC cell lines
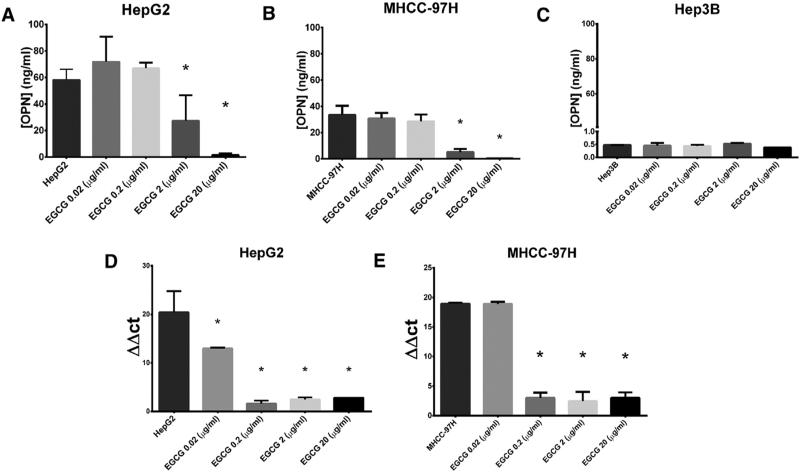
To assess OPN expression in human HCC cell lines, we performed ELISA and RT-PCR analysis. HepG2 and MHCC97-H cell lines expressed high levels of OPN secreted protein relative to the Hep3B cell line (HepG2: 58.1 ± 4.7 ng/mL; MHCC97-H: 33.5 ± 3.9 ng/mL vs Hep3B: 0.48 ± 0.01 ng/mL; all P < .001; Fig 1). RT-PCR demonstrated high levels of OPN mRNA in the MHCC-97 and HepG2 cell lines.
Fig 1.
EGCG decreases OPN mRNA and secreted protein levels. Data are presented as mean ± standard error of the mean (SEM). (A–C) OPN protein levels are significantly greater in HepG2 and MHCC-97H versus Hep3B cell lines. (A and B) OPN secreted protein, measured by ELISA, decreased after EGCG treatment in (A) HepG2 and (B) MHCC-97H cell lines at EGCG concentrations of 2 μg/mL and 20 μg/mL (All P < .001). (D) In HepG2 cells, OPN mRNA measured with RT-PCR decreased after EGCG treatment of 0.02–20 μg/mL (All P < .05). (E) In MHCC-97H cell lines, OPN mRNA decreased after EGCG treatment of 0.2–20 μg/mL (P < .001). ΔΔCT values were defined using β-actin normalization. The figure is representative of 2 experiments (*P < .05).
EGCG treatment decreases OPN expression in a dose-dependent manner
To demonstrate that EGCG decreased the mRNA and secreted protein levels of OPN, we treated MHCC-97H and HepG2 cells with varying concentrations of EGCG (0.02–20 μg/mL) for 48 hours before measuring OPN with ELISA and RT-PCR (Fig 1).
Treatment of MHCC-97H with 2 μg/mL and 20 μg/mL EGCG caused a ~6-fold and ~90-fold decrease in secreted protein levels of OPN compared with no treatment controls (5.2 ± 1.4 ng/mL; 0.4 ± 0.1 ng/mL, vs 33.5 ± 3.9 ng/mL; all P < .001). Similar treatment of HepG2 cells resulted in a ~2-fold and ~40-fold decrease in secreted protein levels of OPN (27.1 ± 11.0 ng/mL; 1.5 ± 0.8 ng/mL vs 58.1 ± 4.7 ng/mL; all P < .001).
Treatment with EGCG at concentrations of 0.02–20 μg/mL in HepG2 cells decreased OPN mRNA (All P < .05). In MHCC-97H cells, EGCG concentrations of 0.2–20 μg/mL resulted in at least a 6.1 × 104 fold decrease in OPN RNA (all P < .001).
