Abstract
A cross-sectional area (CSA) and thickness reduction of the abductor hallucis (AbH) is shown in subjects with hallux valgus (HV). To date, other soft-tissue structures have not been researched in relation with HV. The aim of this study was to compare the CSA and thickness of the intrinsic plantar muscles and fascia (PF) between feet with and without HV. Therefore, a cross-sectional and case-control study was performed using B-mode with an iU22 Philips ultrasound system and a 5 to 17-MHz transducer. The CSA and thickness were measured for the AbH, flexor digitorum brevis (FDB) and flexor hallucis brevis (FHB), and also the thickness for the anterior, middle, and posterior PF portions. A convenience sample of 40 feet, 20 with HV and 20 without HV, was recruited from a clinical and research center. A multivariate regression analysis using linear regression was performed to evaluate the ultrasound imaging measurements (α = 0.05). Consequently, statistically significant differences were observed between the groups (P < 0.05) for the AbH and FHB thickness, and CSA reduction, and also the plantar fascia thickness increase in favor of the HV group. On the contrary, the FDB thickness and CSA did not show statistically significant differences (P ≥ 0.05). In conclusion, the CSA and thickness of the AbH and FHB intrinsic plantar muscles are reduced, whereas the thickness of the anterior, middle, and posterior PF portions are increased, in subjects with HV compared with those without HV.
Keywords: anatomy, cross-sectional, hallux valgus, physical therapy modalities, ultrasonography
1. Introduction
The toe region comprises the 14% of the nontraumatic foot and ankle consultations in primary care. Indeed, hallux valgus (HV) is 1 of the 10 most frequently documented nontrauma conditions.[1] The estimated prevalence for HV reaches the 23% in adults aged 18 to 65 years, and increases with age or female sex.[2] Moreover, HV produces an impact in the quality of life and depression levels, which appears to be associated with their degree of deformity.[3,4]
Furthermore, pronated foot posture and function are associated with the presence of HV.[5] Therefore, this condition modifies foot loading and pressure patterns.[6] The severity of the radiographic first metatarsophalangeal joint osteoarthritis increases with the prevalence of HV, among other demographic and clinical factors.[7] HV shows a reduction in the cross-sectional area (CSA) and thickness of the abductor hallucis (AbH), independently of the degree of deformity. Consequently, morphological changes to the AbH muscle may occur early in the HV development.[8] The toe-spread-out exercise is recommended for subjects with mild to moderate HV degree due to the angle reduction and AbH CSA increase.[9]
Rehabilitative ultrasound imaging (RUSI) has been used to measure the CSA and thickness of the muscles and connective tissue in the locomotor system conditions which influence physical therapy evaluation.[10] Regarding the intrinsic plantar muscles and fascia (PF), the CSA and thickness of the flexor hallucis brevis (FHB), flexor digitorum brevis (FDB), AbH, and fascia can be used to explain the relationship between foot function and clinical conditions (ie, pes planus).[11,12] These RUSI measurements showed an excellent intraclass correlation coefficient (ICC) from 0.91 to 0.98.[11]
To date, the decrease of the CSA and thickness of the AbH in subjects with HV were stated. Nevertheless, RUSI measurements need to be established in the other plantar muscles and fascia of patients with HV. Accordingly, the aim of this study was to compare the CSA and thickness of the FHB and FDB plantar muscles, and also the PF thickness, in feet with and without HV.
2. Methods
2.1. Sample
A convenience sample of 40 feet was recruited at the CARMASALUD clinical and research center, Madrid, Spain (20 feet with HV and 20 feet without HV).[13] Subjects did not receive any treatment of the foot or forefoot regions in the 6 months before measurements.
