Abstract
Severe hypoglycemia is an established risk marker for cardiovascular complications of diabetes, but whether mild hypoglycemia confers similar risks is unclear. We examined the association of self-reported recurrent mild hypoglycemic events with cardiovascular disease (CVD) and all-cause mortality in a prospective cohort of Chinese adults with type 2 diabetes.
From June 2007 to May 2015, 19,019 patients in Hong Kong underwent comprehensive assessment of metabolic and complication status using the Joint Asia Diabetes Evaluation program. Recurrent mild hypoglycemic event was determined by self-report of mild-to-moderate hypoglycemic symptoms at least once monthly in previous 3 months. Incident cardiovascular events were identified using hospital discharge diagnosis codes and death using Hong Kong Death Registry.
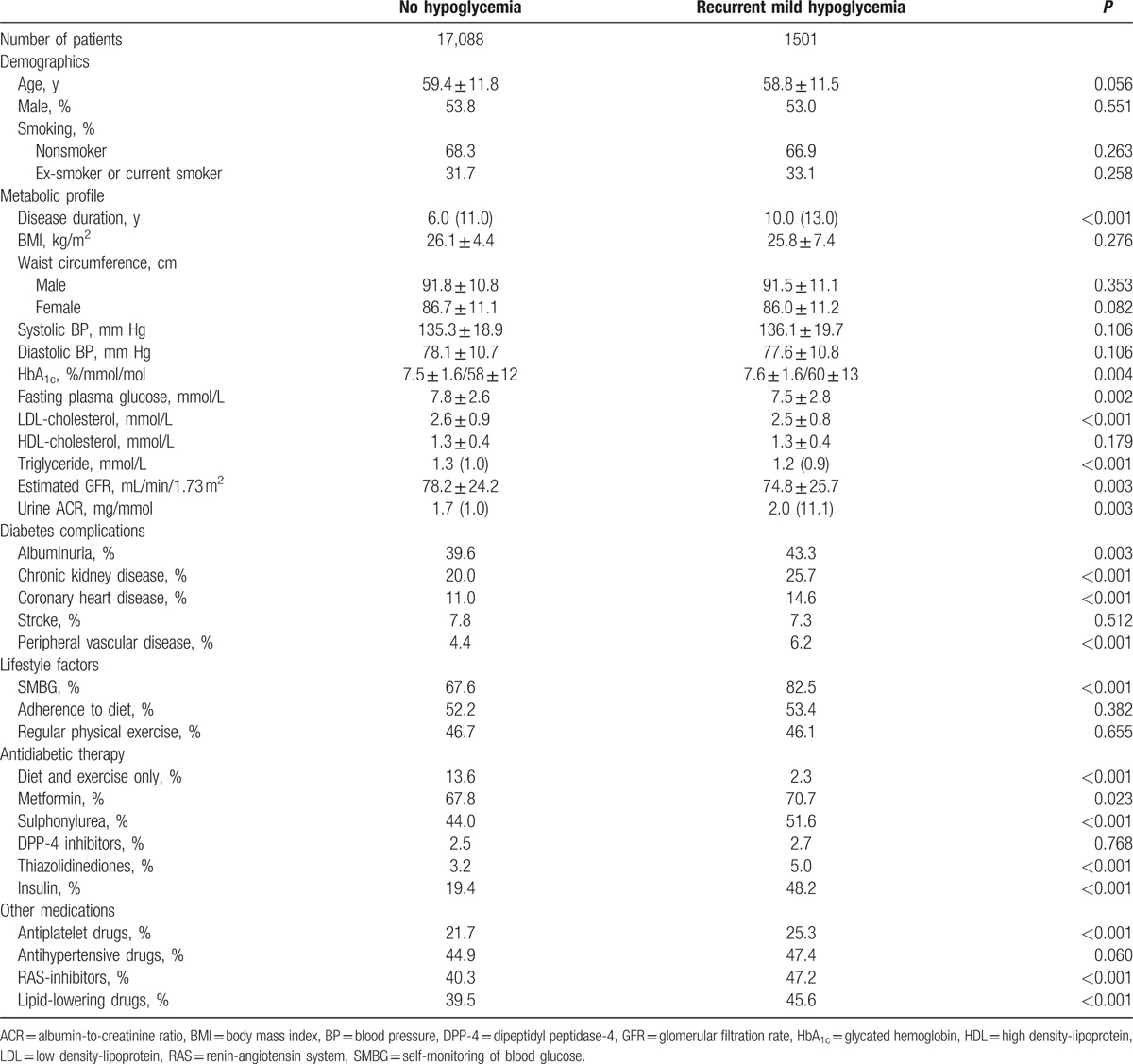
Patients reporting recurrent mild hypoglycemia (n = 1501, 8.1%) were younger, had longer disease duration, worse glycemic control, and higher frequencies of vascular complications at baseline. Over 3.9 years of follow-up, respective incidences of CVD and all-cause death were 18.1 and 10.3 per 1000 person-years and 15.4 and 9.9 per 1000 person-years in patients with and without recurrent mild hypoglycemia. Using multivariate Cox regression analysis, recurrent mild hypoglycemia was not associated with CVD or all-cause mortality. In subgroup analysis, mild hypoglycemia was related to CVD in patients with chronic kidney disease (hazard ratio 1.36, 95% confidence interval 1.01–1.84, P = 0.0435) and those on insulin (hazard ratio 1.37, 95% confidence interval 1.01–1.86, P = 0.0402) adjusted for confounders.
Mild hypoglycemia by self-report was frequent in patients with type 2 diabetes and was associated with increased risk of CVD in susceptible groups.
Keywords: cardiovascular disease, death, mild hypoglycemia, type 2 diabetes
1. Introduction
Intensive glycemic control reduces the risk of diabetic microvascular complications, but whether aggressive pharmacologic management of hyperglycemia has long-term cardiovascular and mortality benefit is less well-established.[1–6] The unexpected finding of higher mortality in patients assigned to intensive glycemic control during the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study has fuelled concerns about possible latent harm of hypoglycemia with respect to cardiovascular risks.[3,4] Critical evaluation of landmark clinical trials have demonstrated that severe hypoglycemia increased the hazards of cardiovascular morbidity and mortality by 2 to 3-folds among patients with type 2 diabetes, where severe hypoglycemia was defined as an event that required third-person assistance.[7,8] Similar findings were reported across several observational cohort studies.[9–11] Acutely, hypoglycemia triggers activation of the sympathetic nervous system, which may cause abnormal cardiac repolarization and prolongation of QT interval to result in ventricular arrhythmia.[12,13] The attendant hemodynamic changes and increased myocardial work may precipitate myocardial ischemia in susceptible individuals with pre-existing coronary artery disease.[14] Mechanistic studies have further suggested that hypoglycemia may induce a range of maladaptive changes to the vasculature including increase in platelet aggregation, endothelial dysfunction, and enhanced subclinical inflammatory response that are pro-atherogenic.[15,16] It was demonstrated that many of these changes can persist for days after an episode of hypoglycemia.[17]
Foregoing literatures provided indisputable evidences for a strong risk relationship between severe hypoglycemia and major events, although the link of mild hypoglycemia with late complication is not as well-defined. Mild hypoglycemia occurs more frequently than severe episodes, but is also under-reported due to under-recognition and impaired hypoglycemia awareness.[18] Only a few studies have specifically addressed the impact of mild hypoglycemia with respect to long-term cardiovascular outcome, in part because of the inconsistencies in defining mild hypoglycemia and the difficulty in accurately capturing minor episodes[10,19] In this study, we investigated the association of self-reported recurrent mild hypoglycemic symptoms and incident cardiovascular disease (CVD) and all-cause death in a prospective cohort of Chinese adults with type 2 diabetes.
2. Methods
2.1. Patients
The Joint Asia Diabetes Evaluation (JADE) program is an electronic disease management program that systematically captures clinical details of patients with diabetes including demographics, significant medical history, medication use, metabolic status, and presence of diabetes-related complications.[20,21] The JADE program has 2-fold objectives: to serve as an enhancement to routine diabetes care through structured complication assessment and personalized information feedback to patients and physicians with automated decision support; and to establish an ongoing Asia Diabetes Database with the aim to advance understanding of the natural history of diabetes and its comorbidities in a real world practice. For this study, we included patients from Hong Kong who were enrolled into the JADE program between July 1, 2007 and May 31, 2015. Referral sources to JADE included both private and public specialists, and also community clinics. Adults aged 18 years or above with type 2 diabetes based on physician diagnosis and who did not report severe hypoglycemia at baseline were included for analysis. We excluded patients with type 1 diabetes or diabetes of unknown type and those with missing information about hypoglycemia. Given that severe hypoglycemia is an established risk factor for CVD and mortality, and that patients with severe hypoglycemia are also more likely to have recurrent mild hypoglycemia,[7–11] those with self-report of severe hypoglycemia at baseline were also removed from analysis as the inclusion of this group may confound the relationship between mild hypoglycemia and outcome. All patients have given written informed consent for the research team to track and analyze their anonymized data. The use of JADE portal for research has been approved by the Joint Chinese University of Hong Kong—New Territories East Cluster clinical research ethics committee. The study was conducted in accordance to Declaration of Helsinki.
2.2. Baseline assessment
All participating patients underwent comprehensive clinical assessment including documentation of sociodemographic parameters, significant past medical history, current medication use, and lifestyle factors based on the JADE protocol. Vitals [blood pressures (BPs) and pulse rates] and anthropometric measurements (body weight, body height, waist circumference) were collected. The presence of diabetic retinopathy was examined using retinal photography, and the retina photos were graded by a trained endocrinologist to no diabetic retinopathy, background diabetic retinopathy, preproliferative diabetic retinopathy, and proliferative diabetic retinopathy. Peripheral sensory neuropathy was determined using graduated tuning fork and monofilament. Sensory neuropathy was defined by fulfilling 2 of three criteria: self-report of abnormal sensation in lower extremities, reduced sensation on monofilament, and reduced sensation to graduated tuning fork. CVD was defined as a history of coronary heart disease, stroke or peripheral artery disease (PAD), the latter defined as nontraumatic lower-extremity amputation, revascularization procedure for PAD, or ankle-to-brachial index (ABI) ≤0.9. Hypoglycemia was assessed by self-report of mild or severe hypoglycemic events in the previous 3 months. Recurrent mild hypoglycemia was defined as an experience of typical hypoglycemic symptoms such as hunger sensation, sweating, tremor, malaise, and dizziness, at least once per month, over the past 3 months. Severe hypoglycemia was defined as hypoglycemic event that required medical or nonmedical third-person assistance at least once during this period. The assessment of hypoglycemia did not include review of self-blood glucose readings.
Blood and spot urine samples were collected for plasma glucose, glycated hemoglobin (HbA1c), total cholesterol, low-density lipoprotein (LDL)-cholesterol, high-density lipoprotein (HDL)-cholesterol, triglyceride, renal function test, and urine albumin-to-creatinine ratio (ACR), after at least 8 hours of fasting. Estimated GFR (GFR) as expressed in mL/min/1.73 m2 was calculated using the abbreviated Modification of Diet in Renal Disease (MDRD) equation recalibrated for Chinese: estimated GFR = 186 × [SCR × 0.011]−1.154 × [age]−0.203 × [0.742 if female] × 1.233, where SCR was serum creatinine in μmol/L and 1.233 is the adjusting co-efficient for Chinese.[22] Chronic kidney disease (CKD) was defined as estimated GFR <60 mL/min/1.73 m2, while end-stage renal disease (ESRD) was defined as estimated GFR <15 mL/min/1.73 m2 or requirement of renal transplant or dialysis. Microalbuminuria was defined as urine ACR ≥2.5 to 25.0 mg/mmoL in men and ≥3.5 to 25 mg/mmoL in women, macroalbuminuria urine ACR ≥25 mg/mmoL.
2.3. Outcome identification and definition
The Hong Kong Hospital Authority (HA) is the governing body of all public-funded hospitals and outpatient clinics. Health care in Hong Kong is heavily subsidized and as such, the majority of Hong Kong residents seek care for acute and chronic illnesses in the public sector. In a recent estimation, the HA provides over 90% of total hospital bed days.[23] Clinical records of patients attending public hospitals and clinics, including diagnoses, laboratory results, and medications, are continuously entered into the HA Central Computer System, which identifies patients by their unique Hong Kong Identity Card number compulsory for all Hong Kong residents. For this study, we retrieved hospital discharge diagnoses coded according to International Classification of Disease, Ninth Revision (ICD-9), of all patients in this cohort from the day of their enrolment until May 31, 2015. Information on death was retrieved from the Hong Kong Death Registry, which is maintained by the Immigration Department, the Hong Kong Government, and linked to the HA Central Computed System.
Diagnostic codes listed as the principal diagnosis were used for outcome identification. Coronary heart disease was defined as myocardial infarction (ICD-9 code 410), ischemic heart disease (ICD-9 code 411–414), or death due to coronary heart disease (ICD-9 code 410–414). Congestive heart failure was defined as nonfatal or fatal heart failure (ICD-9 code 428). Stroke was defined as nonfatal (ICD-9 code 432–434, 436) or fatal ischemic stroke (ICD-9 code 432–438), or haemorrhagic stroke as defined by fatal and nonfatal subarachnoid hemorrhage (ICD-9 code 430) or intracerebral hemorrhage (ICD-9 code 431). Transient ischemic attack was not included in the definition of stroke in the present analysis. PAD was defined as diabetes with peripheral circulatory disorders (ICD-9 code 250.7), gangrene (ICD-9 code 785.4), angiopathy in diseases classified elsewhere (ICD-9 code 443.81), peripheral vascular disease unspecified (ICD-9 code 443.9), other peripheral vascular shunt or bypass (procedure code 39.29), insertion of nondrug-eluting peripheral vessel stents (procedure code 39.90), or amputation of lower limb (procedure code 84.1) without a traumatic amputation diagnosis code (ICD-9 code 895–897). CVDs included coronary heart disease, congestive heart failure, stroke, and PAD.
2.4. Statistical methods
Analysis was performed using the Statistical Analysis System (version 9.3; SAS Institute, Cary, NC). We compared baseline demographics and clinical characteristics of patients with or without recurrent mild hypoglycemia. Continuous variables were expressed as mean ± SD or as median (interquartile range), and categorical variables were expressed as percentages. Chi-square test was used for between-group comparison of categorical variables, t test for normally distributed continuous variables, and Wilcoxon rank-sum test for continuous variables with skewed distribution. Follow-up time was calculated as the period from enrolment to the date of the first event of CVD, death, or censored date of May 31, 2015, whichever came first.
Multivariate Cox proportional-hazards regression was conducted to estimate the hazards of recurrent mild hypoglycemia, compared with the reference group of no or infrequent hypoglycemia, on incident CVD and all-cause death. Four models were constructed sequentially for each outcome: model 1, adjusted for age, sex, and disease duration; model 2, adjusted for smoking, body mass index (BMI), systolic BP, HbA1c, LDL-cholesterol, HDL-cholesterol, estimated GFR, urine ACR, and baseline history of CVD, in addition to variables in model 1; model 3, baseline use of antiplatelet drugs, lipid-lowering drugs, antihypertensive drugs, and renin–angiotensin system inhibitors, in addition to variables in model 2; model 4, baseline use of sulphonylurea, non-sulphonylurea oral antidiabetic drugs, and insulin, in addition to variables in model 3. The variables were selected based on prior knowledge that these factors are associated with either risks of hypoglycemia or outcomes of interest. The proportional-hazard assumption was checked by plotting the scaled Schoenfeld residuals against survival time.[24] In the regression models for the outcome of CVD, as the baseline history of CVD violated the proportional hazard assumption, regression was stratified by baseline history of CVD, and separate baseline hazard functions were fitted. Missing data are not imputed.
We examined the association of recurrent mild hypoglycaemia with CVD and death in the following subgroups of patients: HbA1c ≥8.0% (64 mmol/mol) or <8.0% (64 mmol/mol), presence or absence of baseline history of CVD, presence or absence of CKD, and use or nonuse of insulin., The modifying effects, if any, of these stratifying variables on the relationship between mild hypoglycemia and CVD or death was further tested by introducing interaction terms of mild hypoglycemia with each of the stratifying variables of HbA1c ≥8.0% (64 mmol/mol), baseline history of CVD, presence of CKD, and use of insulin, into the Cox regression model. A P value of less than 0.05 (2-tailed) was considered significant.
3. Results
Between July 1, 2007 and May 31, 2015, 19,019 patients were enrolled into JADE. After excluding 355 patients with type 1 diabetes, 16 patients with missing information on hypoglycemia status, and 59 patients reporting severe hypoglycemia, 18,589 patients were available for analysis. In this cohort (mean age: 59.4 ± 11.8 years, male: 53.8%, mean disease duration: 8.4 ± 7.9 years), 17,088 (91.9%) reported no hypoglycemia or mild hypoglycemia less than once monthly, and 1501 (8.1%) experienced mild hypoglycemia at least once monthly over the previous 3 months based on self-recall. Compared with patients with no or infrequent mild hypoglycemia, patients with recurrent mild hypoglycemia were younger, but had longer disease duration, worse glycemic control, and were more likely to have albuminuria, CKD, and CVD at baseline (Table 1). Patients reporting recurrent mild hypoglycemia were also more likely to use insulin and most classes of noninsulin antidiabetic drugs.
Table 1.
Baseline clinical characteristics of 18,589 Chinese patients with and without recurrent mild hypoglycemia.
Over a mean observation period of 3.9 [95% confidence interval (CI) 0.3–7.8] years, CVD developed in 1060 (5.7%) patients, with incident coronary heart disease in 650 patients, congestive heart failure in 337, stroke in 366, and PAD in 150 patients. During this time, 697 (3.7%) patients died. The incidences of CVD for patients with no or infrequent mild hypoglycemia group and those reporting recurrent mild hypoglycemia were 15.4 (95% CI 14.4–16.3) and 18.1 (95% CI 14.8–22.0) per 1000 person-years, respectively. The corresponding figures for all-cause death were 9.9 (95% CI 9.2–10.7) and 10.3 (95% CI 7.9–13.3) per 1000 person-years. The cumulative incidences of CVD and all-cause death are shown in Figs. 1 and 2.
Figure 1.
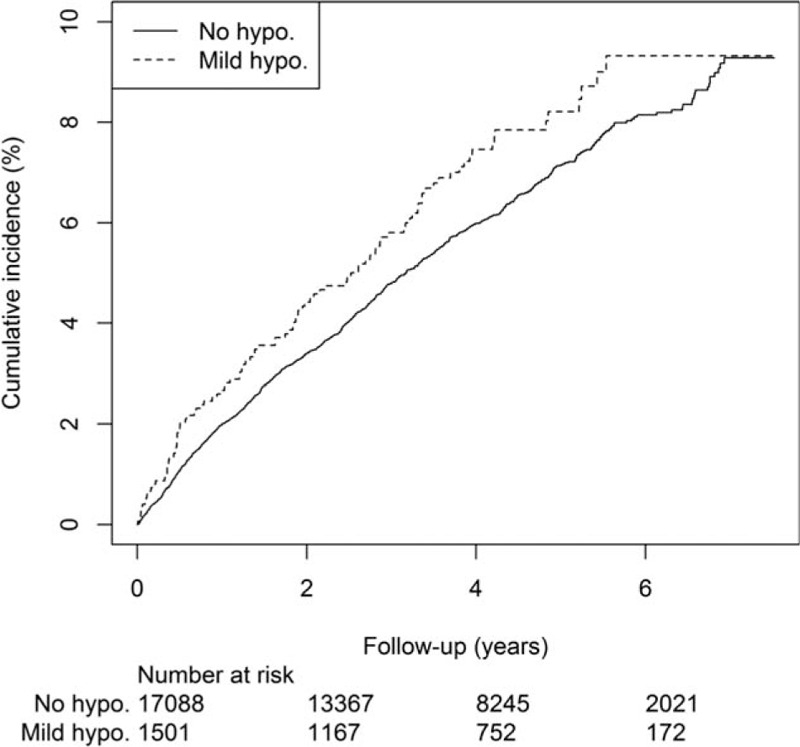
Cumulative incidence of cardiovascular disease in 18,589 Chinese patients with type 2 diabetes with and without self-reported recurrent mild hypoglycemia.
Figure 2.
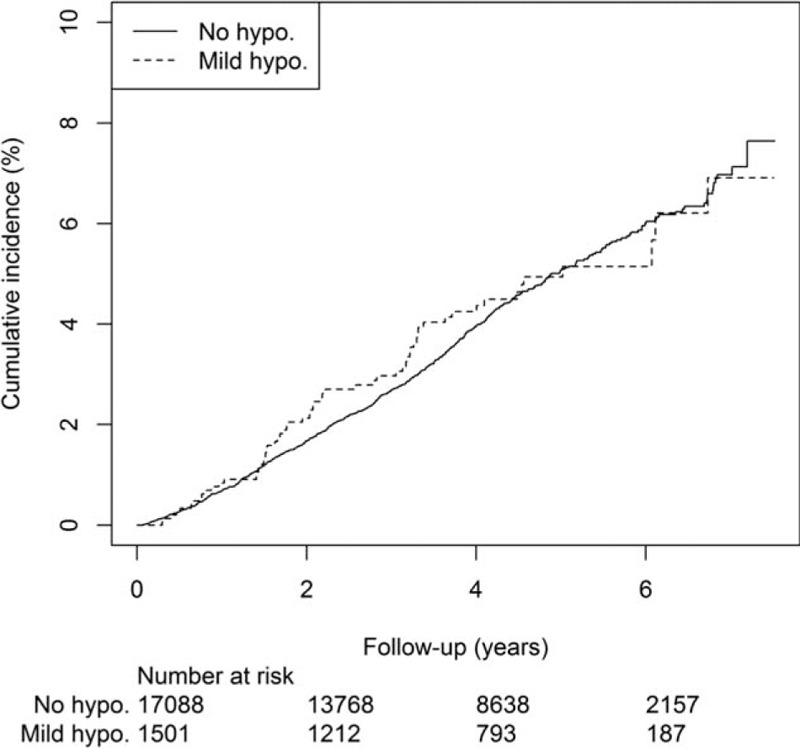
Cumulative incidence of all-cause mortality in 18,589 Chinese patients with type 2 diabetes with and without self-reported recurrent mild hypoglycemia.
We examined the association of self-reported mild hypoglycemia with major clinical events using multivariate Cox regression (Tables 2 and 3). In the model adjusted for age, sex, and disease duration, mild hypoglycemia was related to CVD [hazard ratio (HR) 1.23, 95% CI 1.00–1.51, P = 0.049] at borderline significance, but was not related to all-cause death (HR 1.12, 95% CI 0.86–1.47, P = 0.403). The association of mild hypoglycemia with CVD became nonsignificant when the model was further adjusted for metabolic indices, baseline cardiovascular–renal complications, and drugs.
Table 2.
Multivariate Cox regression model to estimate the association of recurrent mild hypoglycemia with cardiovascular disease and all-cause death in 18,589 Chinese patients with type 2 diabetes.
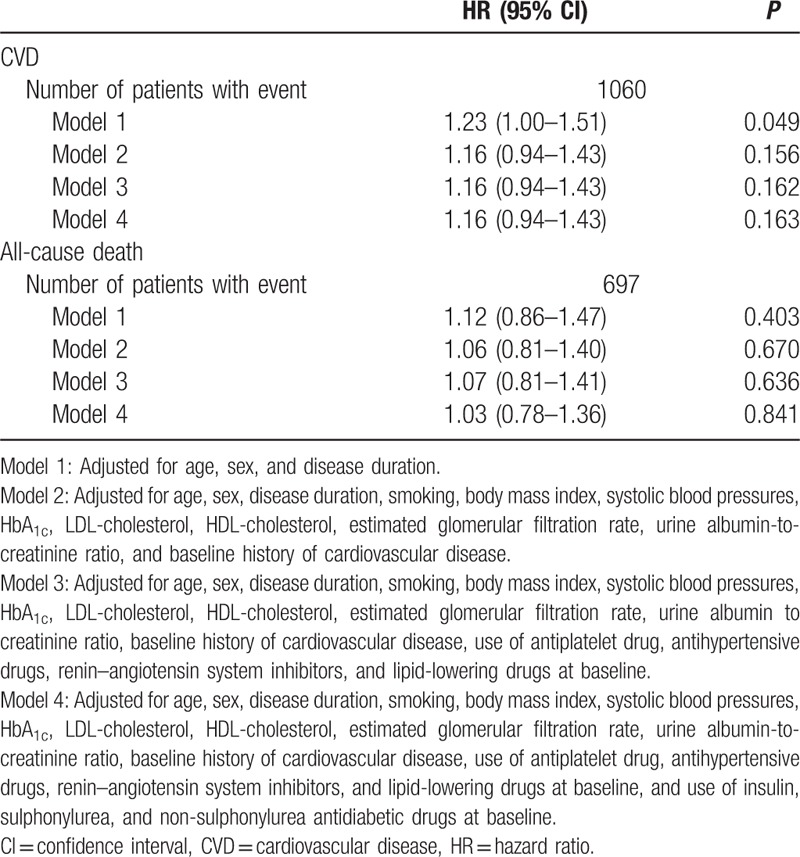
Table 3.
Multivariate Cox regression model on the association of recurrent mild hypoglycemia with cardiovascular disease and all-cause death in patient subgroups.
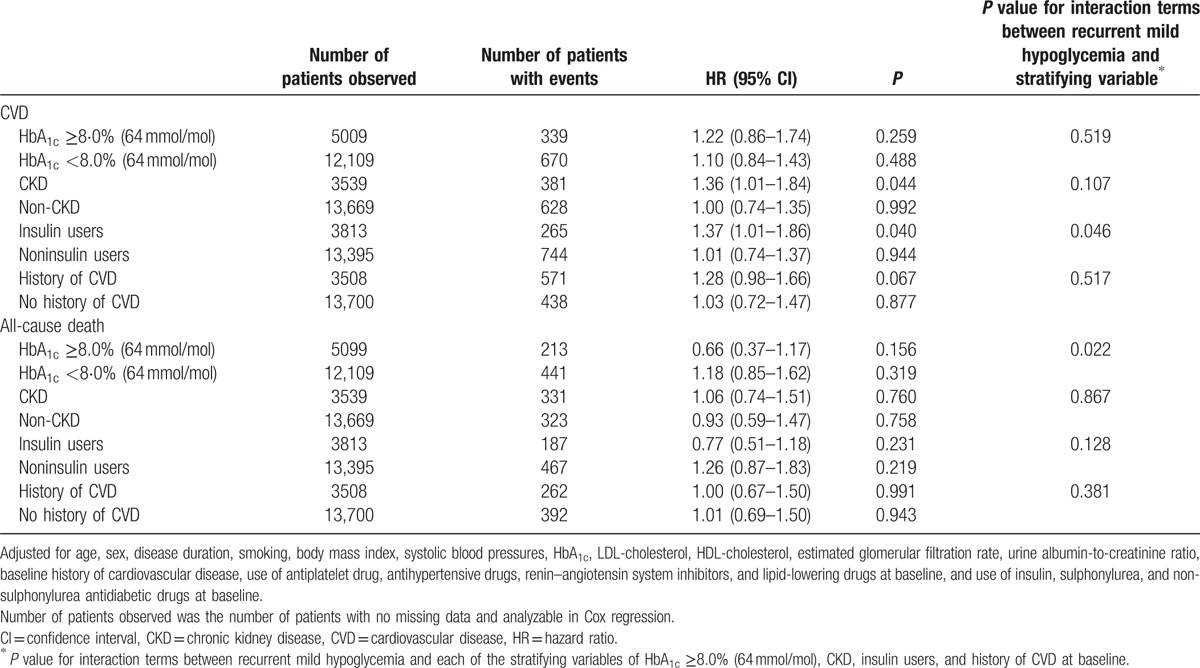
The risk relationship between mild hypoglycemia and clinical events was tested in subgroups of patients stratified by glycemic control, GFR, history of CVD, and insulin use at baseline (Table 3). Self-reported mild hypoglycemia was associated with CVD in patients with estimated GFR <60 mL/min/1.73 m2 (HR 1.36, 95% CI 1.01–1.84, P = 0.044) and in insulin users (HR 1.37, 95% CI 1.01–1.86, P = 0.040). When interaction terms between mild hypoglycemia and each of the stratifying variables were introduced to the full regression model, the interaction between mild hypoglycemia and use of insulin for CVD remained significant (P = 0.046).
Recurrent mild hypoglycemia was not associated with all-cause death in each of the subgroups tested, although the interaction term of mild hypoglycemia with HbA1c ≥8.0% (64 mmol/mol) was tested significant (P = 0.022) with HR of 0.58 (95% CI 0.37–0.93) for all-cause death (Table 3).
4. Discussion
Intensification of antidiabetic drug treatment with the use of insulin and insulin secretagogues is associated with hypoglycemia, which, in turn, is a major obstacle for attainment of glycemic goal, and also contributes to direct and indirect costs of treating diabetes. In this study that have collected data from real world practice, up to 10% of patients with type 2 diabetes reported recurrent mild hypoglycemia, which was not associated with incident CVD or all-cause mortality. In subgroup analysis, we observed increased risk of incident CVD among those with CKD and those on insulin. To our knowledge, this is the largest prospective cohort that examined the risk relationship of recurrent mild hypoglycemia with late diabetes outcome. Our results add to the expanding literature on hypoglycemia with respect to its long-term impact on cardiovascular health.
There is a growing consensus that severe hypoglycemia increases subsequent risks of CVD and death of both vascular and nonvascular causes.[7–11,25] The ACCORD study reported higher death rates in patients assigned to intensive glycemic control,[3] although post hoc analyses of the ACCORD study data suggested that severe hypoglycemia did not account for excess mortality.[8] Among subjects who have experienced severe hypoglycemia during the ACCORD study, those who were assigned to intensive control in fact had lower death rate than those assigned to standard arm, suggesting that the relationship of severe hypoglycemia with outcome may be influenced by the intensity of therapeutic intervention and attained glycemic control.[8] Another subanalysis of the ACCORD study cohort investigated whether mild and severe hypoglycemia differ in their respective correlation with mortality.[26] In the intensive but not the standard arm, recurrent mild hypoglycemia, as defined by episodes of capillary blood glucose ≤3.9 mmol/L with or without symptoms, was associated with reduced mortality. Furthermore, the protective effect of mild hypoglycemia was more pronounced in those who have also experienced severe hypoglycemia during the study. Likewise, in the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) study, which was a randomized controlled trial comparing the long-term impact of intensive versus standard glycemic control, the rates of CVD and death were lower in patients reporting minor hypoglycemia.[7] It has been suggested that exposure to mild hypoglycemia may guard against the adverse impact of acute severe hypoglycemia on myocardium through prior blunting of sympathetic responses.[27] It is also possible that patients who have coped with repeated episodes of mild hypoglycemia became better equipped at preventing or managing more severe events.
In contrast to findings from clinical trials, observational studies showed that mild hypoglycemia had either no association with or even increased the risks of incident CVD. In an analysis conducted by Hsu et al of the Taiwan National Health Insurance Research Database, comprising 77,611 patients with type 2 diabetes, mild hypoglycemia, as identified through insurance claims of hypoglycemia in outpatient settings, was independently predictive of cardiovascular events and death.[10] A limitation with quantifying mild hypoglycemia by physician notification in the Taiwan study is under-recording of minor events as it was likely that only the more significant episodes or episodes that required adjustment to antidiabetic medications were brought to the attention of the physician. McCoy et al examined the relationship between patient-reported mild or severe hypoglycemia and death in 1020 patients with type 1 or type 2 diabetes followed up for 5 years.[19] Corrected for explanatory variables, severe but not mild hypoglycemia was associated with increased mortality. In the current study comprising a large patient sample, we found no relationship between self-reported recurrent mild hypoglycemia and either incident CVD or mortality. Differences in methods of quantifying mild hypoglycemic events may have contributed to discrepancies in results between clinical trials and observational studies.
In subgroup analysis, we did detect a positive association of recurrent mild hypoglycemia with incident CVD among patients with CKD and those who were prescribed insulin. CKD per se is a risk factor for hypoglycemia and patients with renal insufficiency are also more likely to suffer cardiovascular event. Insulin users are similarly prone to experience hypoglycemia and patients who required insulin often have longer disease duration and worse glycemic control compared with noninsulin users. Hemodynamic and metabolic assaults of recurrent episodes of low blood glucose on the vasculatures may have greater impacts in those patients with more complicated diabetes whose internal milieu already favors atherosclerosis. Besides, patients who have frequent mild hypoglycemia may have greater intraday glucose fluctuation, which further increases the predisposition to CVD through increased oxidative stress and endothelial dysfunction. Despite physiological feasibility, whether the excess events were immediate consequences of hypoglycemia is difficult to prove, and it remains conceivable that mild hypoglycemia is an index of vulnerability rather than directly causative. In this instance, recurrent hypoglycemia may be due to recent infection or hospitalization which increases the hazard of an adverse outcome. We further detected a negative association between the interaction term of recurrent mild hypoglycemia and HbA1c above 8% (64 mmol/mol), and all-cause death, suggesting that mild hypoglycemia may be protective in patients with poor glycemic control. Whilst this finding concurs with observations from aforementioned clinical trials, given the smaller sample sizes used in subgroup analysis and the few number of events, we cannot rule out the possibility that findings are by chance and results must be interpreted with caution.
We acknowledge the following limitations of our study. Firstly, the identification of mild hypoglycemic events was based on self-report of typical symptoms only and as such, we were not able to capture asymptomatic events. It has been estimated that 25% to 50% of hypoglycemic episodes occur in the absence of symptoms particularly during night time,[28] and it is likely that we have underestimated the frequency of hypoglycemia in our cohort. Likewise, as home glucose readings were not available, we could not confirm that symptoms reported by patients were genuinely related to hypoglycemia. For instance, patients with poor glycemic control may experience symptoms of hypoglycemia even when blood glucose level has not yet reached hypoglycemic ranges. Secondly, the reliance of self-reported events may be subjected to recall bias, and the accuracy of recall for hypoglycemic events has not been previously established. Younger patients may be more likely to report symptoms than older individuals due to better awareness. Nevertheless, self-reported events are simple to record and it would not be feasible to apply more vigorous procedures such as continuous glucose monitoring to large patient cohort. Thirdly, whereas the sample size was large, the duration of follow-up was relatively short at a mean of 3.9 years, and number of events was few, which could limit the power of the study to detect a significant association, if there is one.
In conclusion, in this large prospective cohort of type 2 diabetes, recurrent mild hypoglycemia was not predictive of CVD or mortality, although an association of mild hypoglycemia with CVD was observed among patients with CKD and insulin users. Whereas the observational nature of the study precludes detailed delineation of the underlying mechanisms for these findings, our results suggested that mild hypoglycemia may have prognostic significance in patients who are inherently more vulnerable to cardiovascular events.
Acknowledgments
The JADE Program was conceptualized, designed and implemented by the Asia Diabetes Foundation (ADF) (www.adf.org.hk), established as a charitable organization under the Chinese University of Hong Kong Foundation to conduct translational research through private public partnership. We thank the nurses and health care assistants of the Diabetes and Endocrine Centre of the Prince of Wales Hospital, the Diabetes Centre of the Alice Ho Miu Ling Nethersole Hospital, and the Yao Chung Kit Diabetes Assessment Centre, for their continuous support and dedication in enrolling patients for the JADE program.
Footnotes
Abbreviations: ABI = ankle-to-brachial index, ACCORD = Action to Control Cardiovascular Risk in Diabetes, ACR = albumin-to-creatinine ratio, ADF = Asia Diabetes Foundation, ADVANCE = Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation, BMI = body mass index, BP = blood pressure, CI = confidence interval, CKD = chronic kidney disease, CVD = cardiovascular disease, ESRD = end-stage renal disease, GFR = glomerular filtration rate, HA = Hospital Authority, HbA1c = glycated hemoglobin, HDL = high-density lipoprotein, HR = hazard ratio, ICD-9 = International Classification of Disease, Ninth Revision, JADE = Joint Asia Diabetes Evaluation, LDL = low-density lipoprotein, MDRD = Modification of Diet in Renal Disease, PAD = peripheral artery disease, SCR = serum creatinine, SD = standard deviation.
Author contributions: All authors have contributed to the conception of the study, revision of the manuscript, and given approval for the present version to be published. In addition, AOL and JCC contributed to results interpretation and drafting of the manuscript; TSH and ESL contributed to statistical analysis; GTK, RO, C-CT, and W-YS contributed to acquisition of data. As the corresponding author, AOL has full access to all the data in the study and takes final responsibility for the decision to submit for publication.
Funding: The ADF has been partially supported by an educational grant from Merck Limited. Merck Limited has no role in the conception of study, analysis, data interpretation, and writing of the manuscript. Merck Limited has no role in the decision to submit the manuscript for publication. None of the authors have been paid to write this article by a pharmaceutical company.
JCC and FCC are Chief Executive Counsellors of the ADF. AOL is the Deputy Medical Director of the ADF, and JCC is the Chief Executive Officer of ADF, all on a pro bono basis.
The remaining authors report no conflicts of interest.
References
- 1.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853. [PubMed] [Google Scholar]
- 2.Holman RR, Sanjoy KP, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589. [DOI] [PubMed] [Google Scholar]
- 3.Gerstein HC, Miller ME, Byrington RP, et al. For The Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerstein HC, Miller ME, Genuth S, et al. for the ACCORD Study Group Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011; 364:818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel A, MacMahon S, Chalmers J, et al. for the ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572. [DOI] [PubMed] [Google Scholar]
- 6.Duckworth W, Abraira C, Moritz T, et al. for the VADT Investigators Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139. [DOI] [PubMed] [Google Scholar]
- 7.Zoungas S, Patel A, Chalmers J, et al. for the ADVANCE Collaborative Group Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010; 363:1410–1418. [DOI] [PubMed] [Google Scholar]
- 8.Bonds DE, Miller ME, Bergenstal RM, et al. The Association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340:b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y, Campbell CR, Fonseca V, et al. Impact of hypoglycemia associated with anti-hyperglycemic medications on vascular risks in veterans with type 2 diabetes. Diabetes Care 2012; 35:1126–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu PF, Sung SH, Cheng HM, et al. Association of clinical symptomatic hypoglycaemia with cardiovascular events and total mortality in type 2 diabetes. Diabetes Care 2013; 36:894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khunti K, Davis M, Majeed A, et al. Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care 2015; 38:316–322. [DOI] [PubMed] [Google Scholar]
- 12.Koivikko ML, Karsikas M, Salmela PI, et al. Effects of controlled hypoglycaemia on cardiac repolarisation in patients with type 1 diabetes. Diabetologia 2008; 51:426–435. [DOI] [PubMed] [Google Scholar]
- 13.Stahn A, Pistrosch F, Ganz X, et al. Relationship between hypoglycemic episodes and ventricular arrhythmias in patients with type 2 diabetes and cardiovascular diseases: Silent hypoglycemias and silent arrhythmias. Diabetes Care 2014; 37:516–520. [DOI] [PubMed] [Google Scholar]
- 14.Hilsted J, Bonde-Petersen F, Nørgaard MB, et al. Haemodynamic changes in insulin-induced hypoglycaemia in normal man. Diabetologia 1984; 26:328–332. [DOI] [PubMed] [Google Scholar]
- 15.Wright RJ, Frier BM. Vascular disease and diabetes: is hypoglycaemia an aggravating factor? Diabetes Metab Res Rev 2008; 24:353–363. [DOI] [PubMed] [Google Scholar]
- 16.Goditidze JN, Hedrington MS, Briscoe VJ, et al. Effects of acute hypoglycaemia on inflammatory and pro-atherothrombotic biomarkers in individuals with type 1 diabetes and healthy individuals. Diabetes Care 2010; 33:1529–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright RJ, Newby DE, Stirling D, et al. Effects of acute insulin-induced hypoglycaemia on indices of inflammation. Diabetes Care 2010; 33:1591–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.UK Hypoglycaemia Study Group Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007; 50:1140–1147. [DOI] [PubMed] [Google Scholar]
- 19.McCoy RG, Van Houten HK, Ziegenfuss JY, et al. Increased mortality of patients with diabetes reporting hypoglycemia. Diabetes Care 2012; 35:1897–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko GT, So WY, Tong PC, et al. From design to implementation: the Joint Asia Diabetes Evaluation (JADE) program: a descriptive report of an electronic web-based diabetes management program. BMC Med Inform Decis Mak 2010; 10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan J, So W, Ko G, et al. The Joint Asia Diabetes Evaluation (JADE) program: a web-based program to translate evidence to clinical practice in type 2 diabetes. Diabet Med 2009; 26:693–699. [DOI] [PubMed] [Google Scholar]
- 22.Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006; 17:2937–2944. [DOI] [PubMed] [Google Scholar]
- 23.Available at: http://www.fhb.gov.hk/beStrong/files/consultation/appendixb_eng.pdf Accessed March 2, 2016. [Google Scholar]
- 24.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994; 81:515–526. [Google Scholar]
- 25.Goto A, Arah OA, Goto M, et al. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ 2013; 347:f4533. [DOI] [PubMed] [Google Scholar]
- 26.Seaquist ER, Miller ME, Bonds DE, et al. The impact of frequent and unrecognized hypoglycaemia on mortality in the ACCORD study. Diabetes Care 2012; 35:409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reno CM, Daphna-Iken D, Chen YS, et al. Severe hypoglycaemia-induced lethal cardiac arrhythmias are mediated by sympathoadrenal activation. Diabetes 2013; 62:3570–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schopman JE, Geedes J, Frier BM. Prevalence of impaired awareness of hypoglycaemia and frequency of hypoglycaemia in insulin-treated type 2 diabetes. Diabetes Res Clin Pract 2010; 87:64–68. [DOI] [PubMed] [Google Scholar]