In previous studies, a mean EGCG plasma concentration of greater than 2 μg/mL was achieved in healthy individuals ingesting high levels of purified green tea extract.13,14 We chose to use an EGCG concentration of 2 μg/mL for subsequent experiments, because this concentration was the least physiologically achievable dose that significantly decreased the mRNA and secreted protein levels of OPN in MHCC-97H and HepG2 cell lines.
Previous work identified OPN protein as the main functional component mediating metastasis and invasion.3 Specifically, our laboratory demonstrated that blockade of OPN protein with an RNA aptamer resulted in a loss of the metastatic effects of OPN.15 In the current study, the Hep3B cell line demonstrated low constitutive expression of OPN protein, which remained unchanged with EGCG treatment. We chose not to do any further experiments on the Hep3B cell lines, because EGCG had no effect on the expression of OPN protein.
EGCG treatment does not induce apoptosis
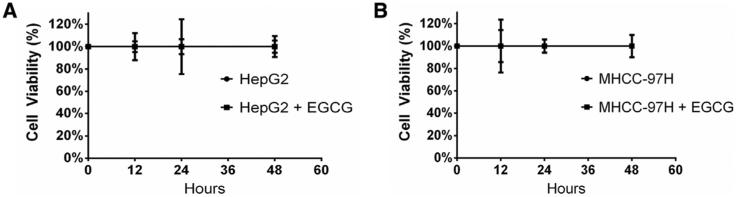
To demonstrate that differences in OPN expression were not attributable to the viability of HCC cells, we conducted an MTT assay on HepG2 and MHCC-97H cells treated with 2 μg/mL EGCG. There were no differences in cell viability between cells treated with EGCG and untreated cells at treatment times of 12, 24, and 48 hours (Fig 2).
Fig 2.
EGCG does not decrease HCC viability. Data are presented as mean ± SEM. MTT assay after EGCG treatment (2 μg/mL) demonstrated no changes in cell viability in EGCG treated versus no treatment controls at times 12, 24, and 48 hours. The figure is representative of 2 experiments.
EGCG does not affect transcriptional regulation of OPN
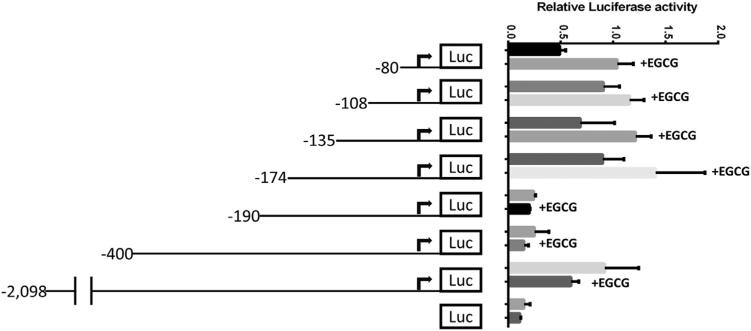
To investigate the role of EGCG in the transcriptional regulation of OPN, we developed a luciferase reporter construct with various deletion fragments of the human OPN promoter (Fig 3). Promoter fragments were cloned into a luciferase reporter plasmid before transfection into HepG2 cells. Transfected cells were treated with 2 μg/mL EGCG for 48 hours, and luciferase reporter activity was measured controlling for constitutive Renilla report. There were no differences in luciferasereport in EGCG-treated versus untreated HCC cell lines between same-length deletion fragments of the OPN promoter (Fig 4). These results suggest that EGCG did not modulate the transcriptional activity of OPN in the immediate 2 kB upstream promoter area.
Fig 3.
EGCG does not modulate the transcription of OPN in the immediate 2 kB upstream promoter area in HepG2 cells. Data are presented as mean ± SEM. There were no differences in relative luciferase report in EGCG treated versus untreated HCC cell lines transfected with plasmids containing same-length deletion fragments of the OPN promoter. The figure is representative of 3 experiments.
