Abstract
Th2 and Th17 cells are both associated with developing ankylosing spondylitis (AS) and asthma. Th2 cells are also associated with allergic rhinitis and atopic dermatitis (AD). The prevalence of such allergic diseases in AS patients is unknown. In this study, we intended to study the risk of allergic diseases in a 10-year follow-up population of newly diagnosed patients with AS. We used a nationwide 10-year population-based database retrieved from the Longitudinal Health Insurance Database 2005 (LHID2005) in Taiwan. The study cohort comprised 857 patients with AS who had at least 1 claim of inpatient admission or at least 2 claims of ambulatory visit. The comparison cohort consisted of 4285 randomly selected subjects matched with AS group at a ratio of 5:1. We used Cox proportional-hazards regression to determine the 10-year disease-free survival rates after adjusting for potentially confounding factors. The AS patients had a 1.31 times greater risk of developing asthma within 10 years of diagnosis when compared with non-AS age- and sex-matched subjects, after adjusting for other risk factors (95% confidence interval = 1.00–1.75). But the difference was not significantly different. The AS patients also had a 1.46 times and a 1.22 times greater risk of developing allergic rhinitis and AD significantly. AS patients also had a lower allergic disease-free survival rate compared to non-AS group. Our results showed that patients with AS had a higher risk of developing allergic diseases later in life.
Keywords: allergic disease, allergic rhinitis, ankylosing spondylitis, asthma, atopic dermatitis, population-based study
1. Introduction
Ankylosing spondylitis (AS) is a systemic inflammatory disease affecting predominantly not only the axial skeleton but also the peripheral joints, uveitis, and aortic root. The primary site of inflammation in AS is in the area where ligaments insert into the bone. AS usually begins in the second or third decade in life and affects more men than women.[1–2] The prevalence of AS is estimated at 0.2% to 0.5% in American and at 0.4% in Taiwan.[3–4]
Anti-tumor necrosis factor (anti-TNF)-α is efficacious to treat AS through counteracting proinflammatory cytokines which are the causes for mediating inflammation and joint destruction. T helper (Th) 1 and cytotoxic T cells predominantly produce interferon-γ and interleukin 2 (IL-2). These cell subsets are primarily involved in cell-mediated immune responses. Th2 mainly expresses in IL-4, IL-5, and IL-13, and play a pivotal role in humoral and atopic responses.[5] Compared to healthy controls, the patients with AS have a higher prevalence of Th2 and Th17 cells to regulate their adaptive immunity.[6]
Asthma which is a common chronic disorder of the airways, is complex and characterized by variable and recurring symptoms, airflow obstruction, bronchial hyper-responsiveness, and an underlying inflammation.[7] The prevalence of asthma is about 7.3% to 8.2% in America and 11.9% in Taiwan.[8–9] T helper cells are involved in the inflammatory process of asthma and play an important role.[10] Th2 and Th17 cells are found to be associated with patients with asthma.[11–13] Th2 cells are also found to be important in causing other allergic diseases, such as allergic rhinitis and atopic dermatitis (AD).[14–15]
Th2 and Th17 cells are both associated with AS and asthma, and Th2 cells are also associated with allergic rhinitis and AD. We wonder whether patients with AS have higher incidence of those allergic diseases. With a population-based cohort database, we intended to examine the risk of those allergic diseases in patients with the diagnosis of AS in Taiwan.
2. Methods
2.1. Research database
The single-payer compulsory National Health Insurance (NHI) program was initiated in Taiwan in 1995. The program provides universal coverage to most Taiwanese residents. The NHI Research Database (NHIRD) provided data of complete outpatient visits, hospital admissions, prescriptions, catastrophic illness information, and vital status of 99% of the 23 million population of Taiwan. The bureau of the NHI released the NHIRD for research purposes. In this study, we adopted the data obtained through the Longitudinal Health Insurance Database 2005 (LHID 2005), from the NHIRD. This LHID 2005 database included registration and medical claims for 1,000,000 randomly sampled patients from the total number of NHRI enrollees. LHID2005 data set allows researchers to follow-up on all the medical services utilized by these 1,000,000 individuals since the initiation of the NHI in 1995.
To protect individual privacy, original identification numbers of the beneficiaries’ data were encrypted for privacy. Encryption procedures were consistent with other datasets and ensured that all claims data could be linked in order to obtain additional medically relevant data. All encrypted data that could be used to identify patients or care providers, including the names of medical institutions and physicians. The study protocol was approved by the institute review board of Chung Shan Medical University Hospital, Taichung, Taiwan without the need of any informed consent.
2.2. Study population
The study cohort consists of patients who were newly diagnosed with AS (ICD-9-Code 720.0) between January 1998 and December 2000. Cases were only included if patients had a diagnosis of AS made by an orthopedist, a neurosurgeon, or a rheumatologist in outpatient clinic visits more than 2 times, or more than once in inpatient service to ensure the diagnostic accuracy. Between January 1998 and December 2000, the initial diagnosis date of AS was assigned as the index date for each patient. We excluded patients with AS before or after the enrollment period and those who had previously experienced asthma (ICD-9-CM 493.X), allergic rhinitis (ICD-9-CM 477.X) or AD (ICD-9-CM 691.0).
Our comparison cohort was randomly sampled from the remaining subjects of the LHID2005 data set. Excluded were those who were diagnosed with or had a history of AS, asthma, allergic rhinitis, or AD. The final comparison cohort was chosen from this representative data set through random selection to match a control-to-case ratio of 5 on the basis of age, sex, and index year. Several comorbidities such as hypertension (ICD-9-CM 401.X-405.X), zoster (ICD-9-CM 053.X, 054.X), gout (ICD-9-CM 274.X), rheumatoid arthritis (ICD-9-CM 714.X), chronic obstructive pulmonary disease (COPD) (ICD-9-CM 490.X-496.X), hyperlipidemia (ICD-9-CM 272.X) and diabetes (ICD-9-CM 250.X) were included in our analytical model.
Each subject was followed for 10 years from index date to identify if they suffered from asthma, allergic rhinitis, or AD. The incidence of asthma was defined if a patient had a diagnosis made by a pediatrician, an otorhinolaryngologist, a general medical physician, or a pulmonologist in outpatient clinic visits more than 2 times, or more than once in inpatient service. The incidence of allergic rhinitis was defined if a patient had a diagnosis made by a pediatrician or an otorhinolaryngologist in outpatient clinic visits more than 2 times, or more than once in inpatient service. The incidence of AD was defined if a patient had a diagnosis assigned by a dermatologist in outpatient visits more than 2 times or more than 1 in inpatient service.
2.3. Levels of urbanization
The Taiwanese NHIRD used cluster analysis to examine urbanization levels. They divided 359 towns in Taiwan into 7 categories, with 1 indicating “most urbanized” and 7 indicating “least urbanized.” The categorization of these urbanization levels into 7 clusters were based on Taiwanese census data from 2000. Because few AS cases were in levels 4 to 7, those 4 levels were combined into a single group. Hence, we have 4 strata in urbanization levels.
2.4. Statistical analysis
We used Pearson chi-square test to compare differences of category variables, and Student t test to compare differences of continuous variables. We also did stratified Cox proportional-hazards regression analysis (stratified by sex, age group, and index year) to assess the risk of subsequent allergic diseases during the 10-year follow-up period. All patients were followed from the index date until presented with one of the three allergic diseases or until the end of the 10-year follow-up period. We adopted hazard ratios (HRs) and 95% confidence intervals (CIs) to show the risk of each allergic disease we analyzed.
All data processing and statistical analyses were performed using the Statistical Package for Social Science software version 17 for Windows (SPSS, Inc., Chicago, IL). The difference between the groups were considered significant if two sided P values were smaller than 0.05.
3. Results
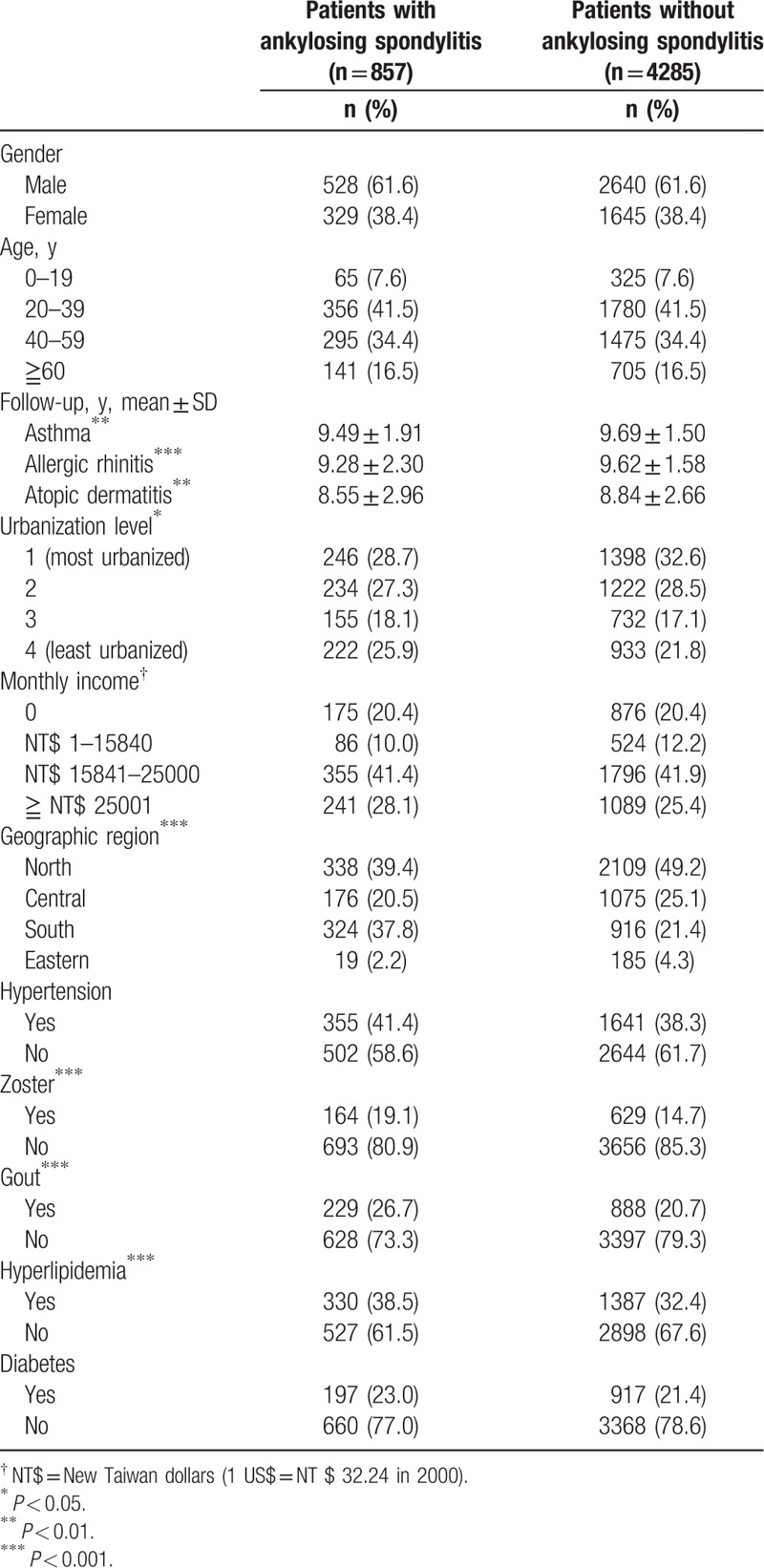
The AS cohort contained 857 patients, and 4285 patients were included in the comparison cohort. Table 1 shows distributions of demographic characteristics and comorbidities of the AS and comparison cohorts. Zoster, gout, rheumatoid arthritis and hyperlipidemia were more prevalent in the AS cohort than the comparison cohort.
Table 1.
Demographic characteristics for the selected patients, stratified by presence/absence of ankylosing spondylitis from 1998 to 2000 (n = 5142).
There were 310 patients developing a new diagnosis of asthma during the 10-year follow-up. 66 patients were in the AS group and 244 patients were in the comparison cohort. Table 2 shows that the crude HR was 1.38 (95% CI: 1.05–1.81). After adjusting for region, urbanization level, and comorbidities, the adjusted HR was 1.31 (95% CI: 1.00–1.75). Figure 1 depicts the Kaplan–Meier survival curves, demonstrating that asthma-free survival rate was significantly lower in the AS cohort than in the comparison cohort. For allergic rhinitis, 10.6% of AS patients developed the disease and 7.1% of comparison group developed it (adjusted HR: 1.46; 95% CI: 1.15–1.85). Patients with AS also showed a higher incidence of developing AD in 10-year follow up (23.9% vs 19.6%; adjusted HR: 1.22; 95% CI: 1.05–1.43). The allergic rhinitis-free survival rate and AD-free survival rate in patients with AS were also significantly lower compared with non-AS patients (Figs. 2 and 3). The patients with AS had a greater risk of developing more than 1 allergic disease. (7.7% vs 3.8%; adjusted HR: 2.07; 95% CI: 1.52–2.82).
Table 2.
HRs of allergic diseases among ankylosing spondylitis patients during the 10-year follow-up period from the index ambulatory visits or inpatient care from1998 to 2000 (N = 5142).
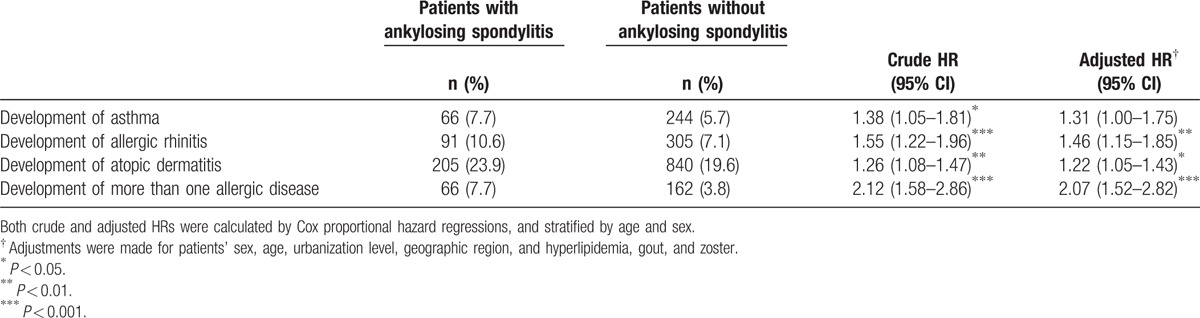
Figure 1.
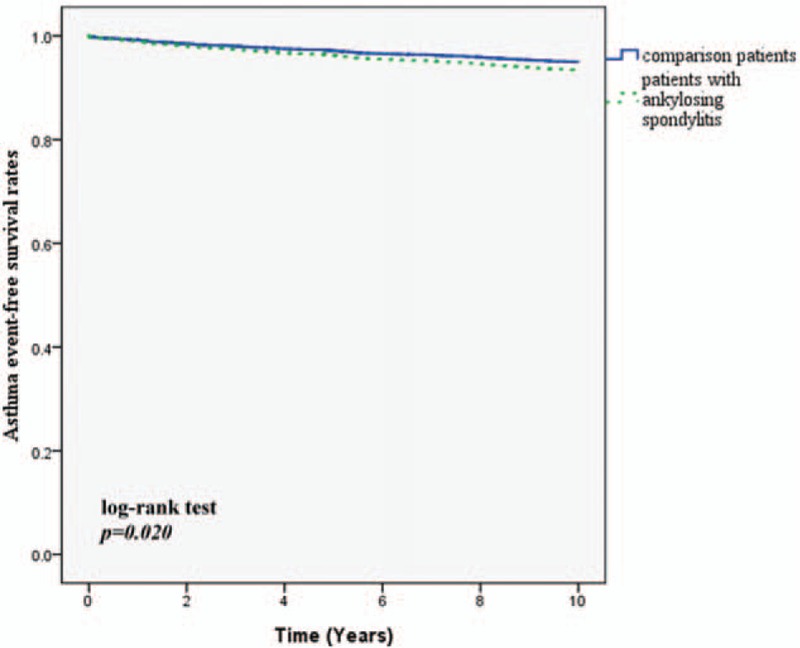
Asthma event free survival rates for subject with ankylosing spondylitis and comparison group from 1998 to 2000.
Figure 2.
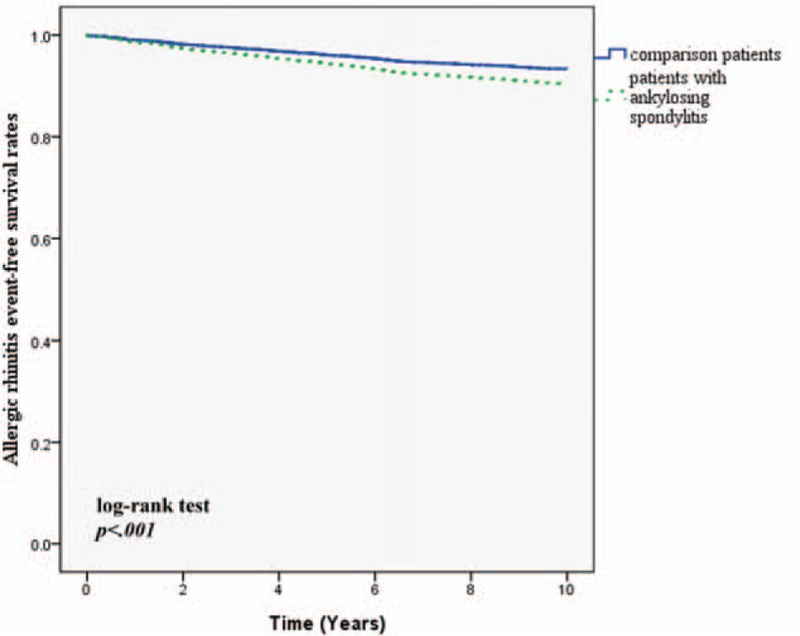
Allergic rhinitis event free survival rates for subject with ankylosing spondylitis and comparison group from 1998 to 2000.
Figure 3.
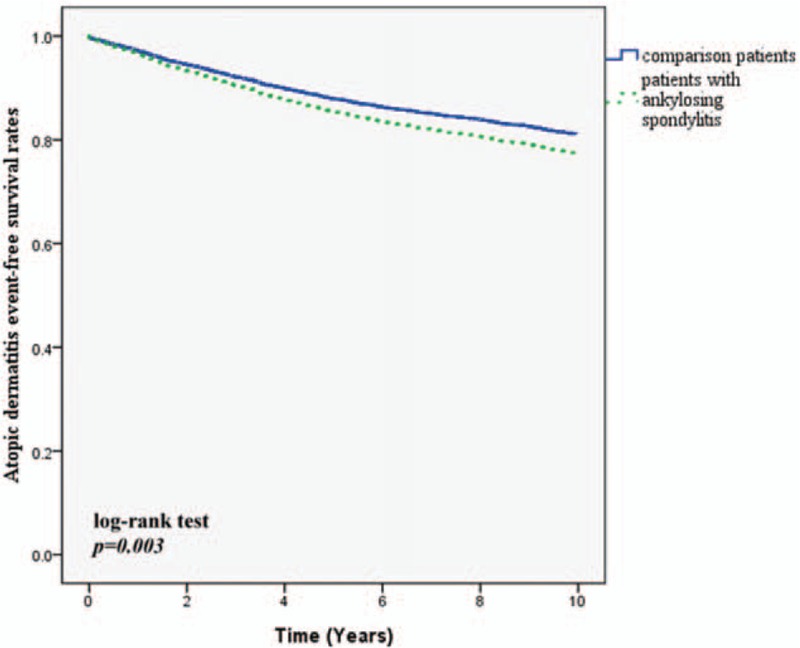
Atopic dermatitis event free survival rates for subject with ankylosing spondylitis and comparison group from 1998 to 2000.
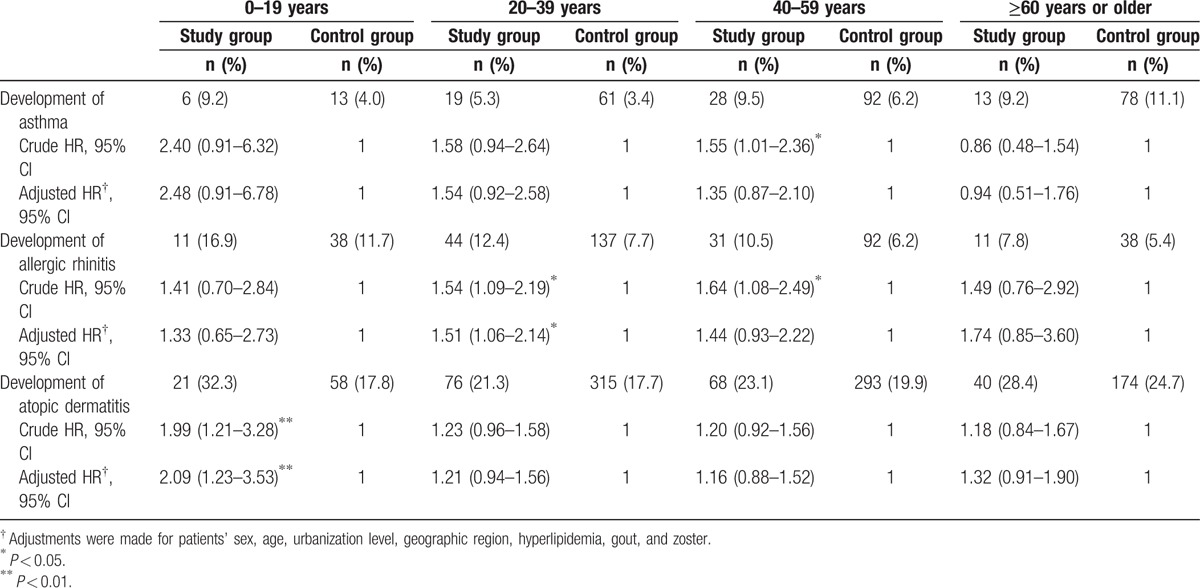
We also analyzed the incidence of 3 diseases according to age group (Table 3). Patients with AS and aged younger than 20-year-old had significantly higher incidence of AD compared to non-AS patients. However, the incidence of these allergic diseases was not different between AS and non-AS groups in other age groups.
Table 3.
Hazard ratios for allergic diseases among patients with ankylosing spondylitis and the comparison cohort by age group.
4. Discussion
To analyze the association between AS and allergic diseases, we did a population-based cohort study with NHIRD in Taiwan. From the study of a 5142-person cohort and 10-year follow-up (Table 2), we found that the incidence of allergic diseases was significantly higher in patients with AS.
Few published papers exist on atopic disorders in patients with AS. In 1 study comparing patients with AS to those with disc prolapse, it has shown a higher prevalence of allergy rhinitis and a trend of higher prevalence of asthma in patients with AS.[16] In another study assessing the prevalence of atopic disorders in AS and rheumatoid arthritis (RA), the prevalence of atopic disorders is lower in patients with RA but not higher in those with AS when compared with control group (13.1% in RA group, 20.7% in control group, and 24.6% in AS group).[1] The prevalence of asthma is also not higher in patients with AS comparing with controls, but case number of the study is limited.
The reason of why patients with AS would have higher risk developing asthma may be illustrated by Th2 and Th17. Increased number of Th2 helper lymphocytes and Th17 cells have been reported in patients with AS.[17–19] Th17 cells express several effector cytokines, including IL-17A, IL-17F, IL-22, IL-26, and granulocyte–macrophage colony-stimulating factor.[20] IL-17 plays an important role in AS. IL-17A is a potentially proinflammatory cytokine which is related with some diseases including AS, multiple sclerosis, psoriasis, and psoriatic arthritis.[21] In a study analyzing the amount of IL-17 in facet joint, it is higher in patients with AS compared with those with osteoarthritis.[22]
Th2 lymphocyte plays a critical role in the pathogenesis of asthma.[23] It can produce several cytokines such as IL-3, IL-4, IL-5, and IL-13. Those cytokines regulate eosinophil, which is related to the severity of asthma.[24] Th17 has been found as a new player in asthma pathogenesis.[25] Airway neutrophilia has been found to be related with disease severity in non-atopic asthma patients.[26] Th17 can produce IL-17, which has a role of neutrophil recruitment.[27] In Winsam et al's[28] study, they found the number of IL-17A+ cells was higher in the patients with severe asthma. In Doe et al's[29] study, they found that the median IL-17A cells is increased in mild to moderate asthma, and IL-17F(+) is increased in severe asthma and mild to moderate asthma compared with healthy controls subjects. Because patients with AS would have more Th2 and Th17 cells and these two cells are important in the pathogenesis of asthma, they may have higher risk to develop asthma. Our data with a large population (Table 2 and Fig. 1) demonstrated that patients with AS had 30% significantly higher risk having a diagnosis of asthma during 10-year follow up (Crude HR = 1.38, 95% CI = 1.05–1.85, P < 0.05).
Th2 cells also play a role in causing allergic rhinitis and AD. Yang et al[30] studied the nasal secretion from patients with allergic rhinitis compared to infections rhinitis. They found that the number of degranulated eosinophils and basophilic cells, clustered epithelial cells, and large granular lymphocytes have been increased in allergic rhinitis. Varga et al[31] studied the nasal biopsy form patients with allergic rhinitis compared to controls. They found that the numbers of cells expressing IL-5 have been higher and the numbers of cells expressing IL-2 are lower in rhinitic patients than controls and suggested that allergic rhinitis is associated with Th2 cells.[31] Jeong et al[32] investigated the expression of various cytokines and assessed the tissue eosinophil counts in skin biopsies from patients with AD. They demonstrated that the expressions of cytokines IL-5, IL-13, and the number of dermal infiltrating eosinophils are increased in patients with elevated IgE levels. Matwally et al[33] assessed IL-13 mRNA expression in peripheral blood of patients with different degrees of AD and compared with healthy subjects. They found that IL-13 mRNA is expressed more in patients with AD as compared to normal control and higher level of IL-13 mRNA expression in severe AD group in comparison with both moderate and mild groups. Because the cytokines are produced by Th2 cell and patients with AS would have higher level of Th2 cell, they may be more likely to develop such allergic diseases. Our population-based data (Table 2, Figs. 2 and 3) also demonstrated that patients with AS had significantly higher risk of developing allergic rhinitis (Crude HR = 1.55, 95% CI = 1.22–1.96, P < 0.001) and AD (Crude HR = 1.26, 95% CI = 1.08–1.47, P < 0.01) during 10-year follow-up since they had a diagnosis of AS.
Our data demonstrated the incidence of AD was significantly higher in patients with AS younger than 20 years old, and a trend of higher incidence rate of allergic diseases in different age groups (Table 3). Due to small case numbers in each age group, it may not be powered to detect the difference. Physicians should monitor related signs and symptoms of AD in younger patients with AS.
There are some strengths of our cohort study. First, the study cohort is highly representative of the general Taiwanese population. Second, claims for each insured person from different medical institutes can be tracked across time in the NHIRD, which can avoid the bias of patients dropping out and minimize the possibility of recall bias.
The readers are warned not to over-interpret our study results because our study has limitations. First, disease-modifying anti-rheumatic drugs may influence affects the expression of symptoms and the diagnosis of asthma. The effects of those drugs, especially anti-TNF therapies, might affect the balance of Th1 and Th2. Although anti-TNF therapies have been available since 2003 in Taiwan, few AS patients have received anti-TNF agents due to the high entry strict criteria for reimbursement. Second, we did not have any information on the severity of AS in the claims data, so we could not analyze if an association existed between the severity of AS and asthma. Third, some patients may have developed mild symptoms of those three allergic diseases but did not seek medical help, so the incidence of these diseases may have been underestimated.
5. Conclusion
We found that the incidence of new diagnosis of allergic diseases (including asthma, allergic rhinitis, and AD) were higher in patients with AS in a nationally representative cohort that was followed-up for 10 years. More attention should be paid to early AS detection and physicians should be aware related signs and symptoms of these allergic diseases and provide managements as early as possible to decrease allergic status.
Footnotes
Abbreviations: AD = atopic dermatitis, anti-TNF = antitumor necrosis factor, AS = ankylosing spondylitis, CI = confidence interval, HR = hazard ratio, IL = interleukin, LHID = Longitudinal Health Insurance Database, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, RA = rheumatoid arthritis, Th = T helper.
W-PC and C-NK contributed equally to this manuscript.
The results and explanations in this study do not represent the views of the Bureau of NHI, Department of Health, or National Health Research Institutes. This study was supported by grants from NSC 102–2314-B-182–053-MY3, CMRPG8B0211 and TMU105-AE1-B05. The authors declare the absence of their competing interests.
The authors have no conflicts of interest to declare.
References
- 1.Rudwaleit M, Andermann B, Alten R, et al. Atopic disorders in ankylosing spondylitis and rheumatoid arthritis. Ann Rheum Dis 2002; 61:968–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei JC, Wong RH, Huang JH, et al. Evaluation of internal consistency and re-test reliability of Bath ankylosing spondylitis indices in a large cohort of adult and juvenile spondylitis patients in Taiwan. Clin Rheumatol 2007; 26:1685–1691. [DOI] [PubMed] [Google Scholar]
- 3.Reveille JD. Epidemiology of spondyloarthritis in North America. Am J Med Sci 2011; 341:284–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou CT, Pei L, Chang DM, et al. Prevalence of rheumatic diseases in Taiwan: a population study of urban, suburban, rural differences. J Rheumatol 1994; 21:302–306. [PubMed] [Google Scholar]
- 5.Eyerich K, Novak N. Immunology of atopic eczema: overcoming the Th1/Th2 paradigm. Allergy 2013; 68:974–982. [DOI] [PubMed] [Google Scholar]
- 6.Szalay B, Meszaros G, Cseh A, et al. Adaptive immunity in ankylosing spondylitis: phenotype and functional alterations of T-cells before and during infliximab therapy. Clin Dev Immunol 2012; 2012:808724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Asthma E, Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol 2007; 120 (5 Suppl):S94–138. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease, C., Prevention Vital signs: asthma prevalence, disease characteristics, and self-management education: United States, 2001–2009. MMWR Morb Mortal Wkly Rep 2011; 60:547–552. [PubMed] [Google Scholar]
- 9.Hwang CY, Chen YJ, Lin MW, et al. Prevalence of atopic dermatitis, allergic rhinitis and asthma in Taiwan: a national study 2000 to 2007. Acta Derm Venereol 2010; 90:589–594. [DOI] [PubMed] [Google Scholar]
- 10.Lloyd CM, Saglani S. T cells in asthma: influences of genetics, environment, and T-cell plasticity. J Allergy Clin Immunol 2013; 131:1267–1274. [DOI] [PubMed] [Google Scholar]
- 11.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 2012; 18:716–725. [DOI] [PubMed] [Google Scholar]
- 12.Wakashin H, Hirose K, Maezawa Y, et al. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med 2008; 178:1023–1032. [DOI] [PubMed] [Google Scholar]
- 13.Wilson RH, Whitehead GS, Nakano H, et al. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med 2009; 180:720–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broide DH. Allergic rhinitis: pathophysiology. Allergy Asthma Proc 2010; 31:370–374. [DOI] [PubMed] [Google Scholar]
- 15.Brandt EB, Sivaprasad U. Th2 cytokines and atopic dermatitis. J Clin Cell Immunol 2011; 2:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zochling J, Bohl-Buhler MH, Baraliakos X, et al. The high prevalence of infections and allergic symptoms in patients with ankylosing spondylitis is associated with clinical symptoms. Clin Rheumatol 2006; 25:648–658. [DOI] [PubMed] [Google Scholar]
- 17.Yang PT, Kasai H, Zhao LJ, et al. Increased CCR4 expression on circulating CD4(+) T cells in ankylosing spondylitis, rheumatoid arthritis and systemic lupus erythematosus. Clin Exp Immunol 2004; 138:342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jandus C, Bioley G, Rivals JP, et al. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum 2008; 58:2307–2317. [DOI] [PubMed] [Google Scholar]
- 19.Szalay B, Mészáros G, Cseh Á, et al. Adaptive immunity in ankylosing spondylitis: phenotype and functional alterations of T-cells before and during infliximab therapy. Clin Dev Immunol 2012; 2012:808724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov 2012; 11:763–776. [DOI] [PubMed] [Google Scholar]
- 21.Schett G, Elewaut D, McInnes IB, et al. How cytokine networks fuel inflammation: toward a cytokine-based disease taxonomy. Nat Med 2013; 19:822–824. [DOI] [PubMed] [Google Scholar]
- 22.Appel H, Maier R, Wu P, et al. Analysis of IL-17(+) cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the Th17-mediated adaptive immune response. Arthritis Res Ther 2011; 13:R95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnes PJ. Th2 cytokines and asthma: an introduction. Respir Res 2001; 2:64–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bousquet J, Chanez P, Lacoste JY, et al. Eosinophilic inflammation in asthma. N Engl J Med 1990; 323:1033–1039. [DOI] [PubMed] [Google Scholar]
- 25.Cosmi L, Liotta F, Maggi E, et al. Th17 cells: new players in asthma pathogenesis. Allergy 2011; 66:989–998. [DOI] [PubMed] [Google Scholar]
- 26.Louis R, Lau LC, Bron AO, et al. The relationship between airways inflammation and asthma severity. Am J Respir Crit Care Med 2000; 161:9–16. [DOI] [PubMed] [Google Scholar]
- 27.Molet SM, Hamid QA, Hamilos DL. IL-11, IL-17 expression in nasal polyps: relationship to collagen deposition and suppression by intranasal fluticasone propionate. Laryngoscope 2003; 113:1803–1812. [DOI] [PubMed] [Google Scholar]
- 28.Al-Ramli W, Préfontaine D, Chouiali F, et al. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol 2009; 123:1185–1187. [DOI] [PubMed] [Google Scholar]
- 29.Doe C, Bafadhel M, Siddiqui S, et al. Expression of the T helper 17-associated cytokines IL-17A and IL-17F in asthma and COPD. Chest 2010; 138:1140–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang PC, Okuda M, Pawankar R, et al. Electron microscopical studies of the cell population in nasal secretions. Rhinology 1995; 33:70–77. [PubMed] [Google Scholar]
- 31.Varga EM, Jacobson MR, Till SJ, et al. Cellular infiltration and cytokine mRNA expression in perennial allergic rhinitis. Allergy 1999; 54:338–345. [DOI] [PubMed] [Google Scholar]
- 32.Jeong CW, Ahn KS, Rho NK, et al. Differential in vivo cytokine mRNA expression in lesional skin of intrinsic vs. extrinsic atopic dermatitis patients using semiquantitative RT-PCR. Clin Exp Allergy 2003; 33:1717–1724. [DOI] [PubMed] [Google Scholar]
- 33.Metwally SS, Mosaad YM, Abdel-Samee ER, et al. IL-13 gene expression in patients with atopic dermatitis: relation to IgE level and to disease severity. Egypt J Immunol 2004; 11:171–177. [PubMed] [Google Scholar]


