ABSTRACT
The mechanisms of intercellular spreading of amyloidogenic proteins involved in neurodegenerative diseases have yet to be fully elucidated. While secretion has been implicated in the transfer of many proteins, including prions and α-synuclein, tunneling nanotubes (TNTs) have also been demonstrated for prions and mutant Huntingtin. Here, we provide further evidence that Tau aggregates, which have been demonstrated to predominantly be transferred via secretion, can also be found in TNTs. Additionally, cells that have taken up Tau have increased TNT formation. Coupled with previous evidence that other amyloidogenic aggregates also induce TNT formation we propose that misfolded protein aggregates can, through a common mechanism, promote the formation of TNTs and thereby their own intercellular transfer, contributing to the propagation of pathology.
INTRODUCTION
The trans-cellular spread of amyloidogenic proteins like prions, α-synuclein and tau appears to be a crucial event in the pathogenesis of neurodegenerative diseases as it could mediate the progressive spread of the pathology observed in the brain of patients.1,2 In vitro studies have shown that protein aggregates may spread between cells via physical connections such as tunneling nanotubes3,4 as proposed for prions and Htt.5,6 Tunneling nanotubes (TNTs) are thin F-actin containing channels between remote cells which have recently been recognized as a mechanism for long-range intercellular communication in many cell types and contexts. Unlike other filamentous bridges (e.g., filopodia, cytonemes), TNTs directly connect cells, creating temporary cytoplasmic continuity between distant cells, of different types, and they do not attach to the substrate in culture. They have different diameters (from 100 nm to 800 nm) and can be very long (over 100 µm).3,5 These dynamic structures selectively transfer a wide variety of cellular materials, such as cytoplasmic molecules, plasma membrane components, vesicles and organelles. In addition TNTs can be hijacked by various pathogens, such as bacteria, viruses and prions5 and prion like proteins, including mutant Huntingtin (mHtt)6 and α-synuclein.7
Prions and prion-like proteins may also be released via secretory mechanisms, such as exosomes8,9 and internalized by neighboring cells. This has been proposed for superoxide dismutase (SOD-1),10 α-synuclein11-14 and polyglutamine aggregates.15 In addition, earlier evidence had shown release and internalization of fibrillar aggregates of the Tau microtubule-binding region (MTBR).16,17 More recently we have shown that full length human tau readily aggregates into low molecular weight (LMW) aggregates and fibrils, which can enter Hela cells and primary neurons through bulk endocytosis.18 Interestingly the uptake of Tau depends on both the conformation and size of the aggregates. In primary neurons internalization can occur not only at the somatodendritic compartment, followed by anterograde transport to axon terminals, but also at axonal terminals followed by retrograde transport to the cell body,18 supporting the hypothesis that tauopathy may spread trans-synaptically in vivo, via cell-to-cell transfer. Moreover, recent data indicate that endogenously formed tau released from primary neurons can transfer to acceptor neurons and seed the formation of tau aggregates suggesting that endogenous tau transfer is secretion dependent. Furthermore by increasing neuronal activity the authors could demonstrate an increase of tau release in vitro and exacerbated tau pathology in murine hippocampus in vivo.19
Here we have extended these findings by analyzing the potential role of tunneling nanotubes as an alternative pathway for intercellular spreading of exogenous recombinant human tau fibrils. We found that both in Hela cells and neuronal CAD cells, internalized fibrillar tau is found in TNTs. Furthermore we show that tau aggregates increase TNT number supporting the hypothesis that this would facilitate its spreading. Coupled with previous evidence that misfolded protein aggregates such as prion, mHtt and α-synuclein increase TNT formation6,7,20 this suggests that TNTs can be induced by toxic protein aggregates and represent a general mechanism of spreading that exists alongside secretion.