The inclusion criteria comprised subjects aged 18 to 65 years with no pain in the leg, ankle, and foot regions (excluding HV region) over the past 6 months.[11] The exclusion criteria were self-reported, or medical record included fractures, surgeries, tears, sprains, tendinopathies, neuropathies, rheumatoid or systemic conditions, and pharmacotherapy.[11–13] Regarding the foot and forefoot region, other specific exclusion criteria included prior medical diagnosis of plantar orthoses use, pes planus and cavus, hallux rigidus, plantar fasciitis, heel spurs, Morton neuroma, Sever disease, tarsal tunnel syndrome, or tibial nerve entrapment.[5,11–14] Considering the anatomical area from the low back to the leg, degeneration or inflammation of the tibial periosteum, meniscopathy, sprains, Baker cysts, bursitis, sciatic nerve entrapments or piriformis syndrome, labral impingement syndrome, or sacroiliac joint dysfunction were also excluded.[5,11,12,14–20] Furthermore, exercise practice for less than 1 or more than 3 hours per week or high-intensity exercise was excluded due to lower limbs CSA modifications could be produced.[21]
2.2. Ethical considerations
The Research and Ethics Committee of University of A Coruña (A Coruña, Spain; record number: CE 06/2014) approved the study. Consent informs were signed by all subjects before the beginning of the study. The ethical standards for human experimentation of the Declaration of Helsinki were respected.[22] The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were applied.[23]
2.3. Sociodemographic and descriptive data
The sociodemographic descriptive characteristics were collected: sex (male or female), age (years), weight (kg), height (cm), body mass index (BMI, kg/m2), pain intensity (Numeric Rating Scale), HV side (left or right), HV angle (°), dominant side (left or right), and foot length (cm). These data were collected to assess their relationship with the CSA and thickness of the intrinsic plantar muscles and fascia.[11,12]
2.4. HV degree
In addition, the same specialized researcher podiatrist (DRS) diagnosed the HV degree using the Manchester Scale.[23] This scale is a noninvasive method of measuring the grade of HV deformity by means of a standardized photograph set, from grade I (no HV deformity) to grade IV (severe HV deformity). An excellent interexaminer repeatability (kappa coefficient κ = 0.86) was showed for this 4-point scale.[24] A high inter-rater reliability and validity of the HV angle between the photographic measurements and radiographs was demonstrated. Their ICC (>0.96) and Pearson correlation coefficient (r = 0.96) were excellent, and also their confidence interval (95% CI) limits of agreement were acceptable. Therefore, the cost and radiation exposure of radiograph use may be avoided.[25]
2.5. Ultrasound imaging
All RUSI imagines were performed by the same physical therapist (CCL) with 4 years of specialization and experience. This rater was not blind to case or control group assignment during RUSI evaluation. A high-quality diagnostic ultrasound system (iU22; Philips Ultrasound, 22100 Bothell-Everett Highway; Bothell, WA), with a 7 to 17.0-MHz-range linear transducer (L 17–5 Broadband Linear Array type; 38-mm footprint), was used to perform resting B-mode ultrasound imaging.
The probe location (Fig. 1) was marked as proposed by Crofts et al[11] and Angin et al.[12] On one hand, the PF was measured in a longitudinal direction between the medial calcaneal tubercle and the second toe. Three different regions were assessed: the calcaneous insertion (PF-1), navicular tubercle (PF-2), and second metatarsal head (PF-3). On the other hand, the thickness (longitudinal) and the CSA (perpendicular) in the thickest part of the AbH, FDB, and FHB were evaluated on 3 different scanning lines. First, the AbH scanning line was placed between the medial calcaneal tuberosity and the navicular tuberosity. Second, the FDB scanning line was drawn from the medial tubercle of the calcaneus to the third toe. Finally, the FHB scanning line was located longitudinally along the shaft of the first metatarsal.[11,12] Subjects for whom the limits of the muscles and PF could not be differentiated were excluded.
Figure 1.