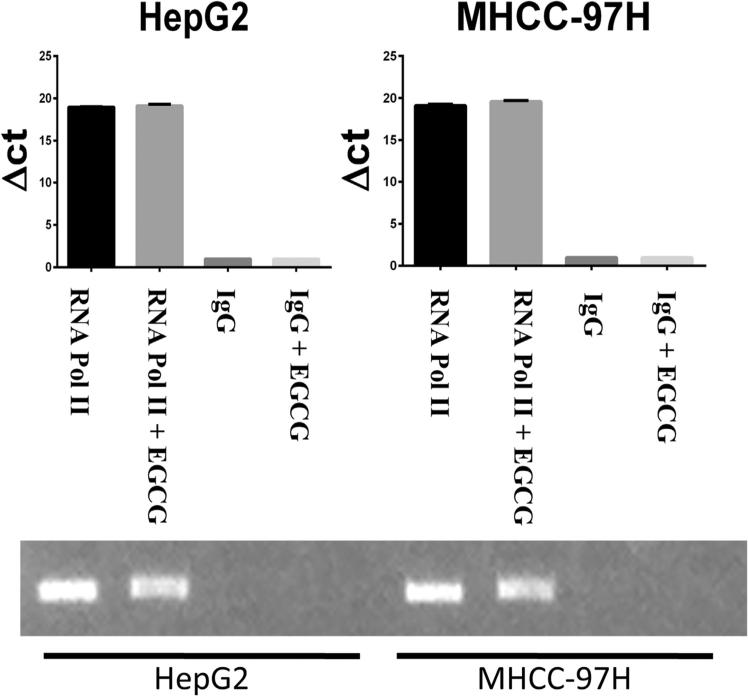
Fig 4.
EGCG does not modulate RNA pol II binding to the human OPN promoter. Data are presented as mean ± SEM. RT-PCR of the OPN promoter after ChIP assay with immunoprecipitation of RNA pol II and IgG demonstrated no difference in RNA pol II binding to the OPN promoter in EGCG treated versus untreated cells. The figure is representative of 2 experiments.
To test whether there was a distant regulatory site or an epigenetic component mediating the effect of EGCG on OPN expression, we performed a ChIP assay for RNA pol II binding to the OPN promoter in HepG2 and MHCC-97H cell lines. After pulldown of RNA pol II, RT-PCR of the OPN TATA box fragment revealed no difference in RNA Pol II binding to the OPN promoter in cells treated with EGCG 2 μg/mL versus untreated cells (Fig 4). These results suggest EGCG did not alter OPN transcription.
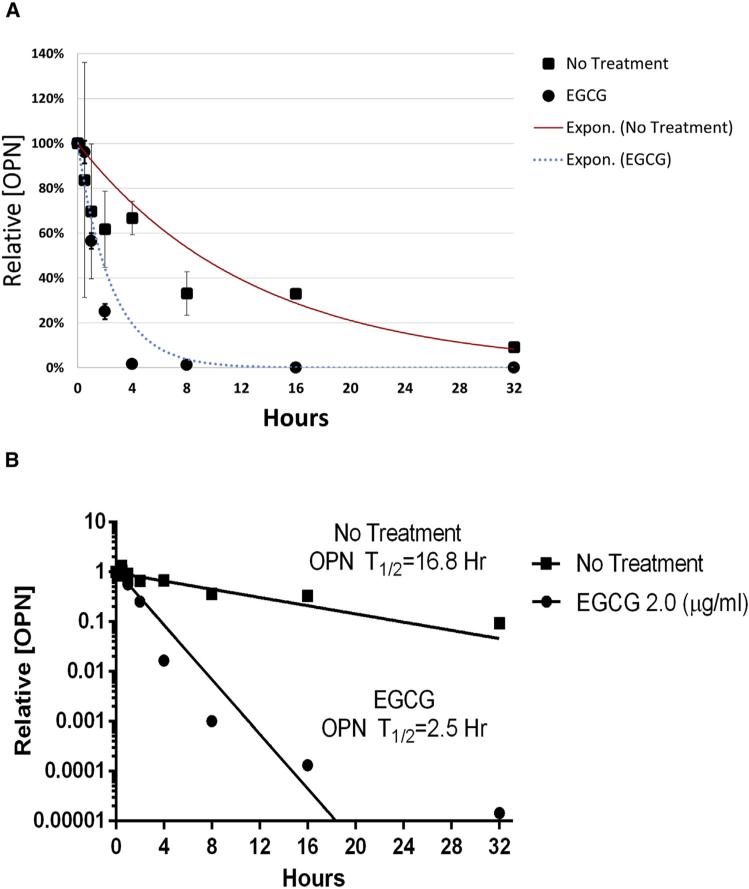
EGCG treatment decreases OPN mRNA half-life
To examine the effect of EGCG on the half-life of OPN mRNA, we exposed EGCG (2 μg/mL) treated MHCC-97H cells to actinomycin D and quantified relative OPN mRNA content at multiple subsequent time points (0–32 hours) using RT-PCR. Actinomycin D halts transcription globally, and the rate of mRNA degradation was determined using the slope of the semi-log plot of OPN mRNA over time relative to starting values. We found EGCG treatment decreased the half-life of OPN mRNA from 16.8 h (95% confidence interval [95% CI] 9.0–125.1 hours) to 2.5 hours (95% CI 2.1–3.2 hours) in MHCC-97H cells (P < .001) (Fig 5).