RESULTS AND DISCUSSION
In order to determine whether recombinant human tau fibrils could be internalized in neuronal catecholaminergic murine CAD cells, which are an excellent model to study TNTs in culture,5 we used fluorescently labeled human tau fibrils, previously shown to be efficiently internalized in Hela cells and primary neurons.18 We compared the rate of internalization after 16 hours incubation in Hela and CAD cells in parallel cultures. By fluorescent microscopy we found that 100% of both cell type internalized tau fibrils. As shown in Fig. 1A, in Hela cells tau fibrils were found to coalesce into several puncta within the cells and to accumulate in the peri-nuclear region. Similarly to HeLa cells, the totality of CADs cells (100%) have taken up fluorescent tau fibrils, however the distribution of tau fibrils appeared to be sparser than in HeLa cells (Fig. 1B).
FIGURE 1.
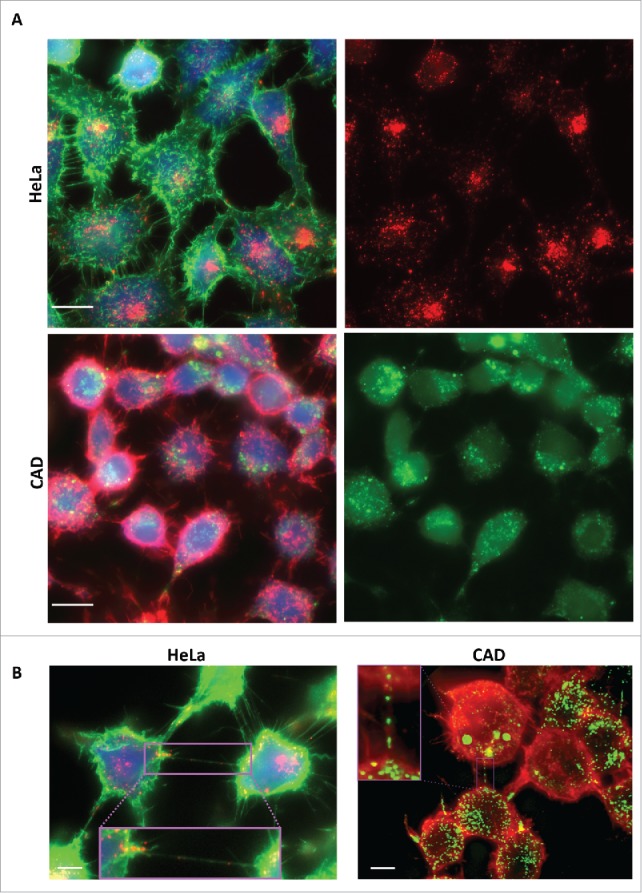
Tau fibrils are internalized in non-neuronal and neuronal cells. A: Representative picture of HeLa (top panel) and CAD (bottom panel) cells respectively incubated with fluorescent Alexa-555 or 488 (red and green) recombinant tau fibrils for 16 hours and stained for the cytosol (in blue) and plasma membrane (WGA, green and red, respectively). Left panels: Merge of WGA and Tau; Right panels: single channel showing fluorescent Tau. Scale bar: 15 and 10 µm for HeLa and CAD respectively. B: Representative deconvolved pictures of HeLa (left, labeled with green WGA to delineate the plasma membrane) and CAD cells (right, labeled with red WGA to delineate the plasma membrane and TNTs) containing fluorescent tau fibrils (red and green, respectively for HeLa and CAD) and connected by TNTs containing several tau fibrils puncta (inset). Scale bar is 15 and 10 µm for HeLa and CAD respectively. Boxed insets show close-ups of the TNTs containing punctate Tau aggregates.
Next, in order to investigate whether tau fibrils could use TNTs as a mechanism to spread between cells, we first analyzed whether tau fibrils could be found inside TNTs. To this end we incubated HeLa and CAD cells with Alexa-fluorophore-tau fibrils. After 16 hours, cells were labeled with fluorescent WGA to delineate the plasma membrane and TNT occurrence.21 By microscopic imaging we could identify several TNTs connecting cells in both HeLa and CAD cultures, respectively (Fig. 1B). Interestingly, among connected cells, we could identify TNTs that contained tau fibrils (Fig. 1B, inset). This suggests that internalized tau fibrils can be transported within TNTs.
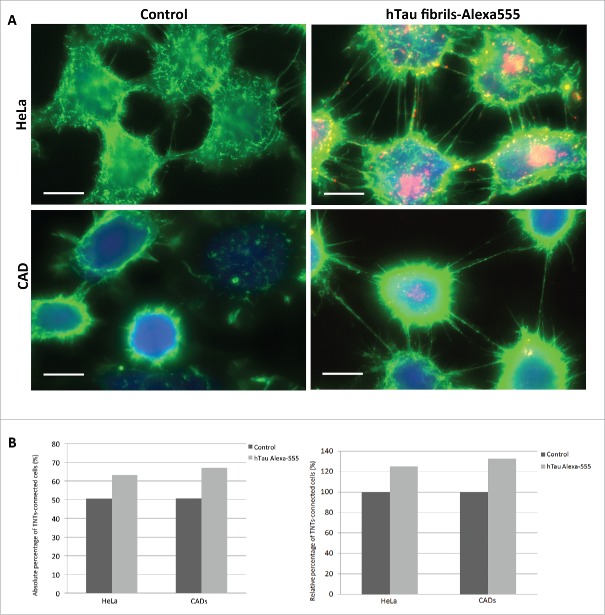
Prions and prion-like proteins aggregates are known to share several similarities in terms of replication and mechanism of propagation.1 Importantly, TNTs were demonstrated to act as a mechanism of intercellular transfer for prion and huntingtin aggregates in neuronal cells and primary neurons.5,6 Since prion, α-synuclein fibrils and huntingtin aggregates were found to increase the amount of TNTs in CAD cells,6,7,20 we investigated whether tau fibrils could similarly affect TNT formation in cultured cells. HeLa or CAD cells were incubated with fluorescent tau fibrils for 16 hours. Then, we analyzed the number of TNTs in the 2 cell populations as previously described.20,21 Interestingly cells exposed to tau appeared to form more TNTs than control cells (Fig. 2A). Based on this observation, we quantified the amount of TNTs based on 2 criteria: i) continuity of TNTs between 2 cells and ii) absence of contact of TNTs with the substratum.21 We found that the number of TNTs was increased for both HeLa and CAD cells containing tau fibrils compared to control cells (25% and 32% increase for HeLa and CAD cells, respectively, Fig. 2B).
FIGURE 2.
TNT number is increased in cells containing tau fibrils. A: Representative pictures of HeLa (top) and CAD (bottom) cells labeled with WGA-Alexa 488 (green) in control untreated conditions (left) or incubated with Alexa-555 tau fibrils (red) for 16 h (right). Scale bar: 15 and 10 µm for HeLa and CAD respectively. B: Quantification of TNT connected cells from experiment done in A. Absolute and relative data are shown in left and right respectively.
These data indicate that similarly to prion, α-synuclein and huntingtin aggregates, tau fibrils are able to enter cells and induce TNT formation. We therefore propose that prion-like proteins, when aggregated, are capable of inducing TNT formation via a common mechanism. While further studies will be necessary to address the specific mechanism leading to TNT increases, we speculate that this is linked to oxidative stress pathways. Indeed while oxidative stress has been shown to increase TNT formation in neuronal cells and primary neurons,5,22 oxidative stress is associated with the presence of aggregated proteins in neurodegeneration.23 Therefore based on these findings, we propose a model where upon aggregation, prion-like proteins would create the formation of reactive oxygen species that would then stimulate TNT formation via an undeciphered mechanism. One possibility could be the up-regulation of membrane biogenetic genes through the unfolded protein response (UPR).24 Alternatively, stress mediated through lysosomes may be involved.25 This hypothesis is especially attractive as prion aggregates20 as well as synuclein aggregates7 are found in lysosomes within the soma and in TNTs and could cause lysosomal damage that may induce a stress-response pathway. The increased number of TNTs would in turn favor the transfer of prion-like aggregate from one cell to another and contribute to the spreading of the pathogenic aggregates (Fig. 3). The mechanism of aggregate-induced TNT formation is an area that requires substantial study in order to decipher the pathways and molecules involved in their formation.
FIGURE 3.
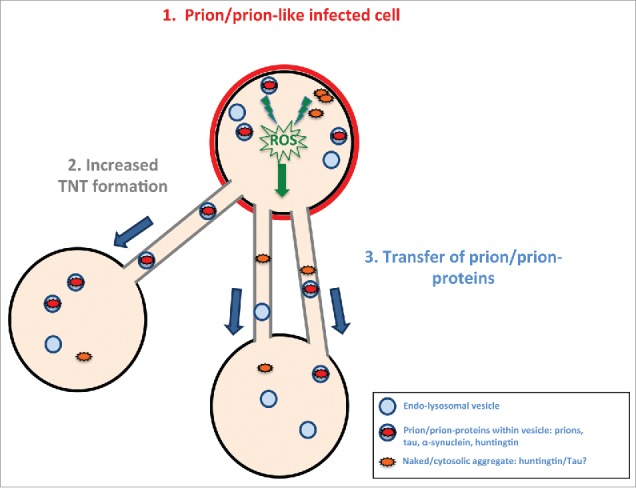
Model of prion/prion-like protein induced formation of TNTs and spreading. Prions or prion-like proteins such as tau fibrils after “infecting” a cell (in red) (1) induce via an unknown (possible common) mechanism (for example, induced via radical oxygen species (ROS) from oxidative stress) an increase in TNT number (2). The prion/prion-like aggregates would then be propagated via TNT-mediated intercellular trafficking from infected cells to naïve cells (3).
Based on the bulk of our in vitro data we suggest that TNT-mediated transfer of prion-like proteins implicated in neurodegenerative diseases could be an efficient mechanism in addition to secretion, exosome release, trans-synaptic spreading, for the propagation of the pathology in the brain. It is possible that these events may occur in different phases of the disease or in propagation of the pathogenic seeds between different cell types during the diseases progression. Deciphering the occurrence and prevalence of these mechanisms will improve our understanding of the pathology progression of neurodegenerative diseases.
Recent studies have provided new insights into the existence of TNTs and TNT-like structures in vivo,26-29 especially in the case of cancer30-33 and more specifically in brain between human astroglioblastoma cells transplanted in mouse brain.34 Nonetheless to assess the contribution of TNTs to disease spreading, it is imperative to find specific markers and use advanced imaging techniques in order to identify this structure in vivo. Therefore in-depth investigation regarding function and mechanism of formation of these conduits in control and diseased brain in model of neurodegenerative disease is required as it might have implications for developing novel therapeutic strategies to halt the progression of these incurable, devastating diseases.
MATERIALS AND METHODS
Cell Lines
Mouse neuron-like CAD cells were a gift from Hubert Laude (Institut National de la Recherche Agronomique, Jouy-en- Josas, France), they were cultured in Opti-MEM (Invitrogen) containing 1% Penicillin-Streptomycin and 10% fetal bovine serum (FBS). HeLa cells were cultured in DMEM (Invitrogen) containing 1% Penicillin-Streptomycin and 10% fetal bovine serum (FBS).
Preparation of Recombinant Tau Fibrils
Recombinant tau protein (tau-441, Protein Data Bank entry 2N4R) was expressed and purified by Dr. Rakez Kayed as previously described.18,19 For fibril assembly, tau solution (6 μM) was incubated with DTT (Invitrogen), heparin (Invitrogen), and sodium azide (0.02%, Invitrogen) for hours up to several days at room temperature and centrifuged at 14,000 × g. Short filaments were prepared by incubating tau with DTT and aracidonic acid (75 μM) in 10 mm HEPES (pH 7.4), 100 mM NaCl as previously described.18 Formation of tau LMW aggregates and fibrils was monitored by electron microscopy.
Tau Fibrils Uptake in Cells
HeLa or CAD were plated in μ-Dish35 mm, high Ibidi (Biovalley, France) 35 mm dishes at a density that would allow sub-confluency to be reached after 10 h. Cells were then treated with 0,1 μM of recombinant human tau fibrils labeled with Alexa-555 or Alexa-488 for 16 h. After the incubation time, cells were washed with PBS, fixed with PFA 4%, stained with WGA-Rhodamine or Alexa-488 (Life technologies), HCS CellMask Blue (ThermoFischer Scientific) (1/1000) and mounted with Aqua-Poly mount (Polysciences, Inc.). Images were acquired on an epifluorecence microscope AxioVision (Zeiss) with a 40x objective.
TNTs Imaging and Quantification
To image TNTs, HeLa and CAD cells were treated with 0,1 μM of Alexa-labeled tau fibrils for 16 h as previously described. Then cells were gently washed several times with PBS. To preserve TNTs, cells were first fixed with fixative solution 1 for 20 min (2% PFA, 0.05% glutaraldehyde and 0.2 M HEPES in PBS) then with fixative solution 2 for another 20 min (4% PFA and 0.2 M HEPES in PBS). Cells were then washed with PBS and incubated for 20 minutes with WGA-Alexa488 or Rhodamine (Life technologies) and mounted. Cells were imaged using AxioVision epifluorescence microscope and serial Z-stacks of 0.25 nm were acquired to image the whole cell volume. After imaging using a fluorescence microscope as described above, the TNT structures connecting remote cells and not touching the substratum were manually counted for both tau fibrils-loaded cells and the unloaded control cells. To better visualize tau fibrils inside TNTs, deconvolution was performed using the Huygens Professional software.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from the Agence Nationale de la Recherche (JPND Neutargets: ANR-14-JPCD-0002-01), the CARNOT-ICSA-PMI and the Equipe FRM (Fondation Recherche Médicale) 2014 (DEQ20140329557) to C.Z. K.D and J.W.W were supported by National Institute of Health NS081555. G.S.V. was supported by Bourse Pasteur-Roux, Institut Pasteur, Paris.
REFERENCES
- [1].Costanzo M, Zurzolo C. The cell biology of prion-like spread of protein aggregates: mechanisms and implication in neurodegeneration. Biochem J 2013; 452(1):1-17; PMID:23614720; http://dx.doi.org/ 10.1042/BJ20121898 [DOI] [PubMed] [Google Scholar]
- [2].Aguzzi A, Falsig J. Prion propagation, toxicity and degradation. Nat Neurosci 2012; 15(7):936-9; PMID:22735515; http://dx.doi.org/ 10.1038/nn.3120 [DOI] [PubMed] [Google Scholar]
- [3].Abounit S, Zurzolo C. Wiring through tunneling nanotubes–from electrical signals to organelle transfer. J Cell Sci 2012; 125(Pt 5):1089-98; PMID:22399801; http://dx.doi.org/ 10.1242/jcs.083279 [DOI] [PubMed] [Google Scholar]
- [4].Rustom A. The missing link: does tunnelling nanotube-based supercellularity provide a new understanding of chronic and lifestyle diseases? Open Biol 2016; 6(6):160057; PMID:27278648; http://dx.doi.org/ 10.1098/rsob.160057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman DT, Chenouard N, de Chaumont F, Martino A, Enninga J, et al.. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol 2009; 11(3):328-36; PMID:19198598; http://dx.doi.org/ 10.1038/ncb1841 [DOI] [PubMed] [Google Scholar]
- [6].Costanzo M, Abounit S, Marzo L, Danckaert A, Chamoun Z, Roux P, Zurzolo C. Transfer of polyglutamine aggregates in neuronal cells occurs in tunneling nanotubes. J Cell Sci 2013; 126(Pt 16):3678-85; PMID:23781027; http://dx.doi.org/ 10.1242/jcs.126086 [DOI] [PubMed] [Google Scholar]
- [7].Abounit S, Bousset L, Loria F, Zhu S, de Chaumont F, Pieri L, Olivo-Marin JC, Melki R, Zurzolo C. Tunneling nanotubes spread fibrillar α-synuclein by intercellular trafficking of lysosomes. EMBO J 2016; pii: e201593411; [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M, Laude H, Raposo G. Cells release prions in association with exosomes. Proc Natl Acad Sci U S A 2004; 101(26):9683-8; PMID:15210972; http://dx.doi.org/ 10.1073/pnas.0308413101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S, Jackson B, McKee AC, Alvarez VE, Lee NC, et al.. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem 2012; 287(6):3842-9; PMID:22057275; http://dx.doi.org/ 10.1074/jbc.M111.277061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Münch C, O'Brien J, Bertolotti A. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc Natl Acad Sci U S A 2011; 108(9):3548-53; PMID:21321227; http://dx.doi.org/ 10.1073/pnas.1017275108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, Kretzschmar H, Hengerer B, Kostka M. Different species of α-synuclein oligomers induce calcium influx and seeding. J Neurosci 2007; 27(34):9220-32; PMID:17715357; http://dx.doi.org/ 10.1523/JNEUROSCI.2617-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Danzer KM, Ruf WP, Putcha P, Joyner D, Hashimoto T, Glabe C, Hyman BT, McLean PJ. Heat-shock protein 70 modulates toxic extracellular α-synuclein oligomers and rescues trans-synaptic toxicity. FASEB J 2011; 25(1):326-36; PMID:20876215; http://dx.doi.org/ 10.1096/fj.10-164624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lee HJ, Suk JE, Bae EJ, Lee JH, Paik SR, Lee SJ. Assembly-dependent endocytosis and clearance of extracellular α-synuclein. Int J Biochem Cell Biol 2008; 40(9):1835-49; PMID:18291704; http://dx.doi.org/ 10.1016/j.biocel.2008.01.017 [DOI] [PubMed] [Google Scholar]
- [14].Lee HJ, Suk JE, Bae EJ, Lee SJ. Clearance and deposition of extracellular α-synuclein aggregates in microglia. Biochem Biophys Res Commun 2008; 372(3):423-8; PMID:18492487; http://dx.doi.org/ 10.1016/j.bbrc.2008.05.045 [DOI] [PubMed] [Google Scholar]
- [15].Ren PH, Lauckner JE, Kachirskaia I, Heuser JE, Melki R, Kopito RR. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat Cell Biol 2009; 11(2):219-25; PMID:19151706; http://dx.doi.org/ 10.1038/ncb1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem 2009; 284(19):12845-52; PMID:19282288; http://dx.doi.org/ 10.1074/jbc.M808759200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI. Trans-cellular propagation of Tau aggregation by fibrillar species. J Biol Chem 2012; 287(23):19440-51; PMID:22461630; http://dx.doi.org/ 10.1074/jbc.M112.346072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wu JW, Herman M, Liu L, Simoes S, Acker CM, Figueroa H, Steinberg JI, Margittai M, Kayed R, Zurzolo C, et al.. Small misfolded Tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J Biol Chem 2013; 288(3):1856-70; PMID:23188818; http://dx.doi.org/ 10.1074/jbc.M112.394528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wu JW, Hussaini SA, Bastille IM, Rodriguez GA, Mrejeru A, Rilett K, Sanders DW, Cook C, Fu H, Boonen RA, et al.. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci 2016; 19(8):1085-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Seng Zhu, Victoria GS, Marzo L, Ghosh R, Zurzolo C. Prion aggregates transfer through tunneling nanotubes in endocytic vesicles. Prion 2015; 9(2):125-135; PMID:25996400; http://dx.doi.org/ 10.1080/19336896.2015.1025189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Abounit S, Delage E, Zurzolo C. Identification and characterization of tunneling nanotubes for intercellular trafficking. Curr Protoc Cell Biol 2015; 67:12.10.1-21; PMID:26061240 [DOI] [PubMed] [Google Scholar]
- [22].Wang Y, Cui J, Sun X, Zhang Y. Tunneling-nanotube development in astrocytes depends on p53 activation. Cell Death Differ 2011; 18(4):732-42; PMID:21113142; http://dx.doi.org/ 10.1038/cdd.2010.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen X, Guo C, Kong J. Oxidative stress in neurodegenerative diseases. Neural Regeneration Res 2012; 7(5):376-85; PMID:25774178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ron D, Hampton RY. Membrane biogenesis and the unfolded protein response. J Cell Biol 2004; 167(1):23-25; PMID:15479733; http://dx.doi.org/ 10.1083/jcb.200408117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Butler D, Bahr BA. Oxidative stress and lysosomes: CNS-related consequences and implications for lysosomal enhancement strategies and induction of autophagy. Antioxidants Redox Signal 2006; 8(1-2):185-96. [DOI] [PubMed] [Google Scholar]
- [26].Chinnery HR, Pearlman E, McMenamin PG. Cutting edge: Membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J Immunol 2008; 180:5779-83; PMID:18424694; http://dx.doi.org/ 10.4049/jimmunol.180.9.5779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pyrgaki C, Trainor P, Hadjantonakis AK, Niswander L. Dynamic imaging of mammalian neural tube closure. Dev Biol 2010; 344(2):941-7; PMID:20558153; http://dx.doi.org/ 10.1016/j.ydbio.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lou E, Fujisawa S, Morozov A, Barlas A, Romin Y, Dogan Y, Gholami S, Moreira AL, Manova-Todorova K, Moore MA. Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. Yang PC, ed. PLoS One 2012; 7(3):e33093; PMID:22427958; http://dx.doi.org/ 10.1371/journal.pone.0033093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Seyed-Razavi Y, Hickey MJ, Kuffová L, McMenamin PG, Chinnery HR. Membrane nanotubes in myeloid cells in the adult mouse cornea represent a novel mode of immune cell interaction. Immunol Cell Biol 2013; 91(1):89-95; PMID:23146944; http://dx.doi.org/ 10.1038/icb.2012.52 [DOI] [PubMed] [Google Scholar]
- [30].Thayanithy V, Dickson EL, Steer C, Subramanian S, Lou E. Tumor-stromal cross talk: direct cell-to-cell transfer of oncogenic microRNAs via tunneling nanotubes. Transl Res 2014; 164(5):359-65; PMID:24929208; http://dx.doi.org/ 10.1016/j.trsl.2014.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Connor Y, Tekleab S, Nandakumar S, Walls C, Tekleab Y, Husain A, Gadish O, Sabbisetti V, Kaushik S, Sehrawat S, et al.. Physical nanoscale conduit-mediated communication between tumour cells and the endothelium modulates endothelial phenotype. Nat Commun 2015; 6:8671; PMID:26669454; http://dx.doi.org/ 10.1038/ncomms9671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ady JW, Desir S, Thayanithy V, Vogel RI, Moreira AL, Downey RJ, Fong Y, Manova-Todorova K, Moore MA, Lou E. Intercellular communication in malignant pleural mesothelioma: properties of tunneling nanotubes. Front Physiol 2014; 5:400; PMID:25400582; http://dx.doi.org/ 10.3389/fphys.2014.00400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Polak R, de Rooij B, Pieters R, den Boer ML. B-cell precursor acute lymphoblastic leukemia cells use tunneling nanotubes to orchestrate their microenvironment. Blood 2015; 126(21):2404-14; PMID:26297738; http://dx.doi.org/ 10.1182/blood-2015-03-634238 [DOI] [PubMed] [Google Scholar]
- [34].Osswald M, Jung E, Sahm F, Solecki G, Venkataramani V, Blaes J, Weil S, Horstmann H, Wiestler B, Syed M, et al.. Brain tumour cells interconnect to a functional and resistant network. Nature 2015; 528(7580):93-8; PMID:26536111 [DOI] [PubMed] [Google Scholar]