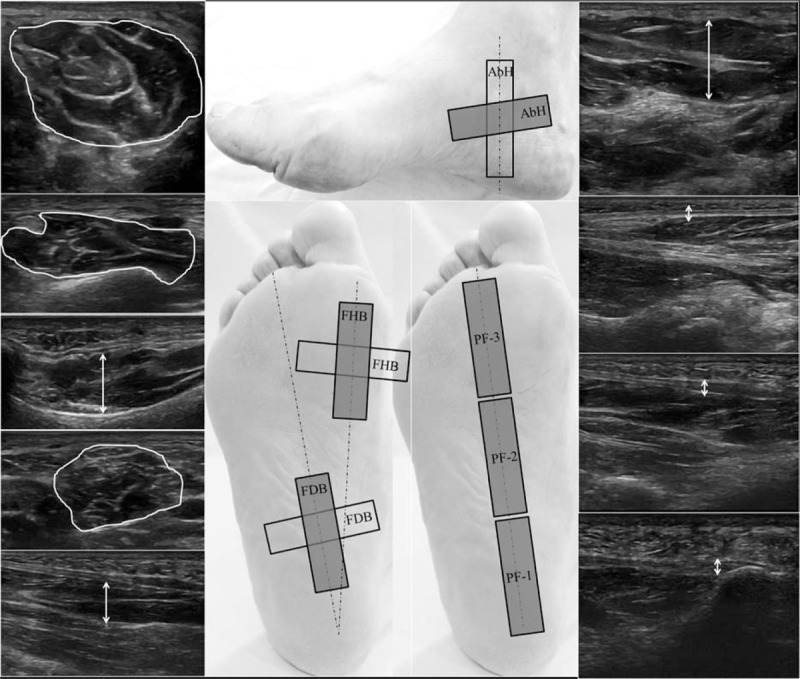
Probe location and ultrasound imaging measurements. AbH = abductor hallucis, CSA = cross-sectional area, FDB = flexor digitorum brevis, FHB = flexor hallucis brevis, HV = hallux valgus, PF-1 = plantar fascia at the calcaneous insertion, PF-2 = plantar fascia at the navicular tubercle, PF-3 = plantar fascia at the second metatarsal head.
The RUSI measurements were carried out by the same physical therapist (AGM) with 4 years of specialization and experience using the software (QLAB advanced quantification software; iSCAN 2D) provided with the ultrasound imaging system (iU22; Philips Ultrasound, 22100 Bothell-Everett Highway; Bothell, WA). The mean of 3 repeated values was obtained for each measure.[11,12]
2.6. Data analysis
The statistical analysis was performed by the SPSS version 22.0 for Windows (IBM SPSS Statistics for Windows; Armonk, NY: IBM Corp) and an α error of 0.05 (95% CI), with a desired power of 80% (β error of 0.2). First, Shapiro-Wilks was carried out to assess normality. Second, all parametric data were analyzed to compare the RUSI measures and the descriptive data (age, weight, height, BMI, pain intensity, HV angle and foot length) by the Student t tests for independent samples. The Fisher exact test was used to compare the sex, HV, and dominant side, and also the chi-square test was used to analyze the HV degree. Box-plots were used to illustrate the CSA and thickness RUSI values from the case and control group characteristics.
In addition, a multivariate predictive analysis was carried out by linear regression. Linear regression was performed using the stepwise selection method and the R2 coefficient to state the quality adjustment. Descriptive data, including age, sex (male = 0; female = 1), weight, height, BMI, pain intensity, foot length, dominant side (left = 0; right = 1), HV angle, HV side (left = 0; right = 1), HV degree (grade I = 0; grade II = 1; grade III = 2; grade IV = 3), and group (control = 0; HV = 1) were considered as dependent variables. The RUSI measures were considered as independent variables.
3. Results
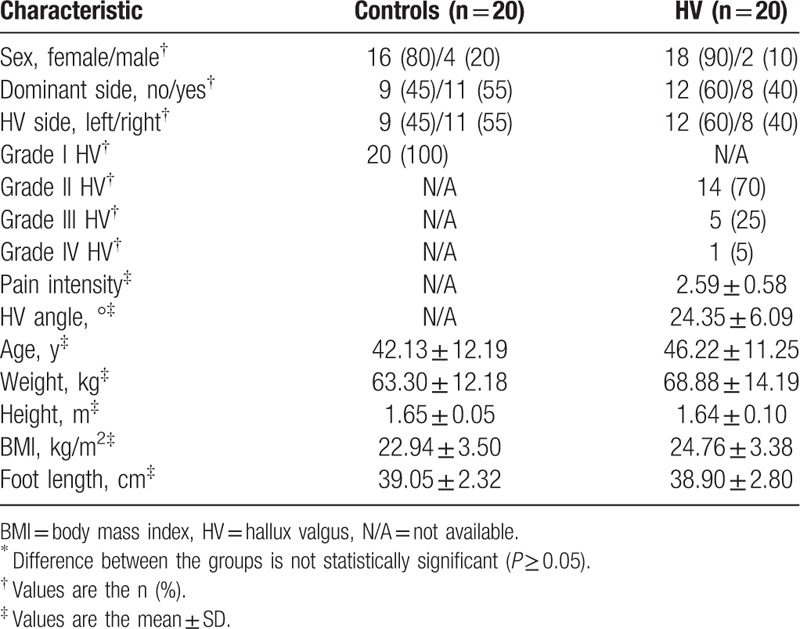
The descriptive data of the sample characteristics are shown in Table 1. The groups did not differ in sex (P = 0.66), age (P = 0.27), dominant side (P = 0.52), HV side (P = 0.52), height (P = 0.65), weight (P = 0.19), BMI (P = 0.10), or foot length (P = 0.85). The pain intensity and HV angle mean ± SD were 2.59 ± 0.58 and 24.35 ± 6.09° in subjects with HV, respectively. The numbers of grades I, II, III, and IV of HV were 20 (100% without HV), 14 (70% with HV), 5 (25% with HV), and 1 (5% with HV), respectively.