Fig 5.
EGCG treatment decreases the half-life of OPN mRNA. (A) The relative amount of OPN over time (0–32 hours) is measured in the MHCC-97H cells in the EGCG treated versus untreated conditions. Data represent n = 3 and are presented as mean ± SEM. (B) The data from (A) is graphed on a semilogarithmic plot to depict the calculation of the half-life of OPN mRNA. EGCG treatment (2 μg/mL) decreased the OPN mRNA half-life from 16.8 h (95% CI 9.0–125.1 hours) to 2.5 hours (95% CI 2.1–3.2 hours) in MHCC-97H cells (P < .001). Depicted is the slope of the semi-logarithmic plot of relative (OPN) over time, which represents the decay rate constant.
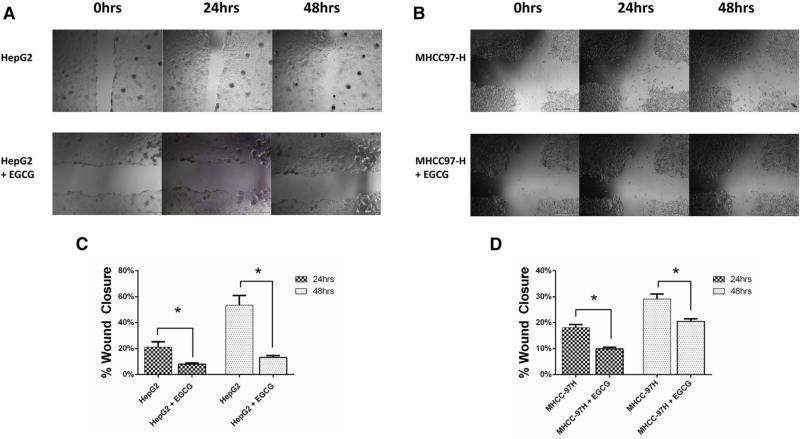
EGCG treatment decreases cell migration
We performed a scratch assay with HepG2 and MHCC-97H cells to investigate the functional effect of EGCG treatment on HCC cell migration (Fig 6). In HepG2 cells, migration was decreased in EGCG-treated group at 24 h (8 ± 2% vs 21 ± 11%, P < .01) and at 48 hours (13 ± 4% vs 54 ± 20%, P < .001). In MHCC-97H cells, migration was decreased in EGCG treated group at 24 h (9.9 ± 1.6% vs 18.1 ± 3.0%) and at 48 hours (21 ± 2% vs 29 ± 5%) (Both P < .01).
Fig 6.
Scratch wound assay in HepG2 and MHCC-97H cells. Data are presented as mean ± SEM. (A and B) A monolayer of (A) HepG2 and (B) MHCC-97H cells was pretreated with EGCG (2 μg/mL) for 24 hours before scratching. EGCG treatment continued and cells were imaged at baseline, 24 and 48 hours after the scratch. (C and D) EGCG treatment resulted in decreased cell migration. Migration was quantified based on the percentage of the wound healed at 24 and 48 hours relative to baseline values in HepG2 (C) and MHCC-97H (D) cell lines. The images are representative of three experiments. (*P < .01).
DISCUSSION
We demonstrated that treatment of the human HCC cell lines HepG2 and MHCC-97H with physiologically achievable concentrations of EGCG, a green tea component, resulted in decreased expression of the mRNA and protein levels of OPN. EGCG treatment at this level did not result in decreased cell viability but did decrease migration. The mechanism of OPN down-regulation appears to be posttranscriptional, and EGCG significantly decreased the half-life of OPN mRNA in MHCC-97H cells.
We found that treatment of the human MHCC-97H cells with EGCG decreased OPN mRNA half-life. In previous studies, our lab found that the low expression of OPN in Hep3B cells is caused by posttranscriptional control of the OPN 5′-UTR.16 Further studies identified elongation translation factor-1A1 (EF1A1) as an actin-dependent regulator of the OPN 5′-UTR that acts to decrease OPN mRNA stability.17 Interestingly, Umeda et al18 identified EF1A as a component responsible for the anticancer activity of EGCG by screening genetic suppressor elements in B16 mouse melanoma cells. In several cell lines including HepG2, EGCG was shown to up-regulate EF1A via EGCG interaction with the extracellular domain of the 67-kDa laminin receptor.18,19
An alternate pathway involves micro-RNA modulation of OPN mRNA stability. In silico analysis revealed mir-20a, mir-93, and mir-221 as targets for decreasing the expression of OPN mRNA (data not published). Interestingly, the expression of these targets increases after EGCG treatment.20 Future studies are required to determine whether EGCG down regulates OPN in HCC via microRNA pathways or the 67-kDa laminin receptor–mediated induction of EF1A.
Previous work in vitro found supraphysiologic levels of EGCG were required to demonstrate anticancer mechanisms. EGCG treatment at concentrations 5–50 times physiologic levels down-regulates EP1 receptor expression to decrease viability in HepG2 cells, down-regulates the VEGF/VEGFR axis in HuH7 cells, and decreases levels of BCL-2 family proteins to induce apoptosis in HLE cells.8,21 Darweish et al22 found EGCG treatment of a rat model of HCC resulted in a decreased number of HCC nodules per liver, decreased serum alpha-fetoprotein, and increased survival. Rat hepatic homogenates demonstrated decreased FGF2 and MMP9 after EGCG treatment. Although the anticancer mechanisms of EGCG described previously are promising, application is limited currently by poor bioavailability.
The EGCG concentration of 2 μg/mL used in this study is physiologically achievable in the plasma of healthy human controls, but only at high doses of purified green tea extract.13,14 Efforts are underway to increase EGCG bioavailability by conjugation with nanoparticles. EGCG conjugation with polylactic acid results in superior pharmacokinetics, including a 10-fold dose advantage in its proapoptotic and antiangiogenic effects after intraperitoneal administration in a mouse model.23
Other work has focused on optimizing oral bioavailability. Chitosan- and lipid-encapsulated EGCG has superior intestinal absorption and demonstrates plasma concentrations 5–6 times unconjugated EGCG.24–26 Tumor targeting with EGCG has been achieved by conjugating EGCG and nanoparticles to organic small molecules with high binding specificity to prostate-specific membrane antigen.27 Although much work remains before clinical application, these studies suggest a role for high concentration EGCG in cancer chemotherapy.
Preliminary evidence suggests EGCG may benefit HCC patients. Baba et al28 gave patients with inoperable HCC a dose of 474 mg of EGCG per day in conjunction with hepatic arterial infusion chemotherapy. Patients demonstrated decreased levels of derivatives of reactive oxygen metabolites, a marker of oxidative stress. Future trials should include serologic markers, such as OPN and alpha-fetoprotein, along with clinical metrics like time to tumor recurrence and survival curves.
There are limitations that may affect the generalizability of our results. While the data was obtained from experiments on certain human HCC cell lines, the mechanism by which EGCG decreases OPN expression was not elucidated in vivo. In addition, we did not prove the functional relevance of EGCG-mediated down-regulation of OPN. Although EGCG treatment resulted in decreased migration in HepG2 and MHCC-97H cells, we did not demonstrate that the decreases in migration were due to lesser levels of OPN. OPN is known to increase HCC motility, migration, and invasion but we did not show that the effect of EGCG is to decrease these processes in vivo.
We provide evidence that EGCG decreases OPN mRNA and secreted OPN protein levels by decreasing the half-life of OPN mRNA in MHCC-97H cells. Functionally, EGCG treatment results in decreased migration. The translatability of EGCG for patients with HCC is promising, because it is an inexpensive, easily accessible chemical with an extensive history of safety.
Acknowledgments
MHCC-97 cells were generously donated by Xin Wei Wang (NIH, Bethesda, MD).
The Loyola University Stritch School of Medicine Student Training in Approaches to Research (STAR) grant supported the work via a medical student stipend.
DISCUSSION
Dr. C. Max Schmidt (Indianapolis, IN): As Matthew eloquently presented, EGCG, a chemical in green tea, suppresses growth and induces apoptosis in HCC in vitro. Their study investigates whether EGCG works through an osteopontin mechanism. Using 3 HCC cell lines, they measure osteopontin levels with multiple measurement techniques: osteopontin promoter binding, transcriptional regulation, and half-life are measured with ChIP analysis, luciferase assay, and actinomycin D techniques. The results suggest that EGCG indeed regulates osteopontin via posttranscriptional modification, which enhances its degradation, dramatically decreasing its functional half-life. I have several questions.
First, one of your premises in your study was that EGCG suppresses growth and induces apoptosis in HCC in vitro. Your study seeks to establish EGCG effects on osteopontin. From your study, it appears that EGCG decreases osteopontin mRNA half-life. It does this, however, without affecting growth On this basis:
Do you hypothesize that the osteopontin effect is NOT the mechanism of the ECGC suppression of growth and induction of apoptosis?
Do you hypothesize that the osteopontin effect must be more pronounced to see the effects on growth and apoptosis?
Do you hypothesize that the osteopontin effect is necessary but not sufficient to see the effects on growth and apoptosis?
Second, EGCG causes decreased osteopontin mRNA, but green tea has a number of other chemicals (and effects) than EGCG. Does green tea cause decreased osteopontin mRNA? If so, at what dose? That is, how much green tea do I need to drink to achieve 2 μg/mL plasma concentration of EGCG to get the effect of lowering osteopontin?
Third, with EGCG treatment, osteopontin is decreased in HCC in vitro.
What is the functional effect of this? By your MTT assay, it appears it does not affect growth.
Have you examined invasiveness or metastatic potential?
Hep3B cells do not express high levels of osteopontin like the other cell lines. What is the effect of EGCG on these cells?
Finally, with ECGC treatment, osteopontin mRNA half-life is decreased in MHCC-97H cells. What is the effect in the high osteopontin expressing HepG2 cells?
Dr Matthew Zapf: The first question addresses the effect of osteopontin on growth in apoptosis. A lot of the focus in our laboratory is actually on the effect of osteopontin in the extracellular environment, specifically in inducing cancer-associated fibroblasts. I'm not quite sure if you could decrease osteopontin to the extent where you would, in fact, induce apoptosis.
As far as the second question about whether green tea alone could decrease osteopontin mRNA, I'm not totally sure. I might expect that it would, based on EGCG being the most abundant of the catechins found in green tea.
Regarding the dosages of regular green tea that would be required to achieve this plasma concentration, the studies that have had healthy subjects achieve this plasma concentration do so by giving them purified green tea extract. To actually achieve this concentration, you would have to drink about 12 or 14 cups of green tea in a somewhat short amount of time, which doesn't seem very reasonable. They have purified and condensed the specific chemical down so that it can be consumed orally.
Regarding the functional effects of EGCG treatment, that's a topic of ongoing research right now. We are assessing the role of EGCG in decreasing osteopontin and the downstream effects of that.
Lastly, we have not yet done an experiment to see if the effect is the same in the HepB2 cell lines.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Fan ST. Hepatocellular carcinoma–resection or transplant? Nat Rev Gastroenterol Hepatol. 2012;9:732–7. doi: 10.1038/nrgastro.2012.158. [DOI] [PubMed] [Google Scholar]
- 3.Wai PY, Kuo PC. Osteopontin: Regulation in tumor metastasis. Cancer Metastasis Rev. 2008;27:103–18. doi: 10.1007/s10555-007-9104-9. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharya SD, Garrison J, Guo H, et al. Micro-RNA-181a regulates osteopontin-dependent metastatic function in hepatocellular cancer cell lines. Surgery. 2010;148:291–7. doi: 10.1016/j.surg.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou C, Zhou HJ, Zhang XF, et al. Postoperative serum osteopontin level is a novel monitor for treatment response and tumor recurrence after resection of hepatitis B-related hepatocellular carcinoma. Ann Surg Oncol. 2013;20:929–37. doi: 10.1245/s10434-012-2749-9. [DOI] [PubMed] [Google Scholar]
- 6.Huang H, Zhang XF, Zhou HJ, et al. Expression and prognostic significance of osteopontin and caspase-3 in hepato-cellular carcinoma patients after curative resection. Cancer Sci. 2010;101:1314–9. doi: 10.1111/j.1349-7006.2010.01524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogunleye AA, Xue F, Michels KB. Green tea consumption and breast cancer risk or recurrence: a meta-analysis. Breast Cancer Res Treat. 2010;119:477–84. doi: 10.1007/s10549-009-0415-0. [DOI] [PubMed] [Google Scholar]
- 8.Nishikawa T, Nakajima T, Moriguchi M, et al. A green tea polyphenol, epigalocatechin-3-gallate, induces apoptosis of human hepatocellular carcinoma, possibly through inhibition of bcl-2 family proteins. J Hepatol. 2006;44:1074–82. doi: 10.1016/j.jhep.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 9.Shirakami Y, Shimizu M, Adachi S, et al. (−)-Epigallocate-chin gallate suppresses the growth of human hepatocellular carcinoma cells by inhibiting activation of the vascular endothelial growth factor-vascular endothelial growth factor receptor axis. Cancer Sci. 2009;100:1957–62. doi: 10.1111/j.1349-7006.2009.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu YC, Liou YM. The anti-cancer effects of (−)-epigallocate-chin-3-gallate on the signaling pathways associated with membrane receptors in MCF-7 cells. J Cell Physiol. 2011;226:2721–30. doi: 10.1002/jcp.22623. [DOI] [PubMed] [Google Scholar]
- 11.Takami Y, Russell MB, Gao C, et al. Sp1 regulates osteopontin expression in SW480 human colon adenocarcinoma cells. Surgery. 2007;142:163–9. doi: 10.1016/j.surg.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CY, Ezzeddine N, Shyu AB. Messenger RNA half-life measurements in mammalian cells. Methods Enzymol. 2008;448:335–7. doi: 10.1016/S0076-6879(08)02617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ullmann U, Haller J, Decourt JP, et al. A single ascending dose study of epigallocatechin gallate in healthy volunteers. J Int Med Res. 2003;31:88–101. doi: 10.1177/147323000303100205. [DOI] [PubMed] [Google Scholar]
- 14.Chow HH, Hakim IA, Vining DR, et al. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of polyphenon E in healthy individuals. Clin Cancer Res. 2005;11:4627–33. doi: 10.1158/1078-0432.CCR-04-2549. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharya SD, Mi Z, Kim VM, Guo H, Talbot LJ, Kuo PC. Osteopontin regulates epithelial mesenchymal transition-associated growth of hepatocellular cancer in a mouse xenograft model. Ann Surg. 2012;255:319–25. doi: 10.1097/SLA.0b013e31823e3a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emani S, Zhang J, Guo L, Guo H, Kuo PC. RNA stability regulates differential expression of the metastasis protein, osteopontin, in hepatocellular cancer. Surgery. 2008;143:803–12. doi: 10.1016/j.surg.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Guo H, Mi Z, et al. EF1A1-actin interactions alter mRNA stability to determine differential osteopontin expression in HepG2 and Hep3B cells. Exp Cell Res. 2009;315:304–12. doi: 10.1016/j.yexcr.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 18.Umeda D, Yano S, Yamada K, Tachibana H. Green tea polyphenol epigallocatechin-3-gallate signaling pathway through 67-kDa laminin receptor. J Biol Chem. 2008;283:3050–8. doi: 10.1074/jbc.M707892200. [DOI] [PubMed] [Google Scholar]
- 19.Fujimura Y, Sumida M, Sugihara K, Tsukamoto S, Yamada K, Tachibana H. Green tea polyphenol EGCG sensing motif on the 67-kDa laminin receptor. PLoS One. 2012;7:e37942. doi: 10.1371/journal.pone.0037942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsang WP, Kwok TT. Epigallocatechin gallate up-regulation of miR-16 and induction of apoptosis in human cancer cells. J Nutr Biochem. 2010;21:140–6. doi: 10.1016/j.jnutbio.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Jin J, Chang Y, Wei W, et al. Prostanoid EP1 receptor as the target of (−)-epigallocatechin-3-gallate in suppressing hepa tocellular carcinoma cells in vitro. Acta Pharmacol Sin. 2012;33:701–9. doi: 10.1038/aps.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darweish MM, Abbas A, Ebrahim MA, Al-Gayyar MM. Chemopreventive and hepatoprotective effects of epigallocatechin-gallate against hepatocellular carcinoma: Role of heparan sulfate proteoglycans pathway. J Pharm Pharmacol. 2014;66:1032–45. doi: 10.1111/jphp.12229. [DOI] [PubMed] [Google Scholar]
- 23.Siddiqui IA, Adhami VM, Bharali DJ, et al. Introducing nanochemoprevention as a novel approach for cancer control: Proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res. 2009;69:1712–6. doi: 10.1158/0008-5472.CAN-08-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith A, Giunta B, Bickford PC, Fountain M, Tan J, Shytle RD. Nanolipidic particles improve the bioavailability and alpha-secretase inducing ability of epigallocatechin-3-gallate (EGCG) for the treatment of alzheimer's disease. Int J Pharm. 2010;389:207–12. doi: 10.1016/j.ijpharm.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dube A, Nicolazzo JA, Larson I. Chitosan nanoparticles enhance the plasma exposure of (−)-epigallocatechin gallate in mice through an enhancement in intestinal stability. Eur J Pharm Sci. 2011;44:422–6. doi: 10.1016/j.ejps.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Hu B, Ting Y, Yang X, Tang W, Zeng X, Huang Q. Nanochemoprevention by encapsulation of (−)-epigallocatechin-3-gallate with bioactive peptides/chitosan nanoparticles for enhancement of its bioavailability. Chem Commun (Camb) 2012;48:2421–3. doi: 10.1039/c2cc17295j. [DOI] [PubMed] [Google Scholar]
- 27.Sanna V, Pintus G, Roggio AM, Punzoni S, Posadino AM, Arca A, et al. Targeted biocompatible nanoparticles for the delivery of ([C0])-epigallocatechin 3-gallate to prostate cancer cells. J Med Chem. 2011;54:1321–32. doi: 10.1021/jm1013715. [DOI] [PubMed] [Google Scholar]
- 28.Baba Y, Sonoda JI, Hayashi S, Tosuji N, Sonoda S, Makisumi K, et al. Reduction of oxidative stress in liver cancer patients by oral green tea polyphenol tablets during hepatic arterial infusion chemotherapy. Exp Ther Med. 2012;4:452–8. doi: 10.3892/etm.2012.602. [DOI] [PMC free article] [PubMed] [Google Scholar]