Supplemental Digital Content is available in the text
Keywords: Breast cancer, mastectomy, surgery, trend
Abstract
The aim of the study was to review the surgical trends in breast cancer treatment in China over the past 15 years and to explore the possible factors related to the choice of surgical modality.
The medical records of 18,502 patients with unilateral early stage breast cancer who underwent surgery from January 1999 to December 2013 at our institute were retrospectively reviewed. The utilization of different surgical modalities and the associated clinicopathological factors were analyzed. Furthermore, the prognostic role of surgical modality was also evaluated.
The median patient age was 50.0 years. According to the pTNM staging system, 12.5% of the patients were classified as stage 0; 30.2% as stage I; 40.0% as stage II; and 17.3% as stage III. In total, 9.3% of the patients could not be staged. Overall, 67.1% of the breast cancer cases were estrogen receptor (ER) positive. The pattern of breast cancer surgery has changed tremendously over the past 15 years (P < 0.001). The pattern of mastectomy has shifted from radical mastectomy to modified radical mastectomy and simple mastectomy + sentinel lymph node biopsy. A total of 81.7% of the patients underwent mastectomy without immediate reconstruction, 15.2% underwent breast-conserving surgery (BCS), and 3.7% received immediate breast reconstruction after mastectomy. Age, TNM staging, and pathological characteristics greatly affected the choice of surgical modality. The 5-year recurrence-free survival (RFS) rates for the mastectomy, BCS, and reconstruction groups were 87.6%, 93.2%, and 91.7%, respectively (P < 0.001); the RFS rate was likely affected by distant recurrence instead of loco-regional recurrence. We also identified improved RFS over time, stratified by surgical modality and tumor stage. Multivariate Cox-regression analysis revealed that time of treatment, tumor stage, tumor grade, LVI status, and ER status were independent prognostic factors for RFS in our cohort, whereas surgical modality was not.
Mastectomy remains the most prevalent surgical modality used to manage early stage breast cancer in China, although the utilization of BCS has increased in the past decade. However, surgical management was not a prognostic factor for RFS. The selection of appropriate patients depended on the assessment of multiple clinicopathological factors, which is essential for making surgical decisions.
1. Introduction
Breast cancer is one of the most commonly observed malignancies among Chinese women. Since the 1990s, the incidence of breast cancer in China has accelerated at twice the rate worldwide.[1] It has been estimated that the incidence of breast cancer in China will increase to 100/100,000 in the 55- to 69-year-old age group and that there will be 2,500,000 breast cancer patients in 2021.[2]
Surgery plays an important role in the multidisciplinary treatment of early-stage breast cancer. With improvements in systemic treatments, the concept of breast surgery has changed over time. Large clinical trials have demonstrated no survival difference between patients who received breast-conserving surgery (BCS) versus mastectomy.[3] Additionally, although the rate of loco-regional recurrence (LRR) of breast cancer decreased from 30% in 1990 to 15% in 2011, there was no interaction with surgical modality.[4] BCS has become the primary surgical treatment for breast cancer worldwide, and ∼60% to 70% of stage 0–II patients in the United States undergo BCS.[5] However, in recent years, some studies have reported an increasing trend for mastectomy, partly due to the increased use of pre-operative magnetic resonance imaging (MRI), breast reconstruction, and patient choice.[5,6] The surgical management of breast cancer in China has also undergone tremendous change over recent decades.[7] A multicenter retrospective study in China indicated that modified radical mastectomy remained the primary strategy for treating breast cancer and that the utilization of BCS remained limited.[8]
The current study retrospectively reviewed the changes and trends in the surgical management of breast cancer at a single center in Shanghai from 1999 to 2013 and analyzed the factors related to the use of different surgical modalities. Furthermore, we analyzed the impact of surgical management on patient outcome.
2. Methods
2.1. Patients
Patients who underwent breast cancer surgery from January 1999 to December 2013 in the Department of Breast Surgery at Fudan University Shanghai Cancer Center (FUSCC) were included in the study. Patients with recurrent disease or bilateral or metastatic breast cancer at initial diagnosis were excluded. Pathology assessments were performed based on the final paraffin embedded section postoperatively according to international guidelines and standard routines at our center. The following data were retrospectively collected: patient age at diagnosis; body mass index (BMI); social-economical features; type and date of surgery; pathological diagnosis; tumor size; lymph node status; tumor grade; lymphovascular invasion (LVI); estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER-2) status; and neoadjuvant chemotherapy (NAC) prior to surgery. The 7th edition of the AJCC pathological TNM (pTNM) was utilized to stage the patients. The protocol for the present study was approved by the Ethics Committee of FUSCC.