Table 1.
Demographic and baseline characteristics of the subjects∗.
3.1. Plantar muscles and fascia RUSI changes in subjects with HV
The descriptive data of the RUSI measurements for the CSA and thickness for the muscles and PF of both groups are summarized in Table 2. On one hand, statistically significant differences were observed between the groups (P < 0.05) for the AbH and FHB thickness and CSA reduction, and also the plantar fascia (PF-1, PF-2, and PF-3) thickness increase in favor of the HV group. On the other hand, the FDB thickness and CSA did not show statistically significant differences (P ≥ 0.05). Box-plots to illustrate the CSA and thickness of the ultrasound imaging measurements of the control and HV groups are shown in Fig. 2.
Table 2.
Ultrasound parameter measurements.
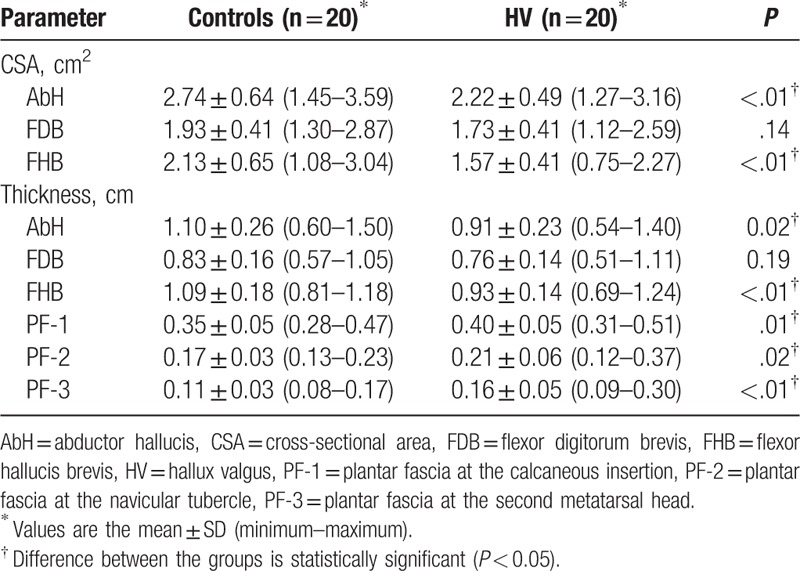
Figure 2.

Box-plot to illustrate the thickness and CSA of the ultrasound imaging measurements of the control and HV groups. AbH = abductor hallucis, CSA = cross-sectional area, FDB = flexor digitorum brevis, FHB = flexor hallucis brevis, HV = hallux valgus, PF-1 = plantar fascia at the calcaneous insertion, PF-2 = plantar fascia at the navicular tubercle, PF-3 = plantar fascia at the second metatarsal head.
3.2. Multivariate predictive analysis of plantar muscles and fascia RUSI changes
Regarding the multivariate regression analysis, the linear regression model (Table 3) determined significant differences (P < 0.05) for each RUSI measurement. Furthermore, the large R2 of the prediction model ranged from 0.224 to 0.595.
Table 3.
Multivariate predictive analysis of plantar muscles and fascia ultrasound imaging.

4. Discussion
To improve the anatomical knowledge, this is the first study that states the resting CSA and thickness of the intrinsic plantar muscles, such as the FDB and FHB, and fascia, at 3 different regions, in subjects with HV.
