Abstract
Highly aggressive, rapidly growing tumors contain significant areas of hypoxia or anoxia as a consequence of inadequate and/or irregular blood supply. During oxygen deprivation, tumor cells withstand a panoply of adaptive responses, including a shift towards anaerobic metabolism and the reprogramming of the transcriptome. One of the major mediators of the transcriptional hypoxic response is the hypoxia-inducible factor 1 (HIF-1), whose stabilization under hypoxia acts as an oncogenic stimulus contributing to chemotherapy resistance, invasion and metastasis. Gene expression analysis by qRT-PCR is a powerful tool for cancer cells phenotypic characterization. Nevertheless, as cells undergo a severe transcriptome remodeling.in response to oxygen deficit, the precise identification of reference genes poses a significant challenge for hypoxic studies. Herein, we aim to establish the best reference genes for studying the effects of hypoxia on bladder cancer cells. Accordingly, three bladder cancer cell lines (T24, 5637, and HT1376) representative of two distinct carcinogenesis pathways to invasive cancer (FGFR3/CCND1 and E2F3/RB1) were used. Additionally, we have explored the most suitable control gene when addressing the influence of Deferoxamine Mesilate salt (DFX), an iron chelator often used to avoid the proteasomal degradation of HIF-1α, acting as an hypoxia-mimetic agent. Using bioinformatics tools (GeNorm and NormFinder), we have elected B2M and HPRT as the most stable genes for all cell lines and experimental conditions out of a panel of seven putative candidates (HPRT, ACTB, 18S, GAPDH, TBP, B2M, and SDHA). These observations set the molecular basis for future studies addressing the effect of hypoxia and particularly HIF-1α in bladder cancer cells.
Introduction
Hypoxia (decreased oxygen partial pressure from <0.1 mmHg (anoxia) to 15 mmHg) is a key microenviromental factor underlying malignant transformation, tumor heterogeneity, disease progression, immune escape, and therapy resistance [1–4]. In solid tumours, the uncontrolled proliferation of cancer cells combined with the structural abnormalities of the tumor vascular network results in the delivery of suboptimal concentrations of oxygen and other nutrients to cancer cells, creating hypoxic microregions [5, 6]. As a survival strategy, hypoxic cancer cells activate major adaptive pathways, and undergo a significant reprogramming of the cell transcriptional activity towards more aggressive phenotypes [7]. The key regulators of the transcriptional hypoxic response are the hypoxia-inducible factors (HIFs), which consist in one alpha subunit, whose expression is oxygen-dependent, and one constitutive beta subunit [6, 8, 9]. HIF-1α is constantly synthesized in the cytosol and rapidly degraded under normoxia; however, it becomes stabilized under low oxygen pressure. After nuclear translocation, HIF-1α directs the expression of multiple genes, promoting the overexpression of angiogenic and anti-apoptotic genes, as well as growth factors, glycolysis, and protein synthesis suppression [2, 10–13]. Moreover, hypoxic stress enhances motility and the invasive capacity of tumor cells via the acquisition of mesenchymal traits [14, 15]. Furthermore, it has been described to favor the establishment of cancer stem cell phenotypes, responsible by recapitulating tumor heterogeneity after treatment [16]. As such, the elimination of hypoxic cells, often responsible by disease relapse and progression, remains a challenging topic in cancer therapeutics.
The transcriptomic analysis of hypoxic cells is of primary importance to understand the roles played by genes in the overall cellular responses and, for ultimately setting the molecular basis for targeted therapeutics. The quantitative reverse-transcription real-time polymerase chain reaction (qRT-PCR) is the gold standard technique to access gene expression through the quantification of mRNA levels [17]. Furthermore, it is frequently used to validate the results from microarray experiments [18]. Even though considered highly sensitive, reproducible and with wide dynamic range, qRT-PCR experiments require the normalization of the results to a reference gene [19, 20]. This internal control is subjected to the same preparation steps as the genes of interest, allowing to refine non-biological variations resulting from sample manipulation [21]. Ideally, it should be uniformly expressed in all experimental conditions of a given systems, and its expression should be similar to the target genes [18]. In particular, exposure to hypoxia poses a hurdle for transcriptomic studies, since it has been shown to modulate the transcription of classical and widely explored reference genes, namely glyceraldehyde-3-phosphate dehydrogenase (GAPDH), β-actin (ACTB), and β-tubulin (TUBB) in several cancer cell lines [22–26]. Still, significant variations have been observed throughout the literature depending on the used model, and many hypoxia-related transcriptomic studies overlooked the careful identification of the most suited reference genes for their particular experimental context.
Recent evidences suggest that hypoxia plays a key role in bladder cancer chemoresistance, invasion and dissemination, which warrants future comprehensive studies towards novel biomarkers and innovative therapeutics [27–30]. Based on these observations, we have devoted to the identification of the most stable reference genes out of a panel of seven candidates, namely Hypoxanthine phosphoribosyltransferase-1 (HPRT), ACTB, 18 ribosomal RNA (18S), GAPDH, TATA-binding protein (TBP), Beta-2 microglobulin (B2M), and Succinate dehydrogenase complex flavoprotein subunit A (SDHA), to address the effect of hypoxia in the context of bladder cancer. We have selected three bladder cancer cell lines (T24, 5637, and HT1376), representative of two distinct molecular pathways to invasive cancer (FGFR3/CCND1and E2F3/RB1), and widely explored by us in the establishment of novel therapeutic schemes. Additionally, we have also explored the most suitable reference gene in the presence of the hypoxia-mimetic Deferoxamine Mesilate salt (DFX), which has been widely applied to disclose HIF-1α-mediated events by avoiding the proteasomal degradation of the transcription factor under normoxia. Altogether we envisage to create the necessary rationale for high throughput transcriptomic and (glyco)proteomic studies towards the identification of novel bladder cancer biomarkers associated with hypoxia.