2.2. Treatment and follow-up
The surgical treatments for breast cancer were grouped as follows: radical mastectomy, modified radical mastectomy, simple mastectomy ± sentinel lymph node biopsy, and breast-conserving surgery (BCS, lumpectomy ± sentinel lymph node biopsy). Cases with immediate breast reconstruction after mastectomy were also included in the study. The choice of surgical modality was based on preoperative imaging (breast ultrasound, mammogram, and MRI), clinical tumor size and lymph node status, contemporary guidelines and patient preferences. When indicated for BCS, patients routinely went for full imaging screening to eliminate occult breast lesions. Immediate breast reconstruction was recommended when tumor size was <5 cm and lymph node was negative upon preoperative evaluation, suggesting these patients were not likely to receive radiotherapy. On the contrary, when patients were prone to receive radiotherapy, delayed reconstruction was recommended.
The follow-up data on the breast cancer patients were acquired from the Department of Clinical Statistics of FUSCC. LRR was defined as any progression in the ipsilateral breast, skin, muscles of the chest wall and/or axillary/supraclavicular lymph nodes. Survival was calculated from the date of surgery to the date of clinical relapse. Patients whose last follow-up was ≤3 months after surgery were regarded as lost to follow-up and were not included in the survival analysis.
2.3. Statistical analysis
The clinicopathological characteristics of different surgical groups were compared using Pearson's χ2 test for categorical variables and the Kruskal–Wallis test for continuous variables. The Cochrane Armitage test was used to examine the trend of new breast cancer cases and surgical trend in our institute over time. Five-year LRR-free survival (LRRFS), distant recurrence-free survival (DRFS) and recurrence-free survival (RFS) were estimated using the Kaplan–Meier method, and differences were assessed with the log-rank test. Univariate and multivariate analyses for RFS was conducted using Cox-regression models. Two-tailed P values were adopted, and P < 0.05 was considered to be significant. All statistical analyses were performed using SPSS version 20.0 and SAS version 9.2.
3. Results
3.1. Baseline characteristics
From January 1999 to December 2013, a total of 18,502 patients underwent breast surgery for primary unilateral breast cancer at FUSCC. The average growth rate per annum was 16.8%. There was an increasing trend in new breast cancer cases every year at our institute (P < 0.001) (Supplementary Table 1).
Among the 18,502 patients, 99.5% were female. The median patient age at the time of surgery was 50.0 years (IQR: 44.0–59.0). The median BMI was 23.1 (IQR: 21.1–25.3), with 25.0% patients categorized as overweight and 3.2% as obese. According to the pTNM staging, 12.5% of the patients were stage 0; 30.2%, stage I; 40.0%, stage II; and 17.3%, stage III. Overall, 9.3% of the patients could not be staged. 67.1% of the breast cancer cases were ER positive, 27.9% were ER negative, and 5.0% were of unknown ER status.
A total of 2210 (11.9%) patients were lost to follow-up. The median follow-up time was 34.1 months (IQR: 15.7–59.8). The 5-year RFS, LRRFS, and DRFS rates were estimated to be 88.6%, 96.1%, and 90.1%, respectively. The 5-year RFS rates for the stage 0–I, stage II, and stage III patients were 96.8%, 90.3%, and 72.5%, respectively. A significant difference was detected among the groups (P < 0.001; Fig. 1). The 5-year LRRFS rates for the stage 0–I, stage II, and stage III patients were 98.1%, 96.7%, and 89.6%, respectively (P < 0.001); and 5-year DRFS rates were 96.1%, 89.8% and 73.5%, respectively (P < 0.001).
Figure 1.
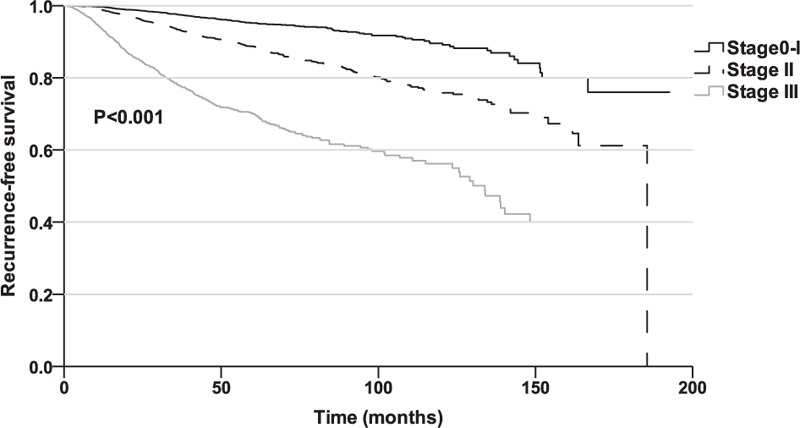
Recurrence-free survival among the breast cancer patients according to the pTNM stage.
3.2. Surgical management of breast cancer at FUSCC
The surgical management pattern for breast cancer has changed remarkably over the past 15 years (P < 0.001) (Supplementary Table 1). The percentage of radical mastectomy has decreased from 50.7% in 1999 to 1.3% in 2008, and it has since remained below 1%. Modified radical mastectomy experienced an increasing and then a decreasing trend, peaking in 2005 at 81.7% of the cases. Simple mastectomy ± sentinel lymph node biopsy increased steadily from 0.3% in 1999 to 31.9% in 2013. The percentage of BCS increased from 7.6% in 1999 to 16.6% in 2007; this percentage has remained at ∼18% in recent years (Fig. 2). Overall, mastectomy (84.8%) was the major surgical strategy used for primary breast cancer; in contrast, the percentage of BCS was only 15.2%.
Figure 2.
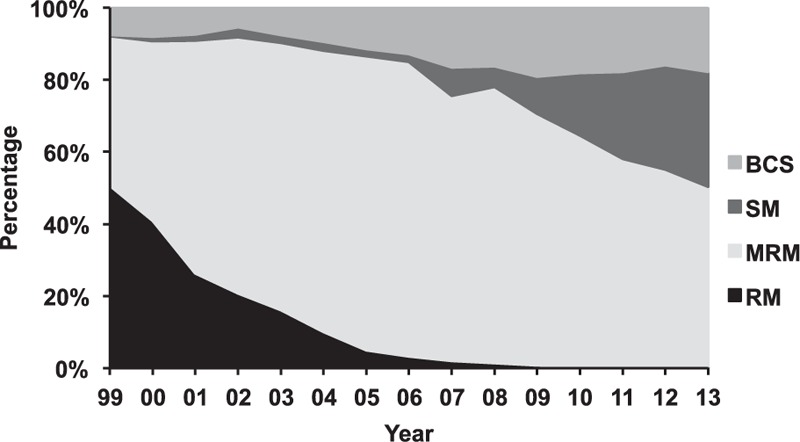
Trends in the surgical management of breast cancer from 1999 to 2013 at FUSCC. BCS = breast-conserving surgery, FUSCC = Fudan University Shanghai Cancer Center, MRM = modified radical mastectomy, RM = radical mastectomy, SM = simple mastectomy.
Among 15,691 mastectomy patients, 573 (3.7%) underwent immediate breast reconstruction. Although the number of such cases has increased over the years (from 1 case in 2001 to 98 cases in 2013), the percentage of immediate breast reconstruction cases has remained as low as 4%.
3.3. The choice of surgical management in different patient subgroups
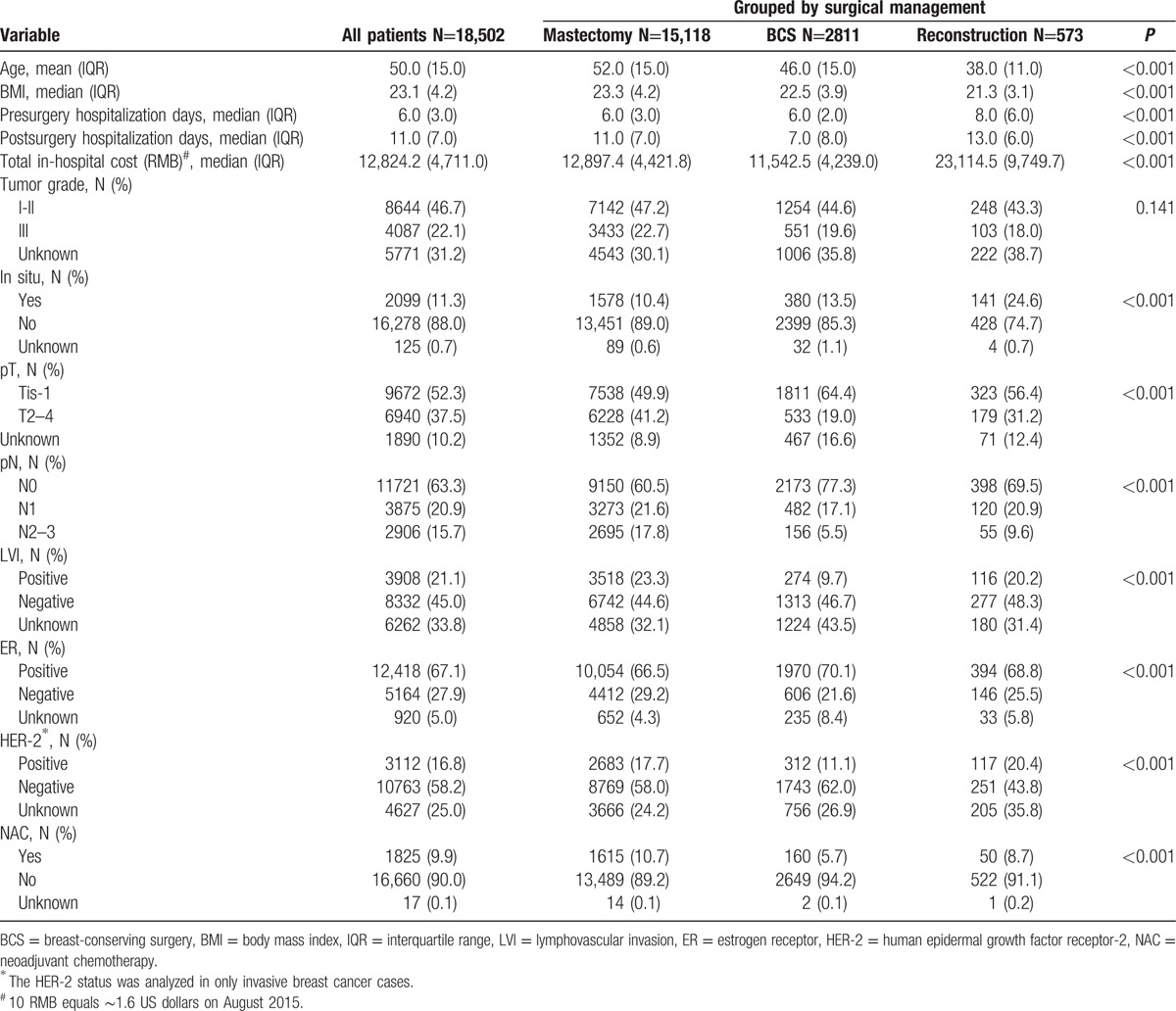
All patients were categorized into 3 surgical management groups: mastectomy without immediate reconstruction (N=15,118; 81.7%), BCS (N=2811; 15.2%), and mastectomy with immediate reconstruction (N=573; 3.7%). Major differences were identified in the clinicopathological characteristics of the patients in the different groups (Table 1). The median ages at the time of surgery in the 3 groups were 52, 46, and 38 years, respectively (P < 0.001). The reconstruction group had the longest pre- and postsurgery hospitalization times and the highest total in-hospitalization cost (P < 0.001). Patients in the BCS group were more likely to have small tumors and node negative diseases (P < 0.001) and to be LVI negative (P < 0.001) and ER positive (P < 0.001). Conversely, patients in the mastectomy group were diagnosed with relatively advanced disease. The reconstruction group had the highest percentage of cases of in-situ and HER-2 positive disease (P < 0.001). In total, 10.7%, 5.7%, and 8.7% of the patients in the 3 groups, respectively (P < 0.001), received NAC prior to surgery.
Table 1.
Clinicopathological characteristics of the different surgical groups.
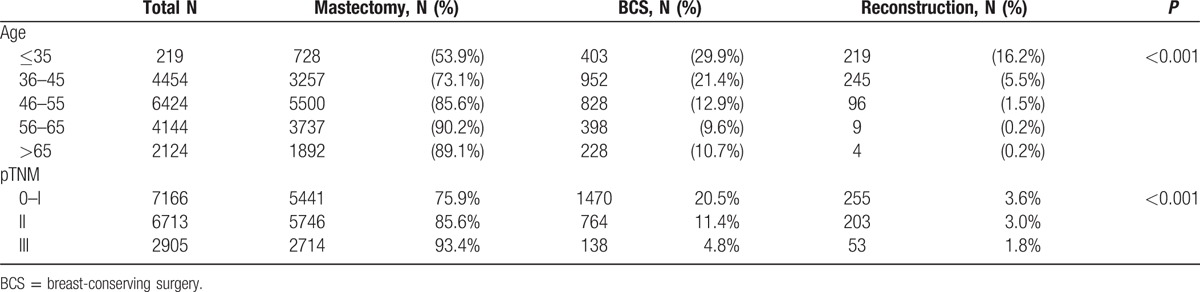
To investigate the correlation between age and surgical modality, the patients were categorized into 5 age groups: 35 years and younger, 36 to 45 years, 46 to 55 years, 56 to 65 years, and >65 years. The choice of surgical modality in the different age groups is shown in Table 2. Pearson's χ2 test revealed a significant difference among the groups (P < 0.001). Patients <35 years formed the highest percentages to be treated with BCS (29.9%) and breast reconstruction (16.2%). There was an increasing trend for mastectomy in older patients. In addition to the effect of age, the pTNM stage also played an important role. For the pTNM 0–I stage patients, 20.5% underwent BCS, and 3.6% underwent breast reconstruction. However, 93.4% of the stage III patients underwent mastectomy. These differences were significant (P < 0.001; Table 2).
Table 2.
Choice of surgical modality in the different ages and pTNM groups.
3.4. RFS and LRRFS in the different surgical groups
To investigate the impact of surgical management on patient survival, RFS, LRRFS and DRFS were compared among the 3 surgical groups. In total, 8.9%, 4.1%, and 5.4% of the patients in the mastectomy, BCS and reconstruction groups, respectively, developed a recurrence during follow-up. The 5-year RFS rates among the mastectomy, BCS, and reconstruction groups were 87.6%, 93.2%, and 91.7%, respectively (P < 0.001; Fig. 3A). The 5-year LRRFS rates for the 3 groups were 96.0%, 96.5%, and 96.0%, respectively, and no differences were detected (P = 0.058; Fig. 3B). The 5-year DRFS rates for the mastectomy, BCS, and reconstruction groups were 89.0%, 94.8%, and 93.6%, respectively (P < 0.001; Fig. 3C). When the analysis was restricted to the stage 0–II patients, no difference in LRRFS was observed (P = 0.434).
Figure 3.
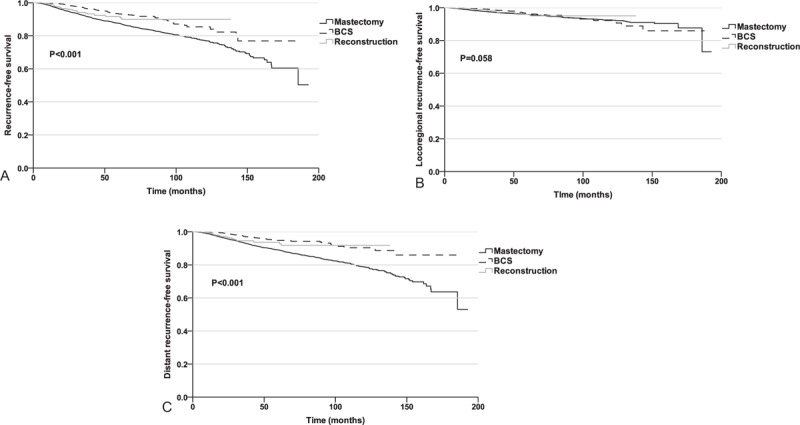
(A) Recurrence-free survival rates in the different surgical groups. (B) Loco-regional recurrence-free survival in the different surgical groups. (C) Distant recurrence-free survival in the different surgical groups.
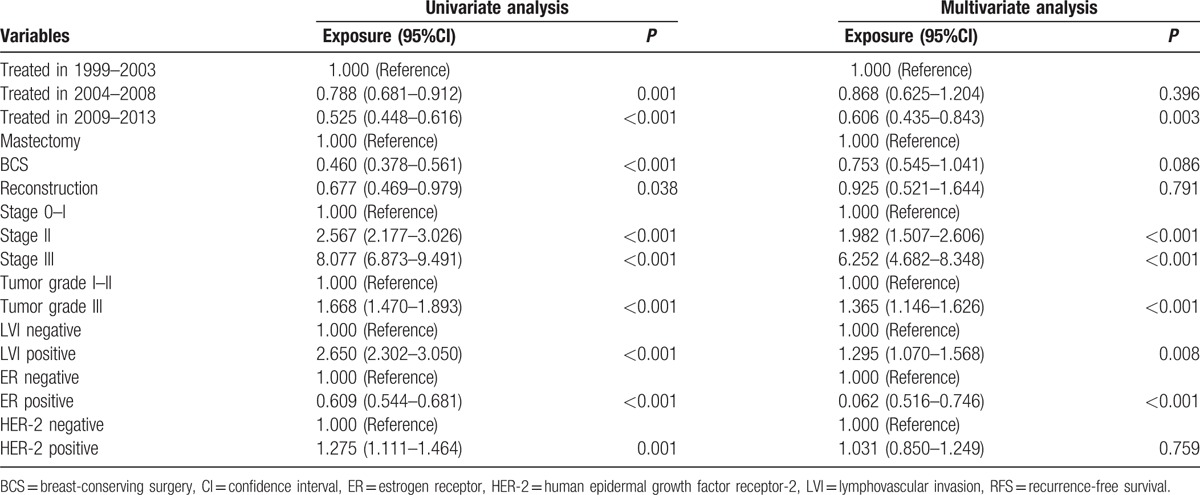
Most interestingly, when we conducted a survival analysis of the temporal trend, we identified improved survival over time (P < 0.001). The 5-year RFS for patients treated between 1999–2003, 2004–2008 and 2009–2013 were 84.4%, 87.3% and 90.3%, respectively (Table 3). This trend remained statistically significant when stratified by surgical management and pTNM stage, excluding only highly selected patients received BCS in the early stage and thus resulted in better survival. Lastly, multivariate Cox-regression analysis revealed that time of treatment, tumor stage, tumor grade, LVI status, and ER status were independent prognostic factors for RFS in our cohort, whereas surgical management was not (Table 4).
Table 3.
The temporal trend of 5-year RFS stratified by surgical management and pTNM stage.
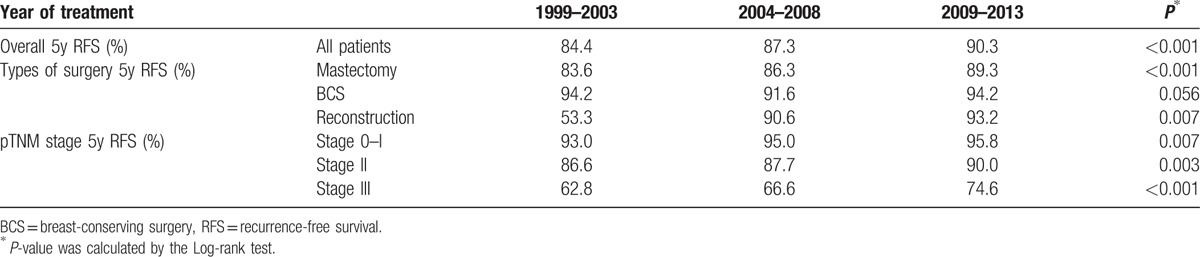
Table 4.
Univariate and multivariate analyses of RFS in breast cancer patients using the Cox regression model.
4. Discussion
At our institute, mastectomy remains the predominant surgical modality for treating primary breast cancer. However, the type of mastectomy has shifted from radical mastectomy to modified radical mastectomy and simple mastectomy + sentinel lymph node biopsy. The remarkable increase in simple mastectomy surgeries at our institute from 2006 to 2013 correlated with the introduction of sentinel lymph node biopsy in 2005. The trend of less extensive surgery relied on the early detection of breast cancer and on improved systemic therapies, such as NAC. Currently, BCS, sentinel lymph node biopsy and breast reconstruction are performed only in leading hospitals in China. The surgical modality pattern may differ between high- and low-economic areas. Modified radical mastectomy may remain predominant in the future before these techniques are embraced by more institutes.
The percentage of BCS remains low, which correlates with other studies from China that have indicated that the percentage of BCS was only 11.2% nationwide.[9] However, BCS is used to treat up to 60% of early breast cancer cases worldwide.[10,11] There are multiple potential reasons for the low rate of BCS. First, in less developed areas, radiotherapy is not available for BCS patients.[12] Second, even if BCS were available, some breast cancer patients worry about cancer recurrence and request a mastectomy because of the lack of patient education and communication. It is reported that some Chinese women were unaware of the health information about breast cancer and they were not confident to the efficacy of BCS.[8] Furthermore, the cost of BCS plus postoperative radiotherapy is greater than that of mastectomy alone, which is a consideration for low-income families.[13] Finally, more than half of female patients are diagnosed with breast cancer before menopause,[9] with dense breast tissue appearing on mammograms. Studies have suggested that patients with dense breasts were more likely to undergo a mastectomy than BCS.[14,15] However, an opposite trend has been observed in the United States; mastectomy has been increasing in recent decades.[16,17] The increasing mastectomy rate might be associated with MRI detection, genetic tests for BRCA1 and BRCA2, and prophylactic mastectomy. Data from SEER suggested that from 1998 to 2003, the percentage of contralateral prophylactic mastectomy increased from 4.2% to 11.0%.[18]
The clinicopathological differences among the mastectomy, BCS and mastectomy + reconstruction groups suggest that several dominant factors affect the choice of surgery, including age. Patients who underwent immediate breast reconstruction were the youngest, and mastectomy patients were the oldest, indicating that age affects the recognition of quality of life in Chinese women. As for tumor burden, the mastectomy group had the latest disease onset, and the BCS group had the earliest disease onset. The disease onset in the reconstruction group was between these 2 groups, which suggested that patients who were not suitable for BCS would consider mastectomy plus reconstruction to decrease the risk of local recurrence and to preserve body image. Since ER, PR, HER-2, and LVI status were not available until postoperatively, it remained unknown whether these factors would affect the choice of surgery in the Chinese population. Furthermore, from the perspective of cost-effectiveness, the reconstruction group had the longest preoperative in-hospital stay, indicating a longer time period for patient–physician communication and higher treatment cost. Families with a limited income and insurance budget were forced to consider the in-hospitalization cost. NAC was reported to affect the trends in surgical modalities because NAC increases the percentage of patients undergoing BCS by shrinking the tumor.[19] However, the current study found that patients who received NAC tended to undergo mastectomy. One possible explanation for this finding is that at our institute, NAC was primarily used for locally advanced patients who would later undergo mastectomy. NAC with different regimens and treatment cycles might influence the choice of surgical modality by achieving better tumor shrinkage and therefore the surgical treatment of this subset of patients should be further investigated in the future. Besides, future studies should also include patients’ family income, literacy, and preoperative imaging pathology results from preoperative biopsy to explore the factors that affect the choice of surgery.
In the survival analysis, there was a difference in patient outcome in RFS and DRFS among BCS, mastectomy, and reconstruction, indicating that the difference in RFS was mainly attributable to DR rather than LRR. DR accounted for 79% of all patients with recurrent breast cancer. The difference in RFS and DRFS was also affected by the heterogeneity among the 3 surgical groups. When the analysis was confined to the stage 0–II patients, BCS, mastectomy, and reconstruction had comparable local control. These results are consistent with previous studies that the BCS survival rate is not inferior to that of mastectomy during long-term follow-up.[20–22] Most interestingly, further analysis suggested that instead of surgical modality, the time of treatment was an independent prognostic factor of RFS. A prior study from FUSCC regarding the status of breast reconstruction also manifested that the disease-free survival of patients who had undergone BCS was better than mastectomy; however, multiple regression analysis revealed that types of surgeries did not affect the breast cancer-specific disease-free survival.[23] As adjuvant therapies have advanced over time, it is likely that improved adjuvant therapies (chemotherapy, radiotherapy, and endocrine therapy) benefited patients’ loco-regional control as well as overall survival. Bouganim et al demonstrated that, the LRR rates of breast cancer decreased from 30% to 15% between 1990 and 2011. Although there was no interaction between type of surgery and the correlation of loco-regional recurrences, both chemotherapy regimen and endocrine therapy were negatively correlated with LRR.[24] Another study reported that in lymph node-negative, HER-2 positive patients with BCS, the 3-year LRR for those who received trastuzumab was 1% compared to 10% for patients who did not receive trastuzumab.[25]
The current study reviewed 18,502 breast cancer patients from 1999 to 2013 and described the surgical trends during this period. Using the patients’ clinicopathological files and follow-up data, we identified major differences in the clinicopathological characteristics among the surgical groups that may have affected the choice of surgery modality. Nevertheless, we failed to demonstrate a correlation between surgical treatment and patient prognosis. The breast cancer outcomes depended on multiple factors, such as systemic therapy. Additionally, overall survival was not included in the analysis because complete data were not available. Due to the gap between China and developed countries, studies with longer follow-up times in China are awaited to demonstrate trends in breast surgery and patient outcome in an Eastern population.
Supplementary Material
Footnotes
Abbreviations: BCS = breast-conserving surgery, BMI = body mass index, DRFS = distant recurrence-free survival, ER = estrogen receptor, FUSCC = Fudan University Shanghai Cancer Center, HER-2 = human epidermal growth factor receptor-2, IQR = interquartile range, LRR = loco-regional recurrence, LRRFS = LRR-free survival, LVI = lymphovascular invasion, MRI = magnetic resonance imaging, NAC = neoadjuvant chemotherapy, PR = progesterone receptor, RFS = recurrence-free survival, RFS = recurrence-free survival.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917. [DOI] [PubMed] [Google Scholar]
- 2.Linos E, Spanos D, Rosner BA, et al. Effects of reproductive and demographic changes on breast cancer incidence in China: a modeling analysis. J Natl Cancer Inst 2008; 100:1352–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002; 347:1227–1232. [DOI] [PubMed] [Google Scholar]
- 4.Bouganim N, Tsvetkova E, Clemons M, et al. Evolution of sites of recurrence after early breast cancer over the last 20 years: implications for patient care and future research. Breast Cancer Res Treat 2013; 139:603–606. [DOI] [PubMed] [Google Scholar]
- 5.McGuire KP, Santillan AA, Kaur P, et al. Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol 2009; 16:2682–2690. [DOI] [PubMed] [Google Scholar]
- 6.Pomahac B, Recht A, May JW, et al. New trends in breast cancer management: is the era of immediate breast reconstruction changing? Ann Surg 2006; 244:282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu KD, Di GH, Wu J, et al. Development and trends of surgical modalities for breast cancer in China: a review of 16-year data. Ann Surg Oncol 2007; 14:2502–2509. [DOI] [PubMed] [Google Scholar]
- 8.Zhang B, Song Q, Zhang B, et al. A 10-year (1999–2008) retrospective multi-center study of breast cancer surgical management in various geographic areas of China. Breast 2013; 22:676–681. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Zhang BN, Fan JH, et al. A nation-wide multicenter 10-year (1999–2008) retrospective clinical epidemiological study of female breast cancer in China. BMC Cancer 2011; 11:364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee MC, Rogers K, Griffith K, et al. Determinants of breast conservation rates: reasons for mastectomy at a comprehensive cancer center. Breast J 2009; 15:34–40. [DOI] [PubMed] [Google Scholar]
- 11.Smith GL, Xu Y, Shih YC, et al. Breast-conserving surgery in older patients with invasive breast cancer: current patterns of treatment across the United States. J Am Coll Surg 2009; 209:425–433.e2. [DOI] [PubMed] [Google Scholar]
- 12.Salminen E, Izewska J, Andreo P. IAEA's role in the global management of cancer-focus on upgrading radiotherapy services. Acta Oncologica 2005; 44:816–824. [DOI] [PubMed] [Google Scholar]
- 13.Zhang B. Current status and development of breast conserving surgery. Chin J Pract Surg 2008; 28:523–524. [Google Scholar]
- 14.Tan YO, Han S, Lu YS, et al. The prevalence and assessment of ErbB2-positive breast cancer in Asia: a literature survey. Cancer 2010; 116:5348–5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapoor NS, Eaton A, King TA, et al. Should breast density influence patient selection for breast-conserving surgery? Ann Surg Oncol 2013; 20:600–606. [DOI] [PubMed] [Google Scholar]
- 16.Katipamula R, Degnim AC, Hoskin T, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol 2009; 27:4082–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones NB, Wilson J, Kotur L, et al. Contralateral prophylactic mastectomy for unilateral breast cancer: an increasing trend at a single institution. Ann Surg Oncol 2009; 16:2691–2696. [DOI] [PubMed] [Google Scholar]
- 18.Tuttle TM, Habermann EB, Grund EH, et al. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol 2007; 25:5203–5209. [DOI] [PubMed] [Google Scholar]
- 19.Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 1997; 15:2483–2493. [DOI] [PubMed] [Google Scholar]
- 20.Fisher B, Jeong JH, Anderson S, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med 2002; 347:567–575. [DOI] [PubMed] [Google Scholar]
- 21.Veronesi U, Saccozzi R, Del Vecchio M, et al. Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med 1981; 305:6–11. [DOI] [PubMed] [Google Scholar]
- 22.Jatoi I, Proschan MA. Randomized trials of breast-conserving therapy versus mastectomy for primary breast cancer: a pooled analysis of updated results. Am J Clin Oncol 2005; 28:289–294. [DOI] [PubMed] [Google Scholar]
- 23.Chen JJ, Huang NS, Xue JY, et al. Current status of breast reconstruction in Southern China: a 15 year, single institutional experience of 20,551 breast cancer patients. Medicine (Baltimore) 2015; 94:e1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouganim N, Tsvetkova E, Clemons M, et al. Evolution of sites of recurrence after early breast cancer over the last 20 years: implications for patient care and future research. Breast Cancer Res Treat 2013; 139:603–606. [DOI] [PubMed] [Google Scholar]
- 25.Kiess AP, McArthur HL, Mahoney K, et al. Adjuvant trastuzumab reduces locoregional recurrence in women who receive breast-conservation therapy for lymph node-negative, human epidermal growth factor receptor 2-positive breast cancer. Cancer 2012; 118:1982–1988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