In addition, previous studies have researched the CSA and thickness of the AbH in this population.[8,9] In addition, the AbH thickness and CSA (mean ± SD) varied from 1.13 ± 0.17 to 1.19 ± 0.14 cm and 2.71 ± 0.61 to 3.00 ± 0.46 cm2 for different HV degrees, respectively. Independently of the degree of deformity, the AbH thickness and CSA were decreased compared with subjects without HV (1.33 ± 0.2 cm and 3.39 ± 0.56 cm2, respectively). Consequently, morphological alterations to the AbH muscle may be developed early in the HV condition. According to Stewart et al,[8] these results and variation ranges are similar with the present study (Table 2 and Fig. 2).
For plantar muscles and fascia RUSI measurements in healthy subjects, the CSA means ± SD of 3.03 ± 0.44, 1.82 ± 0.54, and 3.17 ± 0.50 cm2 for the AbH, FDB, and FHB were determined. In addition, the thickness means ± SD of 1.27 ± 0.14, 1.05 ± 0.19, 1.59 ± 0.29, 0.29 ± 0.05, 0.19 ± 0.03, and 0.13 ± 0.01 cm for the AbH, FDB, FHB, PF-1, PF-2, and PF-3 were established. According to Crofts et al,[11] these measurements coincide with our research (Table 2 and Fig. 2).
Using the same procedure, Angin et al[12] found a similar AbH (2.75 ± 0.34 vs 2.36 ± 0.47 cm2), FDB (2.14 ± 0.59 vs 2.20 ± 0.57 cm2), and FHB (2.97 ± 0.46 vs 2.66 ± 0.48 cm2) CSA in subjects without and with pes planus, respectively. Furthermore, a similar AbH (1.27 ± 0.09 vs 1.18 ± 0.11 cm), FDB (0.89 ± 0.17 vs 0.86 ± 0.16 cm), FHB (1.43 ± 0.20 vs 1.30 ± 0.18 cm2), PF-1 (0.33 ± 0.04 vs 0.32 ± 0.05 cm), PF-2 (0.19 ± 0.03 vs 0.16 ± 0.03 cm), and PF-3 (0.13 ± 0.02 vs 0.10 ± 0.02 cm) thickness were found in subjects without and with pes planus, respectively. Consistent with Angin et al,[12] who showed that the CSA and thickness of the AbH (−12.8% and −6.8%) and FHB (−8.9% and −7.6%) muscles were smaller in feet with pes planus, respectively, the AbH (−18.9% and −17.2%) and FHB (−17.2% and −14.6%) muscles CSA and thickness were also smaller in the feet with HV compared with feet without this condition (Table 2 and Fig. 2). In addition, neither pes planus nor feet with HV showed any statistically significant difference (P ≥ 0.05) in the FDB thickness and CSA. Nevertheless, the middle (−10.6%) and anterior (−21.7%) PF portions were thinner in the pes planus,[12] whereas the anterior (45.4%), middle (23.5%), and posterior (14.2%) PF regions were thicker in the feet with HV (Table 2 and Fig. 2). Consequently, these values may be used as the relevant clinical differences in the RUSI measurements obtained during clinical interventions of subjects with HV.
4.1. Future studies
Further studies are necessary to improve knowledge about the plantar muscles and fascia changes that may occur secondary to the clinical treatments, such as the therapeutic exercise, of subjects with HV. According to a current practice survey of Australian podiatrist, the nonsurgical management of HV is widely recommended.[26] Indeed, the toe-spread-out exercise may reduce the HV angle at rest (−3.41 ± 3.17°) and actively (−6.42 ± 3.42°), and also increase the AbH CSA (0.48 ± 0.28 cm2) in subjects with mild to moderate HV degree.[9]
4.2. Limitations
Several limitations should be considered in the present research. First, a blinded randomized controlled trial was not carried out. Nonsurgical interventional studies in subjects with HV should be considered.[9,26] Second, different age ranges from 18 to 65 years have not been taken into account. Management strategies across patient age groups with updated clinical guidelines should differentiate between adult and juvenile HV.[26] Furthermore, the plantar muscles and fascia RUSI measurements need to be stated in the older adults population due to the high HV prevalence.[2] Third, the bilateral HV may have influenced the quality of life, pain, and related functional status.[27] Fourth, the rater who carried out the ultrasound imaging was not blinded to case or control group. Nevertheless, the examiner who performed the RUSI measurements was blinded to avoid bias. Finally, more diverse subjects and a larger sample size may be useful to improve the research study strength and identify variation across countries.[28]
5. Conclusions
The CSA and thickness of the AbