Abstract
Objective
Diurnal salivary cortisol patterns in healthy adults are well-established but have not been studied in midlife women with hot flashes. We hypothesized that frequent hot flashes are associated with aberrant cortisol patterns similar to sleep deficient individuals.
Design
Cross-sectional
Participants
306 women, ages 40-62, randomized to a behavioral intervention for hot flashes.
Measurements
Baseline comparisons of cortisol geometric means (nmol/L) from 4 daily time-points averaged over 2 consecutive days plus other calculated cortisol measures were made between groups defined by baseline: 1) mean daily hot flash frequency tertile (≥5.5, N=103; >5.5-8.8, N=103; >8.8, N=100), and 2) selected characteristics. Repeated measures linear regression models of log-transformed cortisol evaluated group differences, adjusting for covariates.
Results
Women were 67% White 24% African American, with 7.6 (SD 3.9)hot flashes per day. Salivary cortisol geometric means (nmol/L) among all women were: 75.0 (SD 44.8) total, 8.6 (SD 5.6) wake, 10.0 (SD 7.5) wake +30 minutes, 3.7 (SD 3.3) early afternoon, and 1.6 (SD 1.8) bedtime. Wake + 30 minute values showed an 18% median rise from wake values (interquartile range -24 to 96%), and means varied by hot flash frequency tertile, from lowest to highest: 11.4(SD 7.3), 10.3 (SD 6.5) and 8.6 (SD 7.8), respectively, p=0.003. Beside the early afternoon value (p=0.02), cortisol values did not vary by hot flash frequency.
Conclusion
Taken together, these findings suggest that high frequency of moderate to severe hot flashes may be associated with subtle abnormalities in cortisol concentrations - a pattern consistent with chronic sleep disturbance.
Keywords: salivary cortisol, menopause, hot flash
Introduction
The hypothalamic-pituitary-adrenal (HPA) axis plays a primary role in the body's acute reactions to stress by balancing hormone releases from the epinephrine-producing adrenal medulla, and from the corticosteroid-producing adrenal cortex. Acute stress is associated with an abrupt physiologic rise in cortisol 1,2. Typical daily cortisol concentrations in healthy adults rise abruptly within 30 minutes of awakening (cortisol awakening response), and diminish throughout the day with lowest values in late evening 3,4. Blunted cortisol responses (i.e. diminished awakening response or a lower diurnal variation) as well as lower daily overall concentrations are more apt to reflect chronic illnesses or stressors 1,3-5 and vary by race/ethnicity 6-8. Late evening cortisol may be increased with chronic stress 1,9, insomnia 10, and sleep disturbances 11.
Daily cortisol patterns in midlife women bothered by hot flashes are understudied, but in theory, patterns may vary from normal healthy adults for three reasons. First, hot flashes have been associated with stress and anxiety 12-14. Second, estrogen may affect cortisol secretion 15,16. Increased estrogen variability has been associated with hot flashes (16), and it follows that women with more hot flashes could have aberrant daily cortisol patterns. Third, the majority of women with hot flashes report poor sleep 17, and insomnia 10 and sleep disturbances 11 are associated with abnormal cortisol patterns.
Cortisol can be measured via the urine, serum or saliva, but salivary cortisol measurement has become the gold standard for ambulatory studies of stress and HPA axis regulation 2. Measurement of ambulatory salivary free cortisol concentrations allows for frequent data collection in a natural setting with minimal participant burden and correlates with serum cortisol concentrations 18. We hypothesized that women with more frequent moderate to severe hot flashes have different daily cortisol patterns as compared to women with fewer hot flashes. Secondly, we hypothesized that women with more stress and anxiety would have more hot flashes, and that stress, anxiety and higher hot flash frequency would strongly correlate with abnormal cortisol patterns. Our goal was to describe daily salivary free cortisol values and patterns in midlife women with hot flashes, to evaluate potential differences in cortisol by hot flash frequency and to assess factors that might be associated with cortisol concentrations in midlife women with hot flashes.
Materials and Methods
This study included 306 participants who provided salivary cortisol samples at baseline in a multi-center clinical trial conducted by the Menopausal Strategies: Finding Lasting Answers to Symptoms and Health (MsFlash) network. Details about the network are published 19,20. This study was a 3 by 2 factorial, randomized, controlled trial conducted at 3 sites (Indianapolis, Oakland, and Seattle), with the main results described elsewhere 21-23. Eligible women were randomized to 12 weeks of yoga, exercise, or usual activity, and simultaneously randomized to 1.8 g/day of omega-3 fish oil capsules (EPA 1275 mg, DHA 300 mg) or placebo capsules. Participants were recruited from February 2011 through January 2012, primarily by mass mailing to age-eligible women using purchased lists and health-plan enrollment files.
Women were ages 40-62 years; in the menopausal transition or postmenopausal or had a hysterectomy with FSH >20 mIU/mL and estradiol ≤50 pg/mL; and in general good health. The term “hot flash” is used to represent daytime and nighttime hot flashes or night sweats. The hot flash eligibility criteria were: ≤14 hot flashes per week recorded on daily hot flash diaries for 3 weeks; hot flashes rated as bothersome or severe on 4 or more occasions per week; and the hot flash frequency in week 3 did not decrease >50% from the average weekly levels in weeks 1 and 2. Exclusion criteria included: body mass index (BMI) >37 kg/m2; use of hormonal contraceptives or hormones in the past month; use of prescription or over-the-counter treatments for hot flashes/night sweats in the past month; unstable medical conditions; current user of one of the study interventions or a related activity (i.e., yoga, tai chi, qi gong, or meditation, regular exercise, omega-3 fatty acid supplements, frequent consumption of fish); contraindications to exercise or yoga (e.g., physical limitations), or omega-3 (e.g., allergy to soy or fish); or a major depressive episode in the past 3 months.
