The cellular regulatory system known as autophagy is an essential degradation pathway by which the body removes cellular debris and recycles damaged components. During the process of adipogenesis, which is the formation of adipocytes from the precursor preadipocytes, the extensive cytoplasmic remodeling requires autophagy.1 FoxO1, a forkhead O family protein, is a transcription factor which has been shown to be a central inhibitory molecule for the adipogenesis, where it transrepresses PPARγ by directly interacting with it.2 FoxO1 has been shown to be a direct mediator of autophagy in a transcription-independent manner, whereby acetylated FoxO1 interacts with autophagy-related protein 7 (Atg7), to induce autophagy in cancer cells.3 In this volume of the cell cycle, Liu et.al. 4 have shown that FoxO1 regulates lipid droplet growth and size in adipocytes by enhancing autophagy. Inhibition of FoxO1 reduced the expression of Beclin1 which increased the stability of p62, an inhibitor of autophagy. Pharmacological antagonism of FoxO1 suppressed lipid droplet growth, an essential event during differentiation, by suppressing autophagy (Fig. 1). This effect was dependent on Fat Specific Protein 27 (FSP27), a lipid droplet protein associated with regulation of lipolysis 5 and lipid droplet morphology in adipocytes.6 Consistent with the role of FoxO1-autophagy mediated shrinkage of lipid droplets, the inhibition of either FoxO1 or autophagy downregulated the FSP27 protein expression in adipocytes. The authors followed a rational approach in primary cells as well as white adipose tissue to strengthen their findings showing FSP27 as a downstream effector of the FoxO1-autophagy axis.
Figure 1.
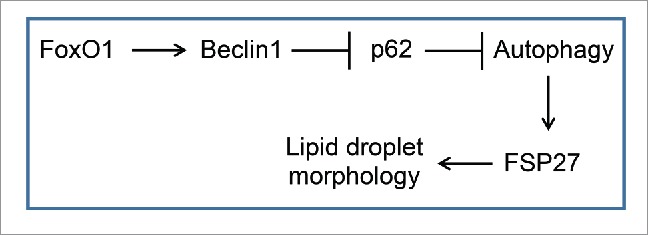
FoxO1 increases the expression of Beclin 1 which then degrades p62 to induce autophagy. Induction of autophagy increases FSP27 expression and lipid droplet growth in adipocytes.
Whether FoxO1 has any direct effect on FSP27 expression independent of autophagy, or FoxO1-autophagy axis affects the stability of FSP27 remains to be answered by future studies. Contrary to the studies showing the inhibitory role of FoxO1 on adipocyte differentiation,2 the study by Liu et al. suggests that a specific inhibitor of FoxO1, AS1842856, has a negative effect on the differentiation of preadipocytes. In differentiated adipocytes, inhibition of FoxO1 leads to reduction in lipid droplet size. It might be that this effect on the lipid droplet size is because inhibition of autophagy leads to shrinkage of lipid droplets caused by conversion of white to brown-like or brite adipocytes.1 Brite adipocytes have smaller lipid droplets with increased β-oxidation.1 Another important question raised by this study is how FoxO1 regulates autophagic machinery? Does it directly control autophagic genes at the transcriptional level or does it change the cellular ATP/AMP ratio to inhibit mTOR or activate AMPK to regulate autophagy, given that FoxO1 can modulate ATP production, and AMPK and mTOR regulates autophagy in response to nutrient signaling.7 Interestingly this work raises a possibility that nuclear FoxO1 and cytosolic FoxO1 might play different roles during adipocyte differentiation. Overall, the study by Liu et al. highlights the role of the FoxO1-autophagy-FSP27 axis in regulating adipocyte differentiation and lipid droplet size in adipocytes. Future studies exploring tissue specific FoxO1 and autophagy knockouts in the adipose tissue of adult mice are needed to determine whether autophagy does contribute to lipid mobilization physiologically.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK, Tang Y, Pessin JE, Schwartz GJ, Czaja MJ. Autophagy regulates adipose mass and differentiation in mice. J Clin Invest 2009; 119:3329-39; PMID:19855132; http://dx.doi.org/ 10.1172/JCI35541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fan W, Imamura T, Sonoda N, Sears DD, Patsouris D, Kim JJ, Olefsky JM. FOXO1 transrepresses peroxisome proliferator-activated receptor gamma transactivation, coordinating an insulin-induced feed-forward response in adipocytes. J Biol Chem 2009; 284:12188-97; PMID:19246449; http://dx.doi.org/ 10.1074/jbc.M808915200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhao Y, Yang J, Liao W, Liu X, Zhang H, Wang S, Wang D, Feng J, Yu L, Zhu WG. Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nat Cell Biol 2010; 12:665-75; PMID:20543840; http://dx.doi.org/ 10.1038/ncb2069 [DOI] [PubMed] [Google Scholar]
- [4].Liu L, Zheng LD, Zou P, Brooke J, Smith C, Long YC, Almeida FA, Liu D, Cheng Z. FoxO1 antagonist suppresses autophagy and lipid droplet growth in adipocytes. Cell Cycle 2016; 15(15):2033-41; PMID:27260854; http://dx.doi.org/ 10.1080/15384101.2016.1192732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Grahn TH, Kaur R, Yin J, Schweiger M, Sharma VM, Lee MJ, Ido Y, Smas CM, Zechner R, Lass A, et al.. Fat-specific protein 27 (FSP27) interacts with adipose triglyceride lipase (ATGL) to regulate lipolysis and insulin sensitivity in human adipocytes. J Biol Chem 2014; 289:12029-39; PMID:24627478; http://dx.doi.org/ 10.1074/jbc.M113.539890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Puri V, Konda S, Ranjit S, Aouadi M, Chawla A, Chouinard M, Chakladar A, Czech MP. Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J Biol Chem 2007; 282:34213-8; PMID:17884815; http://dx.doi.org/ 10.1074/jbc.M707404200 [DOI] [PubMed] [Google Scholar]
- [7].Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 2011; 13:132-41; PMID: 21258367; http://dx.doi.org/ 10.1038/ncb2152 [DOI] [PMC free article] [PubMed] [Google Scholar]


