ABSTRACT
As an inhibitor of apoptosis (IAP) family member, Survivin is known for its role during regulation of apoptosis. More recently its function as a cell cycle regulator has become evident. Survivin was shown to play a pivotal role during embryonic development and is highly expressed in regenerative tissue as well as in many cancer types. We examined the function of Survivin during mouse intestinal organogenesis and in gut pathophysiology. We found high expression of Survivin in experimentally induced colon cancer in mice but also in colon tumors of humans. Moreover, Survivin was regulated by TGF-β and was found to be highly expressed during mucosal healing following intestinal inflammation. We identified that expression of Survivin is essential early on in life, as specific deletion of Survivin in Villin expressing cells led to embryonic death around day 12 post coitum. Together with our recent study on the role of Survivin in the gut of adult mice our data demonstrate that Survivin is an essential guardian of embryonic gut development and adult gut homeostasis protecting the epithelium from cell death promoting the proliferation of intestinal stem and progenitor cells.
KEYWORDS: BIRC5, colorectal cancer, inflammation, intestinal epithelium, mitotic catastrophe
Introduction
Over the past decades Survivin was identified as a molecule highly upregulated in cancer types of various organs including colon,1,2 lung,3 liver4 and breast.5,6 Despite the common assumption that Survivin is rarely expressed in differentiated tissue our and previously published data could show a strong expression of Survivin in the adult intestinal epithelium.7,8,9 Survivin is a member of the inhibitor of apoptosis (IAP) family and is known for its bifunctional role in mitosis and apoptosis respectively.10 Survivin is expressed during the G2/M phase and is part of the chromosomal passenger complex which helps to segregate chromosomes during mitosis.11 We have recently shown that Survivin is responsible for proper cell division of stem and progenitor cells in the crypt compartment of the small and large intestine. Induced genetic deletion of Survivin in intestinal epithelial cells (IECs) led to cell death after failure in proper mitosis which is known as mitotic catastrophe.7,12 Regulation of Survivin is mainly dependent on transcription factors which are involved in cell proliferation and cell survival such as STAT3, P53 and KLF5 and a dysregulated cell cycle control is a hallmark in established tumors and colorectal carcinomas.13 Survivin is overexpressed in many cancers including cancer of the gastrointestinal tract such as esophageal, gastric and colorectal cancer.14 Besides this, the expression of Survivin in inflammatory diseases is not extensively studied, except for rheumatoid arthritis.15,16
In this extra view we extend our data of Survivin in the intestinal epithelium by shedding light on the expression of Survivin during pathophysiologic conditions in the intestine. Our data indicate that Survivin is expressed in colon tumor tissue and during epithelial restitution. Conversely, Survivin expression is strongly suppressed by TGF-β, a cytokine with known proliferation inhibitory functions. We further identify that Survivin is not only required to maintain tissue homeostasis in adult animals but also essential for embryonic development of the gut. Collectively our data highlight an essential role of Survivin for gut development and homeostasis.
Results and discussion
Survivin is increased in intestinal tumor tissue and during resolution of inflammation
We have previously shown that Survivin is important for intestinal epithelial homeostasis by controlling cell division in transit amplifying cells and stem cells. Our data indicated that Survivin is constitutively expressed in the lower intestinal crypt constituting the stem cell niche and early progenitor cells of mice and men under steady state conditions. We here extend our analysis by studying whether Survivin is differentially expressed under pathophysiologic conditions. In contrast to the confined expression pattern of Survivin in the intestinal crypt area in the steady-state colon (Fig. 1A and B, left), analysis of tumor tissue unveiled an extensive amount of Survivin-positive cells throughout the tumor mass in AOM/DSS induced colon tumors in mice and colon tumors of humans (Fig. 1A and B, right). In addition in vitro small intestinal organoids from mice expressed Survivin in crypt buds, suggesting that Survivin is constitutively expressed by epithelial intrinsic factors (Fig. 1C). Since we saw a strong upregulation of Survivin in colon tumors of mice and men we next asked whether tumor environmental factors such as cytokines can affect the expression of Survivin. Of note, when intestinal organoids were stimulated with cytokines known for their expression in the tumor microenvironment we identified TGF-β as a strong negative regulator for Survivin. In contrast TNF-α, IL-6 or IL-10 had no effect on Survivin expression (Fig. 1D).
Figure 1.
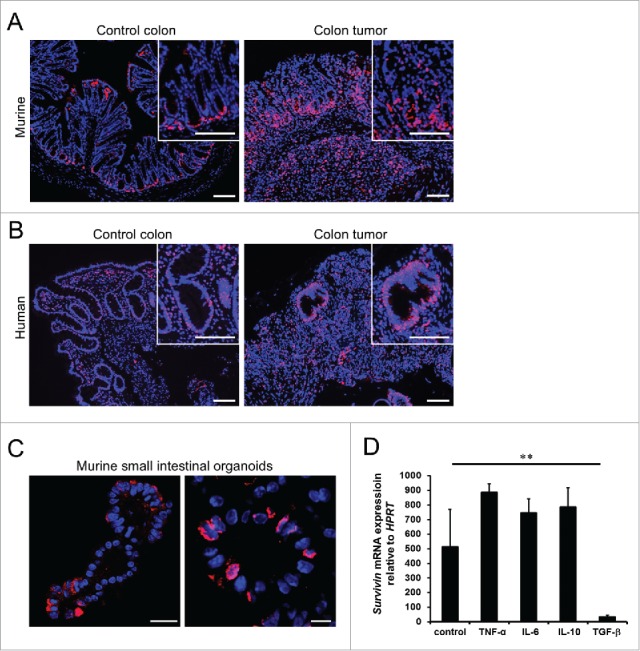
Intestinal Survivin expression in the steady-state colon and colon tumors. (A) Representative picture of the expression of Survivin (red) in murine colon tissue (left) and in murine AOM/DSS induced colon tumor tissue (right). Nuclei were counterstained with Hoechst 33342 (blue). Scale bars 100 µm. (B) Representative picture of the expression of Survivin (red) in human colon tissue (left) and in human colon tumor tissue (right). Nuclei were counterstained with Hoechst 33342 (blue). Scale bars 100 µm. (C) Representative confocal picture showing expression of Survivin (red) in a small intestinal organoid. Nuclei were counterstained with Hoechst 33342 (blue). Scale bars 100 µm, high power field 25 µm. (D) mRNA expression of Survivin relative to HPRT in murine small intestinal organoids stimulated with either TNF-α, IL-6, IL-10 or TGF-β for 16 hours. Data represent mean values + SD (n = 3 per group).
The finding that TGF-β regulates epithelial Survivin expression is surprising given the fact that TGF-β and Survivin are highly expressed in colorectal tumors. TGF-β has a dual role during the development of intestinal tumors. In an early phase of primary tumor development TGF-β inhibits proliferation, whereas in a later phase tumor cells often develop resistance toward TGF-β by altering the TGF-β pathway.17 One might therefore reason that inhibition of proliferation by TGF-β in the early phase is partially due to downregulation of Survivin. At later stages tumor cells become TGF-β resistant allowing high expression of Survivin in advanced tumors. In agreement with this conclusion, in a previous publication our group could show that constitutive TGF-β overexpression suppressed tumor development of mice. Moreover, established experimentally induced tumors (AOM/DSS) in mice showed downregulation of TGF-β receptor I.18,19
In order to investigate a potential role of Survivin during intestinal inflammation we analyzed human and mouse tissue under inflammatory conditions. During acute flares of Crohn´s disease (CD) and Ulcerative Colitis (UC) patients showed no significant changes in Survivin expression compared to healthy controls (Fig. 2A). Of note, infiltrated immune cells in the inflamed tissue showed also positivity for Survivin (Fig. 2A, CD and UC). Interestingly, mice with Dextran Sodium Sulfate (DSS)-induced gut inflammation showed lower expression of Survivin during the active phase of disease, most likely due to loss of intestinal epithelial cells and the excessive infiltration of immune cells associated with this animal model. In contrast, during the phase of mucosal healing and epithelial restitution, the number of Survivin expressing cells exceeded steady state levels presumably due to the high numbers of proliferating IECs during resolution of inflammation (Fig. 2B).
Figure 2.
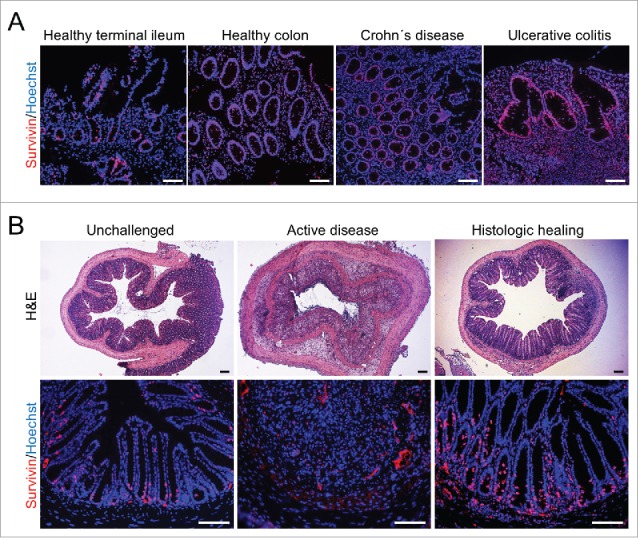
Expression of Survivin during intestinal inflammation in humans and mice. (A) Representative immunohistochemical stainings for Survivin (red) in healthy tissue samples from terminal ileum and colon or from patients suffering from acute Crohn's disease or Ulcerative Colitis. Nuclei were counterstained with Hoechst 33342 (blue). Scale bars 100 µm. (B) Representative histochemical H&E stainings and immunohistochemical Survivin (red) stainings from unchallenged mice and mice suffering from DSS colitis during the active disease and the histologic healing phase. Nuclei were counterstained with Hoechst 33342 (blue). Scale bars 100 µm.
These data implicate that Survivin is not only important for maintaining intestinal turnover in the steady-state. High levels of Survivin as seen in tumors and during resolution of inflammation resemble increased rates of proliferation required during these pathophysiologic conditions.
Intestinal survivin is essential for both early embryonic development and maintenance of gut homeostasis
Given that Survivin is constitutively expressed in the gut under steady state conditions we investigated, whether Survivin expression is essential for embryonic gut development. Therefore we generated mice specifically lacking Survivin in the intestinal epithelium (Fig. 3A). Accordingly, we crossed homozygous Survivin floxed mice (Survivinfl/fl) to mice harboring the Cre recombinase under control of the Villin promoter (Villin-Cre). In contrast to our expectations based on theoretical Mendelian ratios, no mice with floxed Survivin alleles were born which concomitantly expressed the Cre recombinase in epithelial cells (Fig. 3B); strongly suggesting that Survivin expression within the gut epithelium was essential for embryonic development. In fact, when analyzing embryos at 12.5 d post coitum (dpc) we found only encapsulated embryonic remains next to healthy embryos (Fig. 3B). Genomic allele-specific PCR confirmed that these embryonic remains had floxed Survivin alleles and expressed the Cre recombinase in epithelial cells (Fig. 3C). This indicated that mice deficient for Survivin in IECs or embryos die before 12.5 dpc while genotypes that allow expression of Survivin are being born. To overcome embryonic lethality and to study the functional role of Survivin in adult mice, we generated mice with an inducible deletion of Survivin in intestinal epithelial cells (SurviviniΔIEC) in our previous study. When Survivin was deleted by tamoxifen induction, mice rapidly died due to destruction of the intestinal integrity and severe mucosal inflammation. Animals with deficiency for Survivin in IECs showed decreased epithelial proliferation and epithelial cell death was ultimately due to failure in chromosomal segregation during cell division—a cell death modality known as mitotic catastrophe.20 This cell death pathway can be described by polyploidy of cell nuclei, accelerated DNA damage events and high levels of cell-intrinsic apoptosis, features which we could detect in SurviviniΔIEC mice.7 Although we could see an overall decrease in proliferation some IECs continued to proceed in cell cycle and developed giant nuclei in SurviviniΔIEC mice. In support of this we here provide evidence for an initial mitotic active state of the intestinal epithelial cells after Survivin depletion: γ-Tubulin is part of the microtubule-organizing centers such as centrosomes or spindle poles.21 Interestingly, in control mice we detected a regular formation of γ-Tubulin in the form of centrosomes in dividing intestinal epithelial cells, whereas SurviviniΔIEC mice showed asymmetric mitosis with abnormal formation of γ-Tubulin (Fig. 4A). Furthermore, intensified phospho-Histon H3, an epigenetic mitosis marker, could be detected during asymmetric mitosis in SurviviniΔIEC mice (Fig. 4B).22 Mitotic activity in the absence of Survivin provides evidence that some IECs can overcome cell death and continue cycling a process which is known as mitotic slippage.20,23
Figure 3.
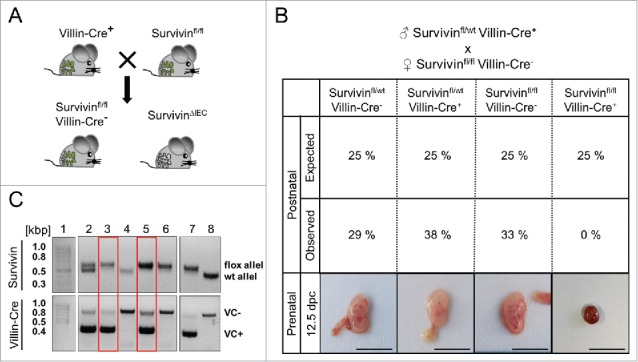
Deletion of Survivin in IECs leads to embryonic lethality. (A) Survivinfl/wt mice expressing the Cre recombinase under the Villin promotor (Villin-Cre+) were crossbred to Survivinfl/fl mice not expressing the Cre recombinase under the Villin promotor (Villin-Cre−) for specific deletion of Survivin in IECs (SurvivinΔIEC). (B) Analysis of observed offspring numbers of altogether 52 mice and expected offspring numbers as well as representative pictures of embryos (Survivinflfl Villin-Cre+) from the crossbreeding of Survivinfl/wt Villin-Cre+ male to Survivinfl/fl Villin-Cre− female mice. Scale bars 1 cm. (C) Genotyping of embryos 12.5 dpc for Survivin and Villin-Cre. Embryonic abort (3 and 5) had 2 Survivin floxed alleles (Survivinfl/fl) and were positive for the expression of the Cre recombinase under the Villin promotor (VC+). Lane 1 = 100 bp marker, lane 2 – 6 = floxed and wildtype alleles for Survivin and Villin-Cre negative (VC-) and Villin-Cre positive (VC+) bands of embryonic tissue samples, lane 7 – 8 = internal controls.
Figure 4.
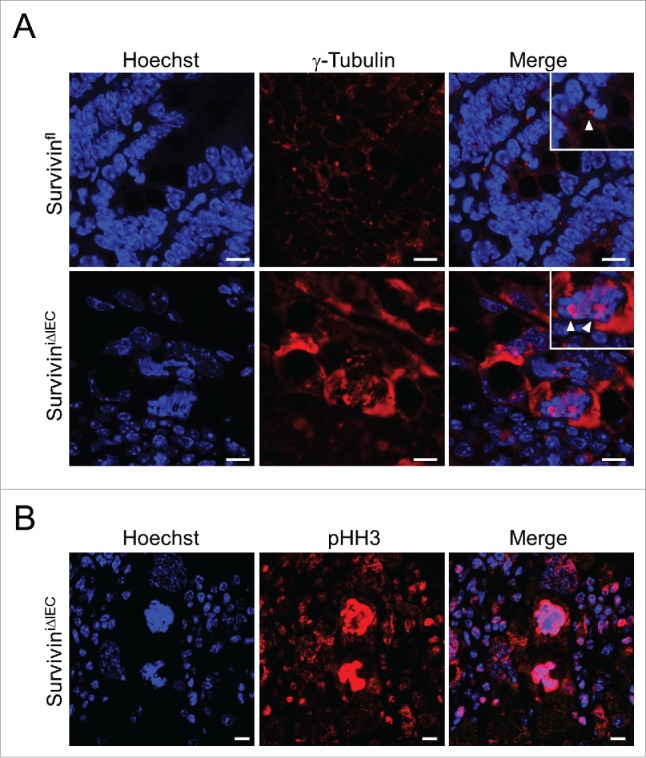
Presence of mitotic activity in Survivin deficient IECs. (A) Representative confocal images of γ-Tubulin (red) in control (Survivinfl) and SurviviniΔIEC mice after 5 d tamoxifen injection. Arrows indicate centrosomes during cell division. Nuclei were counterstained with Hoechst 33342 (blue). Scale bars 10 µm. (B) Representative confocal images of phospho-Histon H3 (pHH3; red) in SurviviniΔIEC mice after 5 d tamoxifen injection. Nuclei were counterstained with Hoechst 33342 (blue). Scale bars 10 µm.
Collectively our data indicate that Survivin is essential for embryonic gut development but also for maintaining the structural integrity of the adult gut. Loss of Survivin in IECs leads to a failure of proper cell division in progenitors and stem cells of the gut epithelium which is characterized by mitotic slippage, DNA damage and ultimately mitotic catastrophe.
Despite the fact that Survivin has been described as an anti-apoptotic protein necessary for embryonic development our data clearly indicate that Survivin is an essential molecule maintaining the structural integrity of the adult gut.
Material and methods
Mice
Mice carrying loxP-flanked Survivin alleles (Survivinfl/fl) were kindly provided by Hitoshi Okada,24 Villin-Cre mice were described earlier.25 Male and female mice were used at an age of 6 to 16 weeks for experiments. Conditional intestinal epithelium specific Survivin knockout mice (SurvivinΔIEC) were generated by breeding Survivinfl/wt mice to Villin-Cre expressing mice. Inducible conditional IEC specific Survivin knockout mice (SurviviniΔIEC) were generated as described before.7 Animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Erlangen. All mice were kept in individually ventilated cages in compliance with the Animal Welfare Act. Mice were housed at a 12 hour day–night cycle. Food and fresh water was provided ad libitum. All mice were kept in mixed cages to avoid cage effects during experiments.
Human patient material
Tissue sections from human biopsies from either patients or healthy individuals were provided by the University Clinic Erlangen. Samples were collected according to the approval by the Ethics Committee of the University Clinic Erlangen, Germany.
Experimental model of inflammation and induced inflammation associated cancer
DSS colitis was induced by treating mice with 2.5 % Dextran Sodium Sulfate (DSS; MP Biomedicals) dissolved in sterile drinking water. Mice were challenged for one week with DSS followed by a 2 week recovery phase with drinking water. DSS was exchanged every third day. To induce inflammation associated carcinomas, mice were intraperitoneally injected with 1mg/100g of the mutagenic agent azoxymethane (AOM; Sigma Aldrich) and subsequently treated with 3 cycles of 2.5 % DSS in sterile drinking water for 1 week followed by sterile drinking water for 3 weeks. Mice were examined by measuring weight loss and monitoring of the intestine by high-resolution mouse video endoscopy as previously described.26 For endoscopic analysis mice were anaesthetized by gassing with 2 % isoflurane (Abbott) mixed with air.
Histology and immunohistochemistry
For histological H&E stainings formalin-fixed, dehydrated and paraffin-embedded tissue was used. Immunofluorescence stainings of cryo- or formalin-fixed, dehydrated and paraffin-embedded tissue sections were performed using anti-Survivin antibody (2808, Cell Signaling Technology), anti-γ-Tubulin antibody (sc-7396, Santa Cruz) or anti-phospho-Histon H3 antibody (ab5176, abcam) over night at 4°C. Slides were then incubated with biotinylated secondary antibody (ebioscience) for 1 hour and signal amplification was performed using Streptavidin conjugated with Alexa 488 (A21311, Invitrogen). Nuclei were counterstained with Hoechst 33342 (62249, Invitrogen). Slides were mounted with mounting medium (S3023, Dako) and analyzed using fluorescence microscopy (Leica DMI 4000B) and / or confocal microscopy (Leica TCS SP5).
Gene expression analysis and genotyping
Total RNA from organoids was extracted using the RNA isolation kit (peqGOLD Total RNA Kit; Peqlab) and cDNA was synthesized by reverse transcription (Script cDNA Synthesis Kit; Jena Bioscience). Quantitative PCR was performed using SYBR Green I Master (Roche) and QuantiTect Primer assays (Qiagen) using the Light Cycler 480 (Roche). Expression signals were normalized to the level of the housekeeping genes hypoxanthine phosphoribosyl-transferase (HPRT). Each independent sample was assayed in duplicates. Genotyping for Survivin was performed by polymerase chain reaction (PCR) of tail DNA using specific primers for Survivin and Villin-Cre.
Crypt isolation, organoid culture and stimulation
Crypts of the small intestine of wildtype mice were isolated as previously described.27 Small intestinal organoids were cultured in Matrigel (356231, Corning) for at least 5 d in R-Spondin conditioned medium (Advanced DMEM/F12, ThermoFisher scientific) supplemented with Pen/Strep and Amphotericin (Sigma Aldrich), HEPES (Sigma Aldrich), GlutaMax (Invitrogen), N2 (Invitrogen), B27 (Invitrogen), recombinant murine EGF (Immunotools), N-Acetyl-Cystein (Sigma Aldrich), recombinant murine Noggin (PeproTech) and recombinalt R-Spondin 1 (PeproTech). Organoids were subsequently stimulated with murine IL-6 (50 ng/ml; PeproTech), murine IL-10 (50 ng/ml; PeproTech), TNF-α (25 ng/ml; Immunotools) and TGF-β (50 ng/ml; R&D Systems) for 16 hours. Organoid growth was monitored via light microscopy (Leica, DMI 4000B). For confocal images organoids were washed, stained as described and embedded in Mowiol (Sigma Aldrich).
Statistical analysis
Statistical analysis was performed using the Student's 2-tailed t-test to compare 2 groups. Significance levels are indicated as follows: *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. Data are presented as mean values + or ± SD. Statistical calculations were performed using standard functions of Microsoft Excel.
Abbreviations
- AOM
Azoxymethane
- bp
base pair
- CD
Crohn´s disease
- DNA
desoxyribonucleic acid
- dpc
days post coitum
- DSS
Dextran Sodium Sulfate
- H&E
haematoxylin and eosin
- HPRT
hypoxanthine-guanine phosphoribosyltransferase
- IL-6
interleukin 6
- IL-10
interleukin 10
- IAP
inhibitor of apoptosis
- IECs
intestinal epithelial cells
- PCR
polymerase chain reaction
- pHH3
phospho-Histon H3
- RNA
ribonucleic acid
- TGF-β
transforming growth factor β
- TNF-α
tumor necrosis factor α
- UC
Ulcerative Colitis
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Eva Podstawa for her excellent technical assistance.
Funding
The research leading to these results has received funding from DFG projects within FOR2438 (TP05), SFB1181 (C05) and the clinical research unit KFO257. Further support was given by the projects SPP1656, SFB796 (B09), BE3686/2, the Interdisciplinary Center for Clinical Research (IZKF) of the University Erlangen-Nürnberg and the European Community's 7th Framework Program (acronym BTCure).
Author contributions
E.M., M.F.N., and C.B. designed the research. E.M. and E.S. performed the experiments. C.N. provided material that made the study possible. E.M. and C.B. analyzed the data and wrote the paper.
References
- [1].Hernandez JM, Farma JM, Coppola D, Hakam A, Fulp WJ, Chen D-T, Siegel EM, Yeatman TJ, Shibata D. Expression of the antiapoptotic protein survivin in colon cancer. Clin Colorectal Cancer 2011; 10:188-93; PMID:21855041; http://dx.doi.org/ 10.1016/j.clcc.2011.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Adamkov M, Výbohová D, Tupá V, Chylíková J, Horáček J, Benčat M. Expression and significance of survivin in colorectal high grade and low grade adenomas. Acta Histochem 2015; 117:590-4; PMID:26095032; http://dx.doi.org/ 10.1016/j.acthis.2015.05.006 [DOI] [PubMed] [Google Scholar]
- [3].Mohamed S, Yasufuku K, Nakajima T, Hiroshima K, Chiyo M, Yoshida S, Suzuki M, Sekine Y, Shibuya K, Agamy G, et al.. Nuclear survivin in pN2 nonsmall cell lung cancer: prognostic and clinical implications. Eur Respir J 2009; 33:127-33; PMID:18715879; http://dx.doi.org/ 10.1183/09031936.00068708 [DOI] [PubMed] [Google Scholar]
- [4].Ye C-P, Qiu C-Z, Huang Z-X, Su Q-C, Zhuang W, Wu R-L, Li X-F. Relationship between survivin expression and recurrence, and prognosis in hepatocellular carcinoma. World J Gastroenterol 2007; 13:6264-8; PMID:18069771; http://dx.doi.org/ 10.3748/wjg.13.6264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brennan DJ, Rexhepaj E, O'Brien SL, McSherry E, O'Connor DP, Fagan A, Culhane AC, Higgins DG, Jirstrom K, Millikan RC, et al.. Altered cytoplasmic-to-nuclear ratio of survivin is a prognostic indicator in breast cancer. Clin Cancer Res 2008; 14:2681-9; PMID:18451232; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yamashita S-I, Masuda Y, Kurizaki T, Haga Y, Murayama T, Ikei S, Kamei M, Takeno S, Kawahara K. Survivin expression predicts early recurrence in early-stage breast cancer. Anticancer Res 27:2803-8; PMID:17695451 [PubMed] [Google Scholar]
- [7].Martini E, Wittkopf N, Günther C, Leppkes M, Okada H, Watson AJ, Podstawa E, Backert I, Amann K, Neurath MF, et al.. Loss of Survivin in Intestinal Epithelial Progenitor Cells Leads to Mitotic Catastrophe and Breakdown of Gut Immune Homeostasis. Cell Rep 2016; 14:1062-73; PMID:26832409; http://dx.doi.org/ 10.1016/j.celrep.2016.01.010 [DOI] [PubMed] [Google Scholar]
- [8].Boman B, Kopelovich L, Siracusa LD, Zhang T, Henderson K, Cofer Z, Buchberg AM, Fields JZ, Otevrel T. A Tcf4-GFP reporter mouse model for monitoring effects of Apc mutations during intestinal tumorigenesis. Mol Carcinog 2009; 48:821-31; PMID:19263440; http://dx.doi.org/ 10.1002/mc.20526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, et al.. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 2002; 111:241-50; PMID:12408868; http://dx.doi.org/ 10.1016/S0092-8674(02)01014-0 [DOI] [PubMed] [Google Scholar]
- [10].Li F, Ackermann EJ, Bennett CF, Rothermela L, Plescia J, Tognin S, Villa a, Marchisio PC, Altieri DC. Pleiotropic cell-division defects and apoptosis induced by interference with survivin function. Nat Cell Biol 1999; 1:461-6; PMID:10587640; http://dx.doi.org/ 10.1038/70242 [DOI] [PubMed] [Google Scholar]
- [11].Vader G, Kauw JJW, Medema RH, Lens SMA. Survivin mediates targeting of the chromosomal passenger complex to the centromere and midbody. EMBO Rep 2006; 7:85-92; PMID:16239925; http://dx.doi.org/ 10.1038/sj.embor.7400562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Castedo M, Perfettini J-L, Roumier T, Andreau K, Medema R, Kroemer G. Cell death by mitotic catastrophe: a molecular definition. Oncogene 2004; 23:2825-37; PMID:15077146; http://dx.doi.org/ 10.1038/sj.onc.1207528 [DOI] [PubMed] [Google Scholar]
- [13].Boidot R, Végran F, Lizard-Nacol S. Transcriptional regulation of the survivin gene. Mol Biol Rep 2014; 41:233-40; PMID:24197699; http://dx.doi.org/ 10.1007/s11033-013-2856-0 [DOI] [PubMed] [Google Scholar]
- [14].Liu Y, Li L, Qi H, Gao Y, Liu S, Xu C. Survivin -31G>C polymorphism and gastrointestinal tract cancer risk: a meta-analysis. PLoS One 2013; 8:e54081; PMID:23405077; http://dx.doi.org/ 10.1371/journal.pone.0054081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jenko B, Praprotnik S, Čučnik S, Rotar Ž, Tomšič M, Dolžan V. Survivin polymorphism is associated with disease activity in rheumatoid arthritis patients. http://dx.doi.org/102217/pgs15147 2015 [DOI] [PubMed] [Google Scholar]
- [16].Turkkila M, Andersson KME, Amu S, Brisslert M, Erlandsson MC, Silfverswärd S, Bokarewa MI. Suppressed diversity of survivin splicing in active rheumatoid arthritis. Arthritis Res Ther 2015; 17:175; PMID:26160473; http://dx.doi.org/ 10.1186/s13075-015-0689-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jakowlew SB. Transforming growth factor-β in cancer and metastasis. Cancer Metastasis Rev 2006; 25:435-57; PMID:16951986; http://dx.doi.org/ 10.1007/s10555-006-9006-2 [DOI] [PubMed] [Google Scholar]
- [18].Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, et al.. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity 2004; 21:491-501; PMID:15485627; http://dx.doi.org/ 10.1016/j.immuni.2004.07.020 [DOI] [PubMed] [Google Scholar]
- [19].Becker C, Fantini MC, Wirtz S, Nikolaev A, Lehr HA, Galle PR, Rose-John S, Neurath MF. IL-6 signaling promotes tumor growth in colorectal cancer. Cell Cycle 2005; 4:217-20; PMID:15655344; http://dx.doi.org/ 10.4161/cc.4.2.1413 [DOI] [PubMed] [Google Scholar]
- [20].Vitale I, Galluzzi L, Castedo M, Kroemer G. Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat Rev Mol Cell Biol 2011; 12:385-92; PMID:21527953; http://dx.doi.org/ 10.1038/nrm3115 [DOI] [PubMed] [Google Scholar]
- [21].O'Toole E, Greenan G, Lange KI, Srayko M, Müller-Reichert T. The Role of γ-Tubulin in Centrosomal Microtubule Organization. PLoS One 2012; 7:e29795; http://dx.doi.org/ 10.1371/journal.pone.0029795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tsuta K, Liu DC, Kalhor N, Wistuba II, Moran CA, Gustafsson B, Kidd M, Chan A, Travis W, Rush W, et al.. Using the mitosis-specific marker anti-phosphohistone H3 to assess mitosis in pulmonary neuroendocrine carcinomas. Am J Clin Pathol 2011; 136:252-9; PMID:21757598; http://dx.doi.org/ 10.1309/AJCPDXFOPXGEF0RP [DOI] [PubMed] [Google Scholar]
- [23].Blagosklonny M V. Mitotic arrest and cell fate: why and how mitotic inhibition of transcription drives mutually exclusive events. Cell Cycle 2007; 6:70-4; PMID:17245109; http://dx.doi.org/ 10.4161/cc.6.1.3682 [DOI] [PubMed] [Google Scholar]
- [24].Okada H, Bakal C, Shahinian A, Elia A, Wakeham A, Suh W-K, Duncan GS, Ciofani M, Rottapel R, Zúñiga-Pflücker JC, et al.. Survivin loss in thymocytes triggers p53-mediated growth arrest and p53-independent cell death. J Exp Med 2004; 199:399-410; PMID:14757745; http://dx.doi.org/ 10.1084/jem.20032092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].el Marjou F, Janssen K-P, Chang BH-J, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, Robine S. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 2004; 39:186-93; PMID:15282745; http://dx.doi.org/ 10.1002/gene.20042 [DOI] [PubMed] [Google Scholar]
- [26].Becker C, Fantini MC, Wirtz S, Nikolaev A, Kiesslich R, Lehr HA, Galle PR, Neurath MF. In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy. Gut 2005; 54:950-4; PMID:15951540; http://dx.doi.org/ 10.1136/gut.2004.061283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al.. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009; 459:262-5; PMID:19329995; http://dx.doi.org/ 10.1038/nature07935 [DOI] [PubMed] [Google Scholar]