Salivary cortisol was collected for 2 consecutive days prior to randomization during the 2 weeks of baseline hot flash data collection, using the Salivette swab (Starstedt AG & Co, Lumbrecht, Germany) at 4 time points on each day: on awakening (wake), 30 minutes later (wake + 30), early afternoon and bedtime. The swab was refrigerated and placed in a home freezer within 4 hours of collection. Specimens were brought in freezer bags to the study appointment within 1-3 weeks of collection and stored at -70 degrees Celsius until assayed. Participants were given written and verbal instructions not to brush their teeth but to rinse their mouth with water 10-15 minutes before collection. They were instructed to write the time and day on the collection form, and to chew the Salivette swab for 45 seconds Participants recorded the timing and presence of alcohol, caffeine, nicotine, intense physical activity and eating within 2 hours of specimen collection.
Salivary free cortisol concentration (nmol/L) was assayed at the University of Washington, School of Nursing Laboratory, using a high-sensitivity cortisol enzyme immunoassay kit (Salimetrics, LLC, Pennsylvania, USA). The intra-assay coefficient of variation (CV) for means 0.16-2.07 (standard deviation [SD] 0.01-0.08) is 3-4%; inter-assay CV is for means 0.43-1.99 (SD 0.01-0.05) is 3-4%.
Statistical analysis
Prior to analysis, cortisol values at each time point were set to missing if they did not meet specimen collection timing requirements. Wake specimens were required to have been collected between 4 and 11AM and within 15 minutes of awakening, wake + 30 between 15 and 60 minutes from wake time, the “early afternoon” specimen between 4 to 8 hours from awakening, and the “bedtime” measurement between 12 and 20 hours from wakening.7 This removed 1, 1, 37, and 10 women from the analyses, from the respective time points. Median and inter-quartile ranges for wake + 30, early afternoon and bedtime, with wake as time 0 were: 30 minutes (30-32), 5.4 hours (4.7-6.1) and 14.2 hours (13.2-15.0). In addition to timing requirements, results were also set to missing if a participant reported possible contamination of the sample due to alcohol, caffeine, or nicotine use, intense physical activity or food intake within 2 hours of specimen collection. We calculated 3 additional HPA axis measures 24: (1) estimated daytime cortisol exposure (area under the curve [AUC]); (2) awakening cortisol response; and (3) diurnal variation. As the AUC and diurnal variation outcomes were dependent on the initial wake time of the participant, both outcomes were scaled to 14 hours between wake and bedtime hour measurement, the median time for the study sample.
Participants were grouped by tertiles of the mean daily hot flash frequency (≤ 5.5, >5.5-8.8, and >8.8), calculated from 2 weeks of baseline hot flash diaries. Tests for trends in baseline characteristics across hot flash categories were estimated via linear (continuous, ordinal) or logistic (dichotomous) regression models. A number of baseline factors were chosen a priori as covariates in models of cortisol by hot flash frequency, including: 1) factors associated with cortisol differences as reported in other populations such as age, race (African American vs. White) (5-7) and BMI (kg/m2) 25; 2) clinical characteristics associated with cortisol or menopause (9,10, 26, 27) as measured by validated scales: depression [Patient Health Questionnaire (PHQ-8, range 0-24)]; anxiety [General Anxiety Disorder Questionnaire (GAD 7, range 0-21)]; stress [Perceived Stress Score (PSS, range 0-40)]; sleep quality [Pittsburgh Sleep Quality Index (PSQI, range 0-21)]; and insomnia [Insomnia Sleep Index (ISI, range 0-28)] 19; 3) menopausal factors that might affect cortisol concentrations including status (postmenopausal vs. menopause transition), hot flash severity (1-3 scale), and duration of hot flashes; and 4) other factors known or suspected to affect cortisol and hot flashes: marital status, full-time employment, and cardiovascular risk factors (systolic and diastolic blood pressure).
The distributions of cortisol values were skewed at the 4 time points, thus values were log transformed. Geometric means and standard deviations (SD) were presented for the 2-day mean cortisol measurements by hot flash tertile. To evaluate the associations of cortisol and hot flash frequency, data from both days of cortisol collection were included as repeated measures in linear regression models of log cortisol values at each time point as a function of hot flash tertile, day (1st or 2nd), and clinical center.
We evaluated associations of: 1) daytime cortisol exposure (AUC), 2) cortisol awakening response, 3) cortisol diurnal variation (wake minus bedtime) with hot flash frequency via a series of repeated measures linear models. These particular measures have been shown to be abnormal in other populations with chronic stress, illness, and sleep disturbances 1,5,9-11. Three models estimated the association of cortisol with hot flash frequency for each of these cortisol measures. The first model adjusted for day and clinical center; the second model additionally adjusted for demographic characteristics and body measurements; and the third model further adjusted for baseline depression, stress, and insomnia.
An additional adjusted model estimated the association of cortisol outcomes with hot flash frequency in the subset of women with moderate to severe insomnia (ISI ≥ 15), a factor known to affect cortisol awakening response 10. Factors evaluated as potential effect modifiers for the association of cortisol measures and hot flash frequency were determined a priori: stress (>13.7), insomnia (< 8, 8-<15, ≥15), and anxiety (>5). Selected baseline factors from the entire study population were evaluated for their association with 2 cortisol measures: awakening response (wake + 30 minutes minus wake) and the log-transformed bedtime value using repeated measures models adjusted for day (1st or 2nd), clinical center, demographic characteristics, body measurements, baseline depression, stress, and insomnia.
All statistical analyses were conducted in SAS for Windows Version 9.4. P values <0.05 were considered statistically significant.
Results
There were 306 women included: the mean and median ages were both approximately 55 years, over 80% were postmenopausal (median 3 years from final menstrual period, two-thirds were White, and almost two-thirds were college graduates (Table 1). Women had on average 7.6 (SD 3.9) hot flashes per day and reported having experienced hot flashes for a median duration of 5 years. Higher baseline hot flash frequency was associated with statistically significant lower age, higher diastolic blood pressure, and higher hot flash bother/severity. Otherwise, the baseline characteristics did not vary by hot flash frequency.
Table 1. Baseline characteristics of all women, and by mean daily hot flash frequency.
| Baseline characteristic | All women | Mean daily hot flash frequency | ||||||
|---|---|---|---|---|---|---|---|---|
| N=306 | ≤ 5.5 N=103 |
>5.5 – 8.8 N=103 |
>8.8 N=100 |
|||||
| N | % | N | % | N | % | N | % | |
| Age at screening (years), mean (SD) 1 | 54.6 | (3.6) | 55.2 | (3.6) | 54.6 | (3.7) | 54.0 | (3.4) |
| ≥ 55 | 149 | 48.7 | 57 | 55.3 | 49 | 47.6 | 43 | 43.0 |
| Race | ||||||||
| White | 204 | 66.7 | 69 | 67.0 | 68 | 66.0 | 67 | 67.0 |
| African American | 72 | 23.5 | 21 | 20.4 | 27 | 26.2 | 24 | 24.0 |
| Other/unknown | 30 | 9.8 | 13 | 12.6 | 8 | 7.8 | 9 | 9.0 |
| Postmenopausal | 250 | 81.7 | 87 | 84.5 | 84 | 81.6 | 79 | 79.0 |
| History of hot flashes (years) | ||||||||
| < 8 | 206 | 67.3 | 73 | 70.9 | 68 | 66.0 | 65 | 65.0 |
| ≥ 8 | 95 | 31.0 | 28 | 27.2 | 34 | 33.0 | 33 | 33.0 |
| Hot flash severity (1-3 scale) 2 | ||||||||
| < Moderate (2) | 181 | 59.2 | 86 | 83.5 | 59 | 57.3 | 36 | 36.0 |
| ≥ Moderate | 125 | 40.8 | 17 | 16.5 | 44 | 42.7 | 64 | 64.0 |
| Married / living as married | 207 | 67.6 | 65 | 63.1 | 73 | 70.9 | 69 | 69.0 |
| Education | ||||||||
| ≤ High school / GED | 13 | 4.2 | 5 | 4.9 | 2 | 1.9 | 6 | 6.0 |
| Some college | 98 | 32.0 | 31 | 30.1 | 33 | 32.0 | 34 | 34.0 |
| College graduate | 194 | 63.4 | 67 | 65.0 | 67 | 65.0 | 60 | 60.0 |
| Full-time employed | 187 | 61.1 | 64 | 62.1 | 68 | 66.0 | 55 | 55.0 |
| Current smoker | 21 | 6.9 | 7 | 6.8 | 5 | 4.9 | 9 | 9.0 |
| BMI (kg/m2), mean (SD) | 26.8 | (4.4) | 27.0 | (4.7) | 27.0 | (4.4) | 26.3 | (4.1) |
| > 30 | 73 | 23.9 | 26 | 25.2 | 28 | 27.2 | 19 | 19.0 |
| Systolic BP, mean (SD) | 117.3 | (13.5) | 115.7 | (12.8) | 117.4 | (13.4) | 119.1 | (14.1) |
| Diastolic BP, mean (SD) 1 | 72.7 | (9.1) | 71.2 | (9.5) | 72.7 | (8.6) | 74.1 | (9.0) |
| PHQ-8 Depression | ||||||||
| < 5 | 194 | 63.4 | 67 | 65.0 | 67 | 65.0 | 60 | 60.0 |
| ≥ 5 (at least mild) | 109 | 35.6 | 33 | 32.0 | 36 | 35.0 | 40 | 40.0 |
| GAD-7 Anxiety | ||||||||
| < 5 | 225 | 73.5 | 70 | 68.0 | 80 | 77.7 | 75 | 75.0 |
| ≥ 5 (at least mild) | 81 | 26.5 | 33 | 32.0 | 23 | 22.3 | 25 | 25.0 |
| PSS Stress | ||||||||
| < 13.7 | 144 | 47.1 | 46 | 44.7 | 57 | 55.3 | 41 | 41.0 |
| ≥ 13.7 (above mean) | 155 | 50.7 | 54 | 52.4 | 46 | 44.7 | 55 | 55.0 |
| ISI Insomnia | ||||||||
| < 8 (none to mild) | 77 | 25.2 | 32 | 31.1 | 25 | 24.3 | 20 | 20.0 |
| 8 - <15 (moderate) | 135 | 44.1 | 44 | 42.7 | 42 | 40.8 | 49 | 49.0 |
| ≥ 15 (severe) | 92 | 30.1 | 27 | 26.2 | 35 | 34.0 | 30 | 30.0 |
| PSQI Sleep | ||||||||
| < 5 | 43 | 14.1 | 18 | 17.5 | 12 | 11.7 | 13 | 13.0 |
| ≥ 5 (at least mild) | 257 | 84.0 | 84 | 81.6 | 91 | 88.3 | 82 | 82.0 |
t-test p-value <0.05 for hot flash frequency in a linear model with baseline characteristic as a function of linear trend hot flash frequency
chi-square p-value <0.05 for hot flash frequency in a logistic model with baseline characteristic as a function of linear trend hot flash frequency
Salivary free cortisol concentration geometric means (nmol/L) for all women were:75.0 (SD 44.8) total, 8.6 (SD 5.6) wake, 10.0 (SD 7.5) wake + 30 minutes, 3.7 (SD 3.3) early afternoon, and 1.6 (SD 1.8) bedtime. The mean daily cortisol values for all women, averaged over the 2 days, followed a normal diurnal pattern (Figure 1). The median percent cortisol rise from awakening was 18% (interquartile range (IQR) -24 to 96%), but did not vary by hot flash frequency (≤ 5.5: 19% increase, IQR -18 to 91%; 5.5-8.8: 24% increase, IQR -20 to 107%; and >8.8: 10% increase, IQR -37 to 98%).
Figure 1. Geometric mean salivary cortisol concentrations in 306 women over time, by mean daily hot flash frequency tertiles.
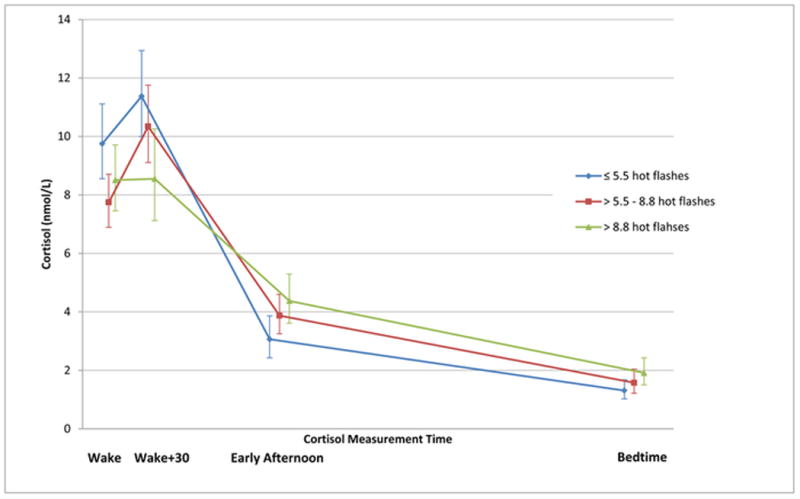
N= 103, ≤ 5.5 mean daily hot flashes; N=103, 5.5-8.8 mean daily hot flashes; and N=100, > 8.8 mean daily hot flashes
Cortisol values did not vary significantly by hot flash frequency, with the exception that the wake + 30 minute cortisol concentration was lowest (p=0.003) and early afternoon was highest among women with the greatest number of mean daily hot flashes (0.02) (Table 2). However,total daytime cortisol, awakening response, diurnal variation, and bedtime cortisol did not vary by frequency of mean daily hot flashes in any of the adjusted models (Table 3). In a subsample of 92 women with ISI ≥ 15 a significant positive association with bedtime cortisol (p=0.03) was found (data not shown). Among this subsample higher hot flash frequency tertiles were associated with decreased diurnal variation (p=0.01). The interactions between insomnia (ISI <8, 8-<15, ≥ 15), hot flashes and cortisol outcomes were not statistically significant, and there was no significant effect modification by stress or anxiety.
Table 2. Cortisol geometric means and standard deviations by mean daily hot flash frequency.
| All women | Mean daily hot flash frequency | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cortisol measure (nmol/L) | N=306 | ≤ 5.5 N=103 |
>5.5 – 8.8 N=103 |
>8.8 N=100 |
p-value1 | ||||
| Geo Mean | Geo SD | Geo Mean | Geo SD | Geo Mean | Geo SD | Geo Mean | Geo SD | ||
| Wake | 8.6 | (5.6) | 9.7 | (6.5) | 7.7 | (4.6) | 8.5 | (5.6) | 0.19 |
| Wake + 30 minutes | 10.0 | (7.5) | 11.4 | (7.3) | 10.3 | (6.5) | 8.6 | (7.8) | 0.003 |
| Early afternoon | 3.7 | (3.3) | 3.1 | (3.2) | 3.9 | (2.9) | 4.4 | (3.7) | 0.02 |
| Bedtime | 1.6 | (1.8) | 1.3 | (1.5) | 1.6 | (1.8) | 1.9 | (2.1) | 0.18 |
p-value from a repeated measures linear model of log cortisol value as a function of linear hot flash tertile, adjusted for consecutive day (1st, 2nd) and clinical center (Indianapolis, Oakland, Seattle)
Table 3. Adjusted models of cortisol outcomes by mean daily hot flash frequency.
| Mean daily hot flash frequency | |||||
|---|---|---|---|---|---|
|
| |||||
| ≤ 5.5 N=1031 |
>5.5 – 8.8 N=1031 |
> 8.8 N=1001 |
p-value3 | ||
|
| |||||
| Cortisol outcome | Model | Estimate (95% CI)2 | Estimate (95% CI)2 | Estimate (95% CI)2 | |
| Total daytime (AUC) 4 | 1 | 0.00 (Ref) | -0.03 (-0.24, 0.17) | -0.01 (-0.21, 0.19) | 0.94 |
| (log nmol/L) | 2 | 0.00 (Ref) | -0.06 (-0.26, 0.14) | 0.01 (-0.21, 0.22) | 0.99 |
| 3 | 0.00 (Ref) | -0.04 (-0.24, 0.17) | -0.01 (-0.24, 0.23) | 0.94 | |
|
| |||||
| Awakening response (wake + 30 minutes minus wake) (nmol/L) | 1 | 0.00 (Ref) | 1.08 (-0.80, 2.97) | -1.02 (-2.92, 0.87) | 0.30 |
| 2 | 0.00 (Ref) | 1.54 (-0.39, 3.47) | -0.59 (-2.58, 1.41) | 0.58 | |
| 3 | 0.00 (Ref) | 1.24 (-0.70, 3.17) | -1.08 (-3.08, 0.93) | 0.30 | |
|
| |||||
| Diurnal variation4 (wake minus bedtime) (nmol/L) | 1 | 0.00 (Ref) | -5.58 (-10.15, -1.00) | -4.23 (-8.72, 0.26) | 0.07 |
| 2 | 0.00 (Ref) | -6.10 (-11.12, -1.09) | -5.26 (-11.12, 0.61) | 0.08 | |
| 3 | 0.00 (Ref) | -4.91 (-9.69, -0.12) | -4.57 (-10.88, 1.74) | 0.15 | |
|
| |||||
| Bedtime (log nmol/L) | 1 | 0.00 (Ref) | 0.18 (-0.16, 0.52) | 0.22 (-0.10, 0.53) | 0.18 |
| 2 | 0.00 (Ref) | 0.20 (-0.12, 0.53) | 0.29 (-0.04, 0.61) | 0.09 | |
| 3 | 0.00 (Ref) | 0.18 (-0.15, 0.51) | 0.29 (-0.05, 0.63) | 0.10 | |
Following exclusions for time window, alcohol, caffeine, nicotine, intense physical activity and eating within 2 hours of specimen collection models included the following numbers of women by hot flash tertile: Total daytime 59, 55, 59; Awakening response 94, 95, 93; Diurnal variation 77, 72, 76; and Bedtime 78, 72, 76.
Estimates and 95% confidence intervals from a repeated measure linear regression model represent the mean cortisol difference between groups with higher baseline hot flash frequency and the ≤5.5 group, after adjustment for other factors as described below
p-values from a repeated measures linear regression model of each outcome as a linear trend over hot flash frequency tertiles
Model 1: Adjusted for day, clinical center
Model 2: Adjusted for factors in Model 1 + age, race, menopause status, marital status, full-time employment, BMI, systolic BP, diastolic BP
Model 3: Adjusted for factors in Models 1 and 2 + depression (PHQ-8), stress (PSS), and insomnia (ISI)
Total AUC and diurnal variation are scaled to the median wake to bedtime measurement time, 14 hours
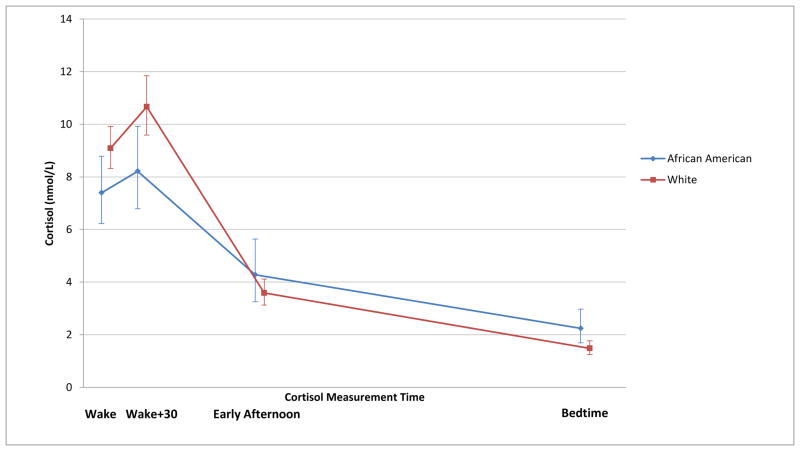
African American women had slightly lower morning cortisol values (both wake and wake + 30 minutes) and higher bedtime cortisol than White women, with a significantly lower awakening response (p=0.05) (Figure 2, Table 4). Higher anxiety was significantly associated with decreased awakening response (p=0.05). None of the other selected baseline factors was significantly associated with cortisol awakening response or bedtime values.
Figure 2. Geometric mean salivary cortisol concentrations over time among 204 White and 72 African American women.
Table 4. Adjusted models of cortisol outcomes by baseline factors other than hot flashes.
| Cortisol outcome | |||||
|---|---|---|---|---|---|
| Baseline characteristic | Subgroups | Awakening response (nmol/L) N=282 | Bedtime (log nmol/L) N=226 | ||
| Estimate (95% CI)1 | p-value1 | Estimate (95% CI)1 | p-value1 | ||
| Race | African American vs. White | -2.19 (-4.35, -0.03) | 0.05 | 0.28 (-0.07, 0.63) | 0.12 |
| Menopause status | Post- vs. Peri-menopausal | 1.24 (-0.64, 3.12) | 0.20 | -0.26 (-0.59, 0.07) | 0.13 |
| History of hot flashes | ≥8 vs. <8 years | 0.25 (-1.82, 2.33) | 0.81 | 0.29 (-0.03, 0.61) | 0.08 |
| Mean hot flash severity | > Moderate vs. ≤ Moderate | 0.14 (-1.76, 2.03) | 0.89 | -0.02 (-0.36, 0.32) | 0.91 |
| BMI (kg/m2) | ≥30 vs. <30 | 0.47 (-1.55, 2.50) | 0.65 | -0.26 (-0.60, 0.09) | 0.15 |
| PHQ-8 Depression | (≥ mild) ≥5 vs. <5 | 0.33 (-1.75, 2.42) | 0.75 | 0.19 (-0.13, 0.50) | 0.25 |
| GAD-7 Anxiety2 | (≥ mild) ≥5 vs. <5 | -2.17 (-4.28, -0.07) | 0.05 | 0.32 (-0.01, 0.64) | 0.06 |
| PSS Stress | (≥mean) ≥13.7 vs. <13.7 | -0.82 (-2.45, 0.81) | 0.32 | -0.11 (-0.39, 0.18) | 0.46 |
| ISI Insomnia3 | (moderate) 8-<15 vs. <8 | 1.95 (0.05, 3.86) | 0.59 | 0.11 (-0.27, 0.49) | 0.54 |
| (severe) ≥15vs. <8 | 0.79 (-1.51, 3.09) | 0.13 (-0.28, 0.54) | |||
| PSQI Sleep Quality4 | (≥ mild) ≥5 vs. <5 | -0.76 (-2.91, 1.39) | 0.49 | 0.08 (-0.30, 0.47) | 0.67 |
Estimates, 95% confidence intervals, and p-values from a repeated measure linear regression model with the outcome of interest as a function of the individual baseline characteristic of interest, adjusted for day, clinical center, hot flash frequency, age, race, menopause status, marital status, full-time employment, BMI, systolic BP, diastolic BP, depression (PHQ-8), stress (PSS), and insomnia (ISI)
GAD-7 Anxiety model not adjusted for PHQ-8 depression because of high correlation between anxiety and depression
For p-value 3-level ISI was treated as a linear variable
PSQI Sleep model not adjusted for ISI insomnia because of high correlation between sleep quality and insomnia
Discussion
We evaluated daily salivary free cortisol patterns in midlife women with hot flashes at 4 time points on 2 consecutive days. Compared with reported normal ranges for salivary free cortisol in the general population 26,27, women in our study appear to have had abnormally low or low range of normal values 8.6 (SD 5.6) wake, 10.0 (SD 7.5) wake +30 minutes, 3.7 (SD 3.3) early afternoon, and 1.6 (SD 1.8) bedtime although direct comparisons cannot be made due to different assay methods. Cortisol values 30-40 minutes after awakening are typically 50-100% higher than awakening values in 75% of normal healthy adults 3. However, in our sample, the median rise was only 18% higher than the wake value.
Our hypothesis that women with greater hot flash frequency would have a different pattern of cortisol compared to women with few hot flashes was supported. Higher hot flash frequency was significantly associated with lower mean cortisol values at the time point of wake + 30 minutes. Women with greater hot flashes also trended towards higher bedtime cortisol, and there was a trend toward a diminished diurnal variation among women with the greatest number of hot flashes, however neither of these were statistically significant.
Researchers have suggested that the cortisol awakening response reflects phasic physiologic processes specific to the sleep-wake transition 5. Given that the majority of women in the study reported poor sleep quality, it is possible that their poor sleep contributed to the decreased wake + 30 minute free cortisol concentrations associated with increased hot flashes, however, this did not translate into a lower awakening response in our study population. Among women with moderate to severe insomnia, both the bedtime cortisol value and diurnal rhythm were associated with the highest tertile of mean daily hot flash frequency, We are not aware of other studies that evaluated sleep, hot flashes and cortisol. However, associations between poor sleep and cortisol were described in the large Whitehall II study, where both sleep duration and sleep disturbances were independently associated with a flatter diurnal slope in cortisol secretion. In the Whitehall II study, evening cortisol secretion was higher in participants who reported short sleep duration and high sleep disturbance 11. Similarly, in a small laboratory-based study of 6 men and 5 women with chronic insomnia, the evening cortisol serum concentrations were high compared to 13 healthy controls without sleep problems 10.
Our hypotheses that women with more stress and anxiety would have more hot flashes and that stress, anxiety and higher hot flashes would strongly correlate with cortisol patterns were not supported. However, we did find that among all women with hot flashes, those with increased anxiety trended toward lower awakening response and higher bedtime cortisol concentrations. One small study correlated self-reported hot flashes with stress, although this was not corroborated when hot flashes were measured objectively 14. Notably psychological stress has been cited as one of the most common triggers of hot flashes. The small number of women in our study who reported high anxiety or high stress made it unlikely to find significant associations.
We are not aware of other reports of associations between daily salivary cortisol patterns and hot flashes in midlife women (search terms: cortisol, menopause, hot flashes, November 2015). Other investigators evaluated overnight urinary free cortisol, a measure of total cortisol production and not daily cortisol patterns 2. However, the findings indicated that midlife women with more severe hot flashes had increased overnight urinary cortisol compared to women who reported fewer hot flashes 28. Similarly another study found increased overnight urinary cortisol in women with higher Greene Climacteric scores in the early postmenopausal period 29. In contrast, a longitudinal study showed higher overnight urinary cortisol was associated with a decrease in the number of hot flashes that same day or on the following day 30. These mixed results suggest that overnight urinary cortisol may not be a good measure of chronic menopausal symptom burden, but instead is a marker of acute hot flash frequency (i.e. hot flashes measured the same day as cortisol measurement).
We did not have sufficient numbers of women in our study to examine the association of hot flashes, cortisol and race. However, African American women in our study, irrespective of hot flashes, had bedtime free cortisol concentrations significantly higher than those of White women and a trend toward morning cortisol concentrations significantly lower than those of White women. These findings are consistent with previous reports from larger population-based studies, where cortisol levels differed significantly between African American and White women 6-8.
Limitations of our study are important to consider. Our study was not designed to identify associations of acute cortisol fluctuations with acute onset of a hot flash. Although a previous laboratory-based study with indwelling catheters in postmenopausal women evaluated acute cortisol response related to hot flashes and showed that an acute cortisol rise followed a hot flash 31, these studies have not been replicated. Another limitation is the lack of a comparison group of women without hot flashes, and subtle differences in cortisol values could have been missed due to sample size. The fact that our results do not confirm prior studies 10,11,25,32 may be related to greater homogeneity among our sample on a variety of health parameters as a result of strict inclusion and exclusion criteria in the MsFLASH02 trial. Lastly, our sample was limited by excluding women who did not take their salivary samples within the specified time ranges or who had potential contamination of their samples. Despite the diminished sample size following these exclusions, this exploratory analysis provides important information for the design and execution of future studies.
Strengths of this study include rigorous protocol used for the collection of salivary samples at multiple time points over 2 consecutive days and the specific parameters used in the selection of samples used for statistical analysis. Salivary cortisol is an excellent measure of unbound cortisol, and it is unaffected by factors that affect serum cortisol binding globulin 18,33. The study included rich covariate data to evaluate associations of numerous potential confounders 24. The sample was community-based and relatively diverse in demographic characteristics, including African American women. Most importantly, the study establishes norms for circadian cortisol concentrations in midlife women with hot flashes.
Taken together, these findings suggest that a greater frequency of moderate to severe hot flashes in midlife women may be associated with subtle abnormalities in free cortisol concentrations in a pattern consistent with chronic sleep disturbance. More than 80% of US women report hot flashes 34 and the majority rate them as moderate to severe, so the potential magnitude of the impact of our findings is not inconsequential. Individuals with chronic sleep disturbance have greater health risks 35 – the long term health consequence of chronic frequent hot flashes and aberrant cortisol patterns warrants further study.
Acknowledgments
Support: This study was supported by a cooperative agreement issued by the National Institute of Aging (NIA), in collaboration with the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD), the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Research and Women's Health (ORWH), and grants U01 AG032656, U01AG032659, U01AG032669, U01AG032682, U01AG032699, U01AG032700 from the NIA and an administrative supplement for the Seattle site U01 AG032682. At the Indiana University site, the project was funded in part with support from the Indiana Clinical and Translational Sciences Institute, funded in part by grant UL1 RR025761 from the National Institutes of Health, National Center for Research Resources, Clinical and Translational Sciences Award.
We appreciate the assistance of Ms. Lisa Temposky and Ms. Lynn Fleckenstein in coordinating salivary data collection. Dr. Joffe has grant support from Cephalon/Teva and Merck, and is an advisor/consultant to Noven, Merck, and Mitsubishi Tanabe. None of the other authors have financial relationships pertinent to this article.
References
- 1.Powell LH, Lovallo WR, Matthews KA, et al. Physiologic markers of chronic stress in premenopausal, middle-aged women. Psychosomatic Medicine. 2002;64:502–509. doi: 10.1097/00006842-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Kudielka BM, Gierens A, Hellhammer DH, et al. Salivary cortisol in ambulatory assessment--some dos, some don'ts, and some open questions. Psychosomatic Medicine. 2012;74:418–431. doi: 10.1097/PSY.0b013e31825434c7. [DOI] [PubMed] [Google Scholar]
- 3.Kunz-Ebrecht SR, Kirschbaum C, Steptoe A. Work stress, socioeconomic status and neuroendocrine activation over the working day. Social Science & Medicine. 2004;58:1523–1530. doi: 10.1016/S0277-9536(03)00347-2. [DOI] [PubMed] [Google Scholar]
- 4.Williams E, Magid K, Steptoe A. The impact of time of waking and concurrent subjective stress on the cortisol response to awakening. Psychoneuroendocrinology. 2005;30:139–148. doi: 10.1016/j.psyneuen.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Wilhelm I, Born J, Kudielka BM, et al. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007;32:358–366. doi: 10.1016/j.psyneuen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 6.DeSantis AS, Adam EK, Doane LD, et al. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. Journal of Adolescent Health. 2007;41:3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Cohen S, Schwartz JE, Epel E, et al. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosomatic Medicine. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- 8.Skinner ML, Shirtcliff EA, Haggerty KP, et al. Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Development and Psychopathology. 2011;23:1167–1186. doi: 10.1017/S095457941100054X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 10.Vgontzas AN, Bixler EO, Lin HM, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. Journal of Clinical Endocrinology and Metabolism. 2001;86:3787–3794. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 11.Kumari M, Badrick E, Ferrie J, et al. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. Journal of Clinical Endocrinology and Metabolism. 2009;94:4801–4809. doi: 10.1210/jc.2009-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swartzman LC, Edelberg R, Kemmann E. Impact of stress on objectively recorded menopausal hot flushes and on flush report bias. Health Psychology. 1990;9:529–545. doi: 10.1037//0278-6133.9.5.529. [DOI] [PubMed] [Google Scholar]
- 13.Freeman EW, Sammel MD, Lin H, et al. The role of anxiety and hormonal changes in menopausal hot flashes. Menopause. 2005;12:258–266. doi: 10.1097/01.gme.0000142440.49698.b7. [DOI] [PubMed] [Google Scholar]
- 14.Thurston RC, Blumenthal JA, Babyak MA, et al. Emotional antecedents of hot flashes during daily life. Psychosomatic Medicine. 2005;67:137–146. doi: 10.1097/01.psy.0000149255.04806.07. [DOI] [PubMed] [Google Scholar]
- 15.Shifren JL, Desindes S, McIlwain M, et al. A randomized, open-label, crossover study comparing the effects of oral versus transdermal estrogen therapy on serum androgens, thyroid hormones, and adrenal hormones in naturally menopausal women. Menopause. 2007;14:985–994. doi: 10.1097/gme.0b013e31803867a. [DOI] [PubMed] [Google Scholar]
- 16.Vamvakopoulos NC, Chrousos GP. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction. Journal of Clinical Investigation. 1993;92:1896–1902. doi: 10.1172/JCI116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kravitz HM, Ganz PA, Bromberger J, et al. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19–28. doi: 10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- 18.Neary JP, Malbon L, McKenzie DC. Relationship between serum, saliva and urinary cortisol and its implication during recovery from training. Journal of Science and Medicine in Sport. 2002;5:108–114. doi: 10.1016/s1440-2440(02)80031-7. [DOI] [PubMed] [Google Scholar]
- 19.Newton KM, Carpenter JS, Guthrie KA, et al. Methods for the design of vasomotor symptom trials: the menopausal strategies: finding lasting answers to symptoms and health network. Menopause. 2014;21:45–58. doi: 10.1097/GME.0b013e31829337a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sternfeld B, LaCroix A, Caan BJ, et al. Design and methods of a multi-site, multi-behavioral treatment trial for menopausal symptoms: The MsFLASH experience. Contemporary Clinical Trials. 2012;35:25–34. doi: 10.1016/j.cct.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sternfeld B, Guthrie KA, Ensrud KE, et al. Efficacy of exercise for menopausal symptoms: a randomized controlled trial. Menopause. 2014;21:330–338. doi: 10.1097/GME.0b013e31829e4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newton KM, Reed SD, Guthrie KA, et al. Efficacy of yoga for vasomotor symptoms: a randomized controlled trial. Menopause. 2014;21:339–346. doi: 10.1097/GME.0b013e31829e4baa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen LS, Joffe H, Guthrie KA, et al. Efficacy of omega-3 treatment for vasomotor symptoms: a randomized controlled trial. Menopause. 2014;21:347–354. doi: 10.1097/GME.0b013e31829e40b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen AM, Garde AH, Persson R. Sources of biological and methodological variation in salivary cortisol and their impact on measurement among healthy adults: a review. Scandinavian Journal of Clinical and Laboratory Investigation. 2008;68:448–458. doi: 10.1080/00365510701819127. [DOI] [PubMed] [Google Scholar]
- 25.Abraham SB, Rubino D, Sinaii N, et al. Cortisol, obesity, and the metabolic syndrome: a cross-sectional study of obese subjects and review of the literature. Obesity. 2013;21:E105–E117. doi: 10.1002/oby.20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ZRT Laboratory. Saliva Reference Range Determination. ZRT Laboratory; 2014. Ref Type: Online Source. [Google Scholar]
- 27.Karlamangla AS, Friedman EM, Seeman TE, et al. Daytime trajectories of cortisol: demographic and socioeconomic differences--findings from the National Study of Daily Experiences. Psychoneuroendocrinology. 2013;38:2585–2597. doi: 10.1016/j.psyneuen.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woods NF, Carr MC, Tao EY, et al. Increased urinary cortisol levels during the menopausal transition. Menopause. 2006;13:212–221. doi: 10.1097/01.gme.0000198490.57242.2e. [DOI] [PubMed] [Google Scholar]
- 29.Cagnacci A, Cannoletta M, Caretto S, et al. Increased cortisol level: a possible link between climacteric symptoms and cardiovascular risk factors. Menopause. 2011;18:273–278. doi: 10.1097/gme.0b013e3181f31947. [DOI] [PubMed] [Google Scholar]
- 30.Woods NF, Mitchell ES, Smith-Dijulio K. Cortisol levels during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women's Health Study. Menopause. 2009;16:708–718. doi: 10.1097/gme.0b013e318198d6b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meldrum DR, Defazio JD, Erlik Y, et al. Pituitary hormones during the menopausal hot flash. Obstetrics & Gynecology. 1984;64:752–756. [PubMed] [Google Scholar]
- 32.Knight JM, Avery EF, Janssen I, et al. Cortisol and depressive symptoms in a population-based cohort of midlife women. Psychosomatic Medicine. 2010;72:855–861. doi: 10.1097/PSY.0b013e3181f4ab87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Putignano P, Dubini A, Toja P, et al. Salivary cortisol measurement in normal-weight, obese and anorexic women: comparison with plasma cortisol. European Journal of Endocrinology. 2001;145:165–171. doi: 10.1530/eje.0.1450165. [DOI] [PubMed] [Google Scholar]
- 34.Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Internal Medicine. 2015;175:531–539. doi: 10.1001/jamainternmed.2014.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parthasarathy S, Vasquez MM, Halonen M, et al. Persistent insomnia is associated with mortality risk. American Journal of Medicine. 2015;128:268–275. doi: 10.1016/j.amjmed.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]