Material and Methods
Cell lines and culture conditions
The T24 (grade III), 5637 (grade II) and HT1376 (grade III) bladder cancer cell lines used in this work were acquired from DSMZ (Düsseldorf, Germany) and recently characterized from the genetic standpoint by our group [31]. Accordingly, the T24 cell line is representative of the FGFR3/CCND1 pathway of invasion, presenting a mutated HRAS and overexpression of CCND1. The 5637 and HT1376 cell lines correspond to the E2F3/RB1 invasion pathway with loss of one copy of RB1 and mutation of the remaining copy. Additionally, HT1376 cells exhibit deletion of the Phosphatase and tensin homolog (PTEN) gene and no alteration of Phosphatidylinositol 3-kinase catalytic subunit alpha (PIK3CA), which in combination with the inactivation of p53, translates into a more invasive and metastatic potential. In contrast, the 5637 cell line presents PIK3CA gene deletion and no PTEN alterations, which translates into a less-invasive phenotype.
The cells were cultured in RPMI 1640+GlutaMAXTM-I medium (Gibco, Life Technologies) supplemented with 10% heat-inactivated FBS (Gibco, Life Technologies) and 1% penicillin-streptomycin (10,000 Units/mL P; 10,000 μg/mL S; Gibco, Life Technologies). Cell lines were cultured as a monolayer at 37°C in a 5% CO2 humidified atmosphere (normoxia), and were routinely subcultured after trypsinization. The cells were also grown under hypoxic atmosphere for 24h (T24 and 5637) or 6h (HT1376) at 37°C with 5% CO2, 99.9% N2 and 0.1% O2 in a BINDER C-150 incubator (BINDER GmbH). Additionally, cells were grown under normoxia in the presence of 500 μM Deferoxamine Mesilate CRS (DFX, Sigma-Aldrich), a hypoxia-mimetic agent that promotes HIF-1α stabilization [32]. Cell viability was determined using the Trypan Blue Exclusion Test of Cell Viability. Of note, cells maintained nearly 100% viability for all experimental conditions.
Expression of hypoxia makers
Growth under hypoxia was confirmed by evaluation of hypoxia marker HIF-1α by western blot using the rabbit anti-human HIF-1α clone [16H4L13] (1:250 in PBS; Invitrogen) as primary antibody. The Abcam’s fluorometric L-Lactate assay kit (Abcam) was used to determine the concentration of L-Lactate in culture media as described by the supplier. A significant increase in lactate concentration was considered as a surrogate marker of anaerobic metabolism.
RNA isolation and cDNA conversion
Total RNA was isolated from T24, 5637 and HT1376 cells, grown under normoxia, hypoxia and DFX exposure, using TriPure isolation Reagent (Roche Diagnostics GmbH, Mannheim, Germany), according to the manufacturer’s instructions. The quality and quantity of the extracted RNA was estimated using a Nanodrop (ND1000, Nano Drop Technologies Inc. Wilmington, DE, USA). Before cDNA synthesis, the integrity of RNA samples was confirmed by electrophoresis on 1% agarose gels. RNA (2μg) was reverse transcribed with random primers, using the “High Capacity cDNA Reverse Transcription Kit” (Applied Biosystems, Foster City, CA).
mRNA expression analysis
Candidate reference genes were selected according to the following criteria: (i) high frequency of use (ACTB and GAPDH); (ii) used for normalization in other hypoxia studies, cancer types and models (HPTR, TBP, B2M, SDHA, 18S). CA9 was also evaluated as a control since it is a transcript that is regulated by hypoxia/DFX. Real-time PCR amplification of cDNA samples was performed in a ABIPrism 7500™ Real-Time PCR System (Applied Biosystems) using a TaqMan® Gene Expression Master Mix, primers and probes provided by Applied Biosystems. The S1 Table shows the manufacturer references for each evaluated genes. The thermal cycling conditions comprised an initial denaturation step at 95°C for 30 s, 40 cycles at 95°C for 5 s, a 34 s period at 65°C, and a final dissociation stage at 95°C for 15 s, 60°C for 1 min, and 95°C for 15 s [33]. All experiments were performed in duplicates. For each experiment, two conversion replicates were done and for every conversion replicate three amplification replicates were performed, resulting a 12 replicates total for each experimental condition.
Analysis of gene expression stability by RT-qPCR
The stability of the reference genes expression was assessed using two different statistical programs: geNorm [34] and NormFinder [35]. The geNorm software analyses the stability of reference genes transcripts taking into account the expression stability value (MgeNorm) [36]. The this stability value is calculated for each gene of a panel of candidate reference genes based on pairwise variation analysis. Moreover, and according to Vandesompele et al., lower values of MgeNorm correspond to higher gene expression stability [34]. Furthermore, geNorm is also capable to determine the ideal number of reference genes needed for accurate normalization [34]. Similarly, NormFinder, is a mathematical algorithm used to identify the best normalization gene amongst a set of reference candidates according to their expression stability (M NormFinder), analyzing both intra- and inter-group variation [22]. Like GeNorm, lower average M NormFinder values indicate more stable genes expression [35].
Results
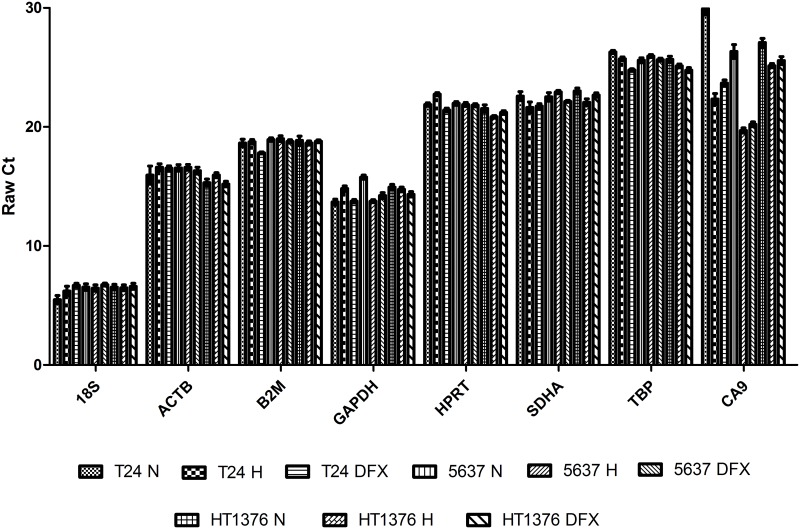
The present work was devoted to the identification of references genes for accessing gene transcription alterations associated with hypoxia in bladder cancer. Three of the most studied bladder cancer cell lines (T24, 5637, and HT1376), reflecting different molecular pathways for cell invasion (FGFR3/CCND1and E2F3/RB1), were chosen envisaging the identification of a ubiquitous reference gene. Accordingly, seven of the most commonly used reference genes (ACTB, GAPDH, HPRT, B2M, SDHA, TBP, and 18S) were amplified in the three bladder cancer cell lines exposed to normoxia and hypoxia. In addition, experiments were conducted in the presence of DFX to disclose HIF-1α mediated events. All cell lines showed increased levels of HIF-1α and lactate under hypoxia and DFX in relation to normoxia, denoting a shift towards anaerobic metabolism, thereby confirming a cellular response to hypoxic stress (S1 Fig). The variation between the maximum and minimum Ct (cycle threshold) values for each tested reference gene was less than 3 cycles for all experimental conditions and cell lines (Fig 1). Particularly, 18S was the most expressed reference gene regardless the cell line and experimental condition, with a mean Ct value of approximately 6.5. GAPDH was the second most transcribed gene with a mean Ct of 14.5, and TBP was the least expressed gene with Ct values of 25.5. Moreover, our control gene CA9 presented a mean expression level of 24.5. As it can be seen the CA9 Ct values in hypoxia and DFX conditions are lower than the normoxia in all cell lines, showing that this HIF-regulated gene is robustly upregulated in these conditions.
Fig 1. Expression of the evaluated genes.
Raw mean Ct values of the reference genes (HPRT, ACTB, 18S, GAPDH, TBP, B2M, SDHA) and CA9 for T24, 5637 and HT1376 cell lines under different experimental conditions (Normoxia (N), Hypoxia (H), and DFX). Results are presented as mean + SD. Twelve replicates were used.
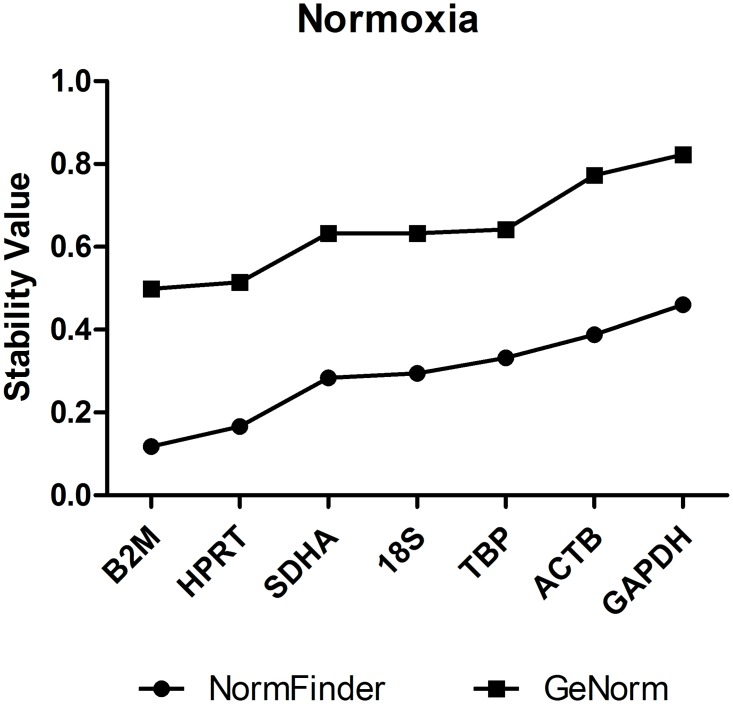
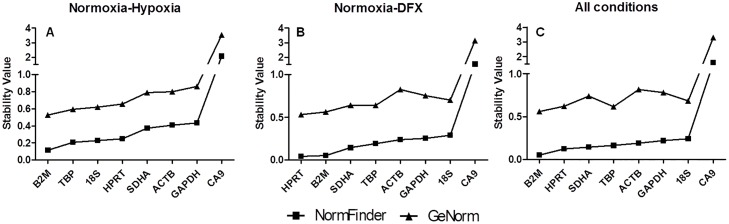
To reliably identify the most stably expressed genes across all cell lines and experimental conditions, both geNorm and NormFinder statistical programs were used. Of note, the two software originated highly comparable results (S2 Fig). geNorm and NormFinder retrieved B2M and HPRT as the most stable reference genes under normoxic conditions for all cell lines, followed by SDHA, 18S and TBP. ACTB and GAPDH were found to be the most unstable genes among the studied candidates (Fig 2). Moreover, when comparing normoxic and hypoxic conditions, the most stable candidate gene for each cell line was B2M, followed by TBP, 18S and HPRT, which presented similar stability values. This results suggest that all of the above reference genes could be suitable controls in combination with B2M (Fig 3). Again, GAPDH and ACTB were shown to be the most unstable genes under hypoxic conditions. Reinforcing the consistent stability of a good reference gene, when comparing normoxia with DFX exposure conditions, B2M and HPRT were again shown to be the most stable genes, while ACTB, GAPDH, and 18S were the less stable (Fig 3B).
Fig 2. Ranked expression stability of the reference genes (geNorm and NormFinder analysis) under normoxic conditions for all cell lines.
Under normoxia, B2M and HPRT presented the lower stability values, meaning they are the most stable reference genes in this condition. On the other hand, ACTB and GAPDH presented the higher stability values, being the least stable genes. Of note, both analytical tools provided similar outcomes.
Fig 3. geNorm and NormFinder analysis of the stability values of reference genes and CA9.
Samples were exposed to (A) normoxia and hypoxia, (B) normoxia and DFX, and (C) all experimental conditions (normoxia, hypoxia and DFX). B2M and HPRT are constitutively top ranked while ACTB and GAPDH are low ranked regardless of the experimental condition.
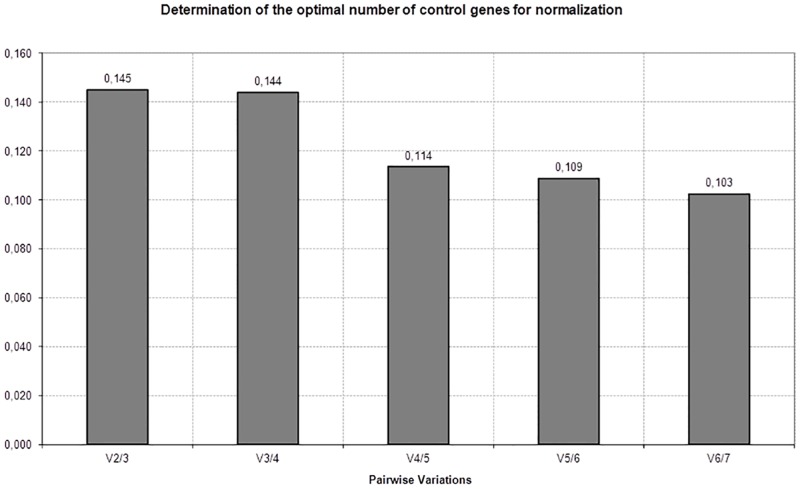
Finally, all experimental conditions were compared in order to identify a common suitable reference gene for further comprehensive studies (Fig 3C). This approach highlighted B2M and HPRT as the most stable genes for all cell lines among all control genes tested. Nevertheless, geNorm analysis showed a stability value for TBP similar to HPRT, while NormFinder analyses ranked TBP only as the fourth most stable. Again, GAPDH, 18S and ACTB were ranked the least stable genes of the evaluated panel. We have also analyzed the stability value of CA9, a gene which expression is known to be affected under hypoxia. Has expected the stability values for CA9 were much more higher, reinforcing the idea that this cell are really in hypoxia (Fig 3). Since the proper normalization of expression data determines the accuracy of RT-qPCR quantification, we have determined the optimal number of reference genes for data normalization through a pairwise variation (Vn/n+1) analysis using GeNorm, (Fig 4). As suggested by Vandesompele, et al, we have adopted a cut-off of Vn/n+1<0.15 as an appropriate selection criterion for estimating the optimal number of reference genes [34]. Of note, this cut-off value has been widely used as a criterion for selection of reference genes [37–41]. As shown in Fig 5, the value V2/3 is 0.145, indicating that the inclusion of a third reference gene would not improve the accuracy obtained with only two reference genes.
Fig 4. Determination of the optimal number of control genes for accurate normalization using geNorm software.
Each bar Vn/n+1 represents the variation between the means of the n most stable genes versus the group of n+1 most stable genes. For example, column 1 represents the variation between the mean of the two most stable genes, B2M and HPRT, versus the three most stable genes B2M, HPRT and SDHA. Because the 0.15 threshold is not exceeded at any point, the use of two reference genes would be sufficient under these conditions.
Fig 5. Examples of gene expression calculations.
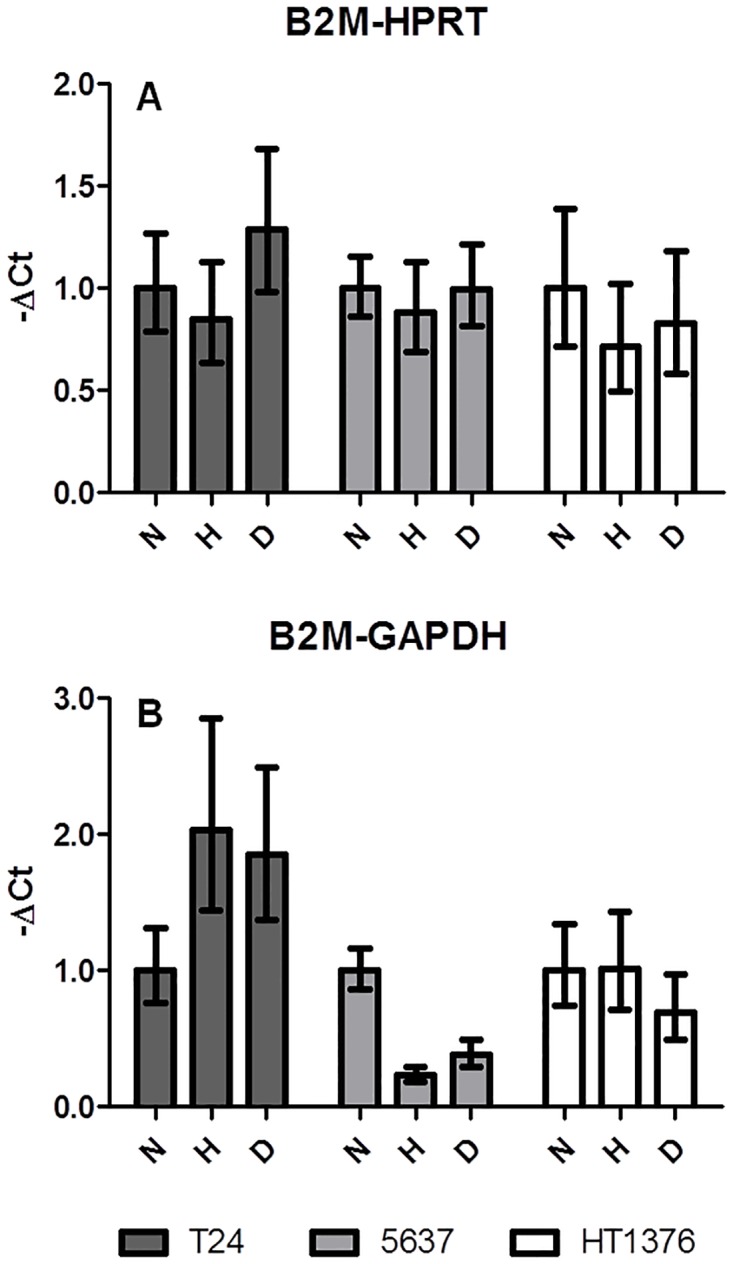
Relative mRNA expression calculated by normalization of a stably expressed gene (B2M) to another stable gene (HPRT, A) or to an unstable gene (GAPDH, B) under different experimental conditions: normoxia (N), hypoxia (H), DFX (D).
To demonstrate that the selection of an inappropriate reference gene for normalization might lead to erroneous data interpretations we have normalized B2M using HPRT. Accordingly, B2M expression was demonstrated to be reasonably stable in all cell lines and experimental conditions (Fig 5A), highlighting the high stability of both genes. On the other hand, when B2M was normalized to GAPDH, one of the less stable genes of the tested panel, significant variations could be observed. Namely, B2M showed increased expression in T24 cells exposed to hypoxia and DFX, and lower expression in 5637 cells exposed to the same conditions. Moreover, DFX-exposed HT1376 cells presented a downregulation of B2M (Fig 5B). These results highlighted that an appropriate normalization across samples determines the magnitude of relative expression levels of the genes tested.
Discussion
Significant amount of evidences support the notion that hypoxia enhances the malignant nature of bladder cancer cells by promoting tumor cell migration, invasion, chemoresistance, metastasis and immune modulation [28, 30, 42–44]. Furthermore, many of the transcriptome remodeling events underlying these transformations are mediated by HIF-1α [45]. Interestingly, many studies addressing gene expression in hypoxic cancer cells have disregarded the adaptations of classical and widely explored reference genes to microenvironmental challenges, which lead to the flawed election of targets for downstream analysis [46]. A common example includes GAPDH, which is significantly upregulated under low oxygen tension in many models, making it an unsuitable reference gene for hypoxic studies [23, 46, 47]. Accordingly, we have also found that GAPDH was among the least stable genes in the studied bladder cancer cells under hypoxia. Still, some reports support GAPDH as an excellent reference gene in hypoxic hepatocellular, colonic and lung carcinoma models [24]. In addition, hypoxic cells frequently undergo epithelial-to-mesenchymal transition (EMT), a plastic process in which fully differentiated epithelial cells are converted into poorly differentiated, migratory and invasive mesenchymal cells [48]. This comprehends a profound remodeling of the cytoskeleton, which includes a downregulation of ACTB, also a widely used reference gene [49, 50]. Likewise, we have observed that ACTB gene was unstable in bladder cancer cell lines exposed to hypoxia. Nevertheless, contradictory evidences have emerged regarding the election of ACTB as a reference gene for hypoxic studies. For instance, ACTB has been considered a stable control gene in breast (MCF-7 cell line) [46] and prostate cancer (LNCaP, 22Rv1, PC3, and DU14) cell lines under hypoxia [25]. These observations highlight that gene stability under hypoxia is highly dependent on the origin of cells as well as the necessity to undergo careful transcriptome evaluation before more in depth molecular studies.
As previously mentioned, RT-qPCR data quantification still remains challenging due to the random selection and number of reference genes used for data normalization in most studies. Moreover, many reports still rely just on a single reference gene for data normalization, despite the existence of more robust statistical methods for the evaluation of several controls. Herein, we have employed geNorm, first developed by Vandesompele, et al [34], and NormFinder statistical programs to access the stability of candidate reference genes. Both retrieved highly comparable results, especially in terms of gene stability ranking [51]. Accordingly, we have identified B2M and HPRT as the most stable reference genes to address the impact of oxygen shortage in hypoxic bladder cancer cells, irrespectively of their molecular nature. This was also verified in cells exposed to DFX [32], suggesting these genes are not regulated by HIF-1α. Moreover, both analytical programs have elected GAPDH and ACTB as the most unstable genes for all conditions.
Our study is in agreement with previous studies describing the stability of B2M in hypoxic cultured human chondrocytes [52]. Contrastingly, it has been reported that B2M expression is significantly altered in hypoxic prostate cancer cells out of a panel of 16 reference genes for qRT-PCR [25]. Similarly to bladder cancer cells, hypoxic prostate cancer and neural stem cells have also demonstrated a stable HPRT expression using geNorm and NormFinder [53, 54]. In contrast, Tan et al., using a model of endogenous cardiac stem cells, suggested that HPRT transcription is not stable under hypoxic conditions [55]. In summary, these findings reinforce the notion that the stable expression of a reference gene is context-dependent and may significantly vary between models. Which poses a limitation of this work, the results stated herein only apply to the three bladder cancer cell lines used in our experiments. Even though these cell lines are the most studied regarding bladder cancer in vitro research, only represents a subset of all bladder cancer cell lines available.
The identification of an optimal number of reference genes is also important for accurate normalization of RT-qPCR data, especially when differences in expression levels are subtle. According to geNorm software, two is the minimal number of reference genes, namely B2M and HRPT, for obtaining an accurate normalization under the hypoxic conditions in the studied cell bladder cancer lines.
In summary, we have highlighted possible problems when translating results from different models in the context of hypoxia, and reinforced the need for careful evaluation of reference genes prior to more in depth molecular studies. To the best of our knowledge this study is the first to analyze and evaluate the stability of a set of reference genes in the hypoxic context for these three bladder cancer cell lines. This information is important for other researchers that will need to evaluate mRNA expression on these cell lines and in this context, using the most widely used tool for gene expression analysis. Moreover, we have set the molecular basis to comprehensively address the transcriptional programs and molecular nature of bladder cancer cells under hypoxia, as well as the role of HIF-1α, towards novel biomarkers and therapeutic strategies.
Supporting Information
Western Blot analysis of HIF-1α and CAIX proteins, 24h (T24 and 5637 cell lines) and 6h (HT1376 cell line) after treatment (A). B2M was used as loading control. The western blot samples appear in the following order: T24, 5637 and HT1376 Normoxia (N); T24, 5637 and HT1376 Hypoxia (H); T24, 5637 and HT1376 DFX treatment (D). Molecular weight markers (MWM) are expressed in kDa. Bladder cancer cell lines overexpressed HIF-1α (B) and CAIX (C) hypoxia biomarkers when exposed to hypoxia. Concomitantly, the metabolic shift from aerobic to anaerobic metabolism, a critical event underlying hypoxia, was also confirmed by increased lactate levels in hypoxia treated cell culture mediums (D). The stabilization of HIF-1α with DFX resulted in similar behaviours suggesting that this transcription factor might regulate this events.
(TIF)
The individual ranks provided by both softwares for each cell line under all studied conditions are summarized: (A-C) T24 cell line comparing (A) normoxia and hypoxia, (B) normoxia and Dfx, (C) and three studied conditions. (D-F) 5637 cell line comparing (D) normoxia and hypoxia, (E) normoxia and Dfx, (F) and three studied conditions. (G-I) HT1376 cell line comparing (G) normoxia and hypoxia, (H) normoxia and Dfx, (I) and all studied conditions.
(TIF)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Some authors have postdoctoral grants SFRH/BPD/66288/2009 (JAF), SFRH/BPD/101827/2014 (LL), and PhD grants SFRH/BD/111242/2015 (AP) supported by Portuguese Foundation for Science and Technology (FCT - www.fct.pt). FCT is co-financed by European Social Fund (ESF) under Human Potential Operation Programme (POPH) from National Strategic Reference Framework (NSRF).
References
- 1.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. Journal of the National Cancer Institute. 2001;93(4):266–76. . [DOI] [PubMed] [Google Scholar]
- 2.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nature reviews Cancer. 2008;8(12):967–75. 10.1038/nrc2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferreira JA, Peixoto A, Neves M, Gaiteiro C, Reis CA, Assaraf YG, et al. Mechanisms of cisplatin resistance and targeting of cancer stem cells: Adding glycosylation to the equation. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy. 2016;24:34–54. 10.1016/j.drup.2015.11.003 . [DOI] [PubMed] [Google Scholar]
- 4.Chouaib S, Noman MZ, Kosmatopoulos K, Curran MA. Hypoxic stress: obstacles and opportunities for innovative immunotherapy of cancer. Oncogene. 2016. 10.1038/onc.2016.225 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nature reviews Cancer. 2002;2(1):38–47. 10.1038/nrc704 . [DOI] [PubMed] [Google Scholar]
- 6.Unruh A, Ressel A, Mohamed HG, Johnson RS, Nadrowitz R, Richter E, et al. The hypoxia-inducible factor-1 alpha is a negative factor for tumor therapy. Oncogene. 2003;22(21):3213–20. 10.1038/sj.onc.1206385 . [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. 10.1016/j.cell.2011.02.013 . [DOI] [PubMed] [Google Scholar]
- 8.Semenza GL. HIF-1 and human disease: one highly involved factor. Genes & development. 2000;14(16):1983–91. . [PubMed] [Google Scholar]
- 9.Marxsen JH, Stengel P, Doege K, Heikkinen P, Jokilehto T, Wagner T, et al. Hypoxia-inducible factor-1 (HIF-1) promotes its degradation by induction of HIF-alpha-prolyl-4-hydroxylases. The Biochemical journal. 2004;381(Pt 3):761–7. 10.1042/BJ20040620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohwer N, Cramer T. Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy. 2011;14(3):191–201. 10.1016/j.drup.2011.03.001 . [DOI] [PubMed] [Google Scholar]
- 11.Schmid T, Zhou J, Brune B. HIF-1 and p53: communication of transcription factors under hypoxia. Journal of cellular and molecular medicine. 2004;8(4):423–31. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuwai T, Kitadai Y, Tanaka S, Onogawa S, Matsutani N, Kaio E, et al. Expression of hypoxia-inducible factor-1alpha is associated with tumor vascularization in human colorectal carcinoma. International journal of cancer Journal international du cancer. 2003;105(2):176–81. 10.1002/ijc.11068 . [DOI] [PubMed] [Google Scholar]
- 13.Giatromanolaki A, Koukourakis MI, Sivridis E, Turley H, Talks K, Pezzella F, et al. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. British journal of cancer. 2001;85(6):881–90. 10.1054/bjoc.2001.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semenza GL. Targeting HIF-1 for cancer therapy. Nature reviews Cancer. 2003;3(10):721–32. 10.1038/nrc1187 . [DOI] [PubMed] [Google Scholar]
- 15.Lu X, Kang Y. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16(24):5928–35. 10.1158/1078-0432.CCR-10-1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer cell. 2009;15(6):501–13. 10.1016/j.ccr.2009.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nascimento CS, Barbosa LT, Brito C, Fernandes RP, Mann RS, Pinto AP, et al. Identification of Suitable Reference Genes for Real Time Quantitative Polymerase Chain Reaction Assays on Pectoralis major Muscle in Chicken (Gallus gallus). PloS one. 2015;10(5):e0127935 10.1371/journal.pone.0127935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riemer AB, Keskin DB, Reinherz EL. Identification and validation of reference genes for expression studies in human keratinocyte cell lines treated with and without interferon-gamma—a method for qRT-PCR reference gene determination. Experimental dermatology. 2012;21(8):625–9. 10.1111/j.1600-0625.2012.01537.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maltseva DV, Khaustova NA, Fedotov NN, Matveeva EO, Lebedev AE, Shkurnikov MU, et al. High-throughput identification of reference genes for research and clinical RT-qPCR analysis of breast cancer samples. Journal of clinical bioinformatics. 2013;3(1):13 10.1186/2043-9113-3-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gadkar VJ, Filion M. Validation of endogenous reference genes in Buglossoides arvensis for normalizing RT-qPCR-based gene expression data. SpringerPlus. 2015;4:178 10.1186/s40064-015-0952-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Kok JB, Roelofs RW, Giesendorf BA, Pennings JL, Waas ET, Feuth T, et al. Normalization of gene expression measurements in tumor tissues: comparison of 13 endogenous control genes. Laboratory investigation; a journal of technical methods and pathology. 2005;85(1):154–9. 10.1038/labinvest.3700208 . [DOI] [PubMed] [Google Scholar]
- 22.Wu C, Wang X, Zhong M, Liu H, He Q, Yang X, et al. Evaluation of potential reference genes for qRT-PCR studies in human hepatoma cell lines treated with TNF-alpha. Acta biochimica et biophysica Sinica. 2013;45(9):780–6. 10.1093/abbs/gmt072 . [DOI] [PubMed] [Google Scholar]
- 23.Higashimura Y, Nakajima Y, Yamaji R, Harada N, Shibasaki F, Nakano Y, et al. Up-regulation of glyceraldehyde-3-phosphate dehydrogenase gene expression by HIF-1 activity depending on Sp1 in hypoxic breast cancer cells. Archives of biochemistry and biophysics. 2011;509(1):1–8. 10.1016/j.abb.2011.02.011 . [DOI] [PubMed] [Google Scholar]
- 24.Said HM, Polat B, Hagemann C, Anacker J, Flentje M, Vordermark D. Absence of GAPDH regulation in tumor-cells of different origin under hypoxic conditions in—vitro. BMC research notes. 2009;2:8 10.1186/1756-0500-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vajda A, Marignol L, Barrett C, Madden SF, Lynch TH, Hollywood D, et al. Gene expression analysis in prostate cancer: the importance of the endogenous control. The Prostate. 2013;73(4):382–90. 10.1002/pros.22578 . [DOI] [PubMed] [Google Scholar]
- 26.Ferguson RE, Carroll HP, Harris A, Maher ER, Selby PJ, Banks RE. Housekeeping proteins: a preliminary study illustrating some limitations as useful references in protein expression studies. Proteomics. 2005;5(2):566–71. 10.1002/pmic.200400941 . [DOI] [PubMed] [Google Scholar]
- 27.Theodoropoulos VE, Lazaris AC, Kastriotis I, Spiliadi C, Theodoropoulos GE, Tsoukala V, et al. Evaluation of hypoxia-inducible factor 1alpha overexpression as a predictor of tumour recurrence and progression in superficial urothelial bladder carcinoma. BJU international. 2005;95(3):425–31. 10.1111/j.1464-410X.2005.05314.x . [DOI] [PubMed] [Google Scholar]
- 28.Theodoropoulos VE, Lazaris A, Sofras F, Gerzelis I, Tsoukala V, Ghikonti I, et al. Hypoxia-inducible factor 1 alpha expression correlates with angiogenesis and unfavorable prognosis in bladder cancer. Eur Urol. 2004;46(2):200–8. 10.1016/j.eururo.2004.04.008 . [DOI] [PubMed] [Google Scholar]
- 29.Azevedo R, Ferreira JA, Peixoto A, Neves M, Sousa N, Lima A, et al. Emerging antibody-based therapeutic strategies for bladder cancer: A systematic review. J Control Release. 2015;214:40–61. 10.1016/j.jconrel.2015.07.002 . [DOI] [PubMed] [Google Scholar]
- 30.Lima L, Oliveira D, Tavares A, Amaro T, Cruz R, Oliveira MJ, et al. The predominance of M2-polarized macrophages in the stroma of low-hypoxic bladder tumors is associated with BCG immunotherapy failure. Urol Oncol. 2014;32(4):449–57. 10.1016/j.urolonc.2013.10.012 . [DOI] [PubMed] [Google Scholar]
- 31.Pinto-Leite R, Carreira I, Melo J, Ferreira SI, Ribeiro I, Ferreira J, et al. Genomic characterization of three urinary bladder cancer cell lines: understanding genomic types of urinary bladder cancer. Tumour Biol. 2014;35(5):4599–617. 10.1007/s13277-013-1604-3 . [DOI] [PubMed] [Google Scholar]
- 32.Woo KJ, Lee TJ, Park JW, Kwon TK. Desferrioxamine, an iron chelator, enhances HIF-1alpha accumulation via cyclooxygenase-2 signaling pathway. Biochem Biophys Res Commun. 2006;343(1):8–14. 10.1016/j.bbrc.2006.02.116 . [DOI] [PubMed] [Google Scholar]
- 33.Lima L, Ferreira JA, Tavares A, Oliveira D, Morais A, Videira PA, et al. FASL polymorphism is associated with response to bacillus Calmette-Guerin immunotherapy in bladder cancer. Urol Oncol. 2014;32(1):44 e1–7. 10.1016/j.urolonc.2013.05.009 . [DOI] [PubMed] [Google Scholar]
- 34.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome biology. 2002;3(7):RESEARCH0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer research. 2004;64(15):5245–50. 10.1158/0008-5472.CAN-04-0496 . [DOI] [PubMed] [Google Scholar]
- 36.Tong Z, Gao Z, Wang F, Zhou J, Zhang Z. Selection of reliable reference genes for gene expression studies in peach using real-time PCR. BMC molecular biology. 2009;10:71 10.1186/1471-2199-10-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Die JV, Roman B, Nadal S, Gonzalez-Verdejo CI. Evaluation of candidate reference genes for expression studies in Pisum sativum under different experimental conditions. Planta. 2010;232(1):145–53. 10.1007/s00425-010-1158-1 . [DOI] [PubMed] [Google Scholar]
- 38.Maroufi A, Van Bockstaele E, De Loose M. Validation of reference genes for gene expression analysis in chicory (Cichorium intybus) using quantitative real-time PCR. BMC molecular biology. 2010;11:15 10.1186/1471-2199-11-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strube C, Buschbaum S, Wolken S, Schnieder T. Evaluation of reference genes for quantitative real-time PCR to investigate protein disulfide isomerase transcription pattern in the bovine lungworm Dictyocaulus viviparus. Gene. 2008;425(1–2):36–43. 10.1016/j.gene.2008.08.001 . [DOI] [PubMed] [Google Scholar]
- 40.Van Hiel MB, Van Wielendaele P, Temmerman L, Van Soest S, Vuerinckx K, Huybrechts R, et al. Identification and validation of housekeeping genes in brains of the desert locust Schistocerca gregaria under different developmental conditions. BMC molecular biology. 2009;10:56 10.1186/1471-2199-10-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nygard AB, Jorgensen CB, Cirera S, Fredholm M. Selection of reference genes for gene expression studies in pig tissues using SYBR green qPCR. BMC molecular biology. 2007;8:67 10.1186/1471-2199-8-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Humar R, Kiefer FN, Berns H, Resink TJ, Battegay EJ. Hypoxia enhances vascular cell proliferation and angiogenesis in vitro via rapamycin (mTOR)-dependent signaling. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2002;16(8):771–80. 10.1096/fj.01-0658com . [DOI] [PubMed] [Google Scholar]
- 43.Ruan K, Song G, Ouyang G. Role of hypoxia in the hallmarks of human cancer. Journal of cellular biochemistry. 2009;107(6):1053–62. 10.1002/jcb.22214 . [DOI] [PubMed] [Google Scholar]
- 44.Zhao J, Du F, Luo Y, Shen G, Zheng F, Xu B. The emerging role of hypoxia-inducible factor-2 involved in chemo/radioresistance in solid tumors. Cancer treatment reviews. 2015;41(7):623–33. 10.1016/j.ctrv.2015.05.004 . [DOI] [PubMed] [Google Scholar]
- 45.Mucaj V, Shay JE, Simon MC. Effects of hypoxia and HIFs on cancer metabolism. International journal of hematology. 2012;95(5):464–70. 10.1007/s12185-012-1070-5 . [DOI] [PubMed] [Google Scholar]
- 46.Caradec J, Sirab N, Keumeugni C, Moutereau S, Chimingqi M, Matar C, et al. 'Desperate house genes': the dramatic example of hypoxia. British journal of cancer. 2010;102(6):1037–43. 10.1038/sj.bjc.6605573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhong H, Simons JW. Direct comparison of GAPDH, beta-actin, cyclophilin, and 28S rRNA as internal standards for quantifying RNA levels under hypoxia. Biochem Biophys Res Commun. 1999;259(3):523–6. 10.1006/bbrc.1999.0815 . [DOI] [PubMed] [Google Scholar]
- 48.Jiang J, Tang YL, Liang XH. EMT: a new vision of hypoxia promoting cancer progression. Cancer biology & therapy. 2011;11(8):714–23. . [DOI] [PubMed] [Google Scholar]
- 49.Wang H, Yang B, Geng T, Li B, Dai P, Chen C. Tissue-specific selection of optimal reference genes for expression analysis of anti-cancer drug-related genes in tumor samples using quantitative real-time RT-PCR. Experimental and molecular pathology. 2015;98(3):375–81. 10.1016/j.yexmp.2014.10.014 . [DOI] [PubMed] [Google Scholar]
- 50.Sun Y, Li Y, Luo D, Liao DJ. Pseudogenes as weaknesses of ACTB (Actb) and GAPDH (Gapdh) used as reference genes in reverse transcription and polymerase chain reactions. PloS one. 2012;7(8):e41659 10.1371/journal.pone.0041659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Spiegelaere W, Dern-Wieloch J, Weigel R, Schumacher V, Schorle H, Nettersheim D, et al. Reference gene validation for RT-qPCR, a note on different available software packages. PloS one. 2015;10(3):e0122515 10.1371/journal.pone.0122515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foldager CB, Munir S, Ulrik-Vinther M, Soballe K, Bunger C, Lind M. Validation of suitable house keeping genes for hypoxia-cultured human chondrocytes. BMC molecular biology. 2009;10:94 10.1186/1471-2199-10-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yao L, Chen X, Tian Y, Lu H, Zhang P, Shi Q, et al. Selection of housekeeping genes for normalization of RT-PCR in hypoxic neural stem cells of rat in vitro. Molecular biology reports. 2012;39(1):569–76. 10.1007/s11033-011-0772-8 . [DOI] [PubMed] [Google Scholar]
- 54.Ohl F, Jung M, Xu C, Stephan C, Rabien A, Burkhardt M, et al. Gene expression studies in prostate cancer tissue: which reference gene should be selected for normalization? Journal of molecular medicine. 2005;83(12):1014–24. 10.1007/s00109-005-0703-z . [DOI] [PubMed] [Google Scholar]
- 55.Tan SC, Carr CA, Yeoh KK, Schofield CJ, Davies KE, Clarke K. Identification of valid housekeeping genes for quantitative RT-PCR analysis of cardiosphere-derived cells preconditioned under hypoxia or with prolyl-4-hydroxylase inhibitors. Molecular biology reports. 2012;39(4):4857–67. 10.1007/s11033-011-1281-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Western Blot analysis of HIF-1α and CAIX proteins, 24h (T24 and 5637 cell lines) and 6h (HT1376 cell line) after treatment (A). B2M was used as loading control. The western blot samples appear in the following order: T24, 5637 and HT1376 Normoxia (N); T24, 5637 and HT1376 Hypoxia (H); T24, 5637 and HT1376 DFX treatment (D). Molecular weight markers (MWM) are expressed in kDa. Bladder cancer cell lines overexpressed HIF-1α (B) and CAIX (C) hypoxia biomarkers when exposed to hypoxia. Concomitantly, the metabolic shift from aerobic to anaerobic metabolism, a critical event underlying hypoxia, was also confirmed by increased lactate levels in hypoxia treated cell culture mediums (D). The stabilization of HIF-1α with DFX resulted in similar behaviours suggesting that this transcription factor might regulate this events.
(TIF)
The individual ranks provided by both softwares for each cell line under all studied conditions are summarized: (A-C) T24 cell line comparing (A) normoxia and hypoxia, (B) normoxia and Dfx, (C) and three studied conditions. (D-F) 5637 cell line comparing (D) normoxia and hypoxia, (E) normoxia and Dfx, (F) and three studied conditions. (G-I) HT1376 cell line comparing (G) normoxia and hypoxia, (H) normoxia and Dfx, (I) and all studied conditions.
(TIF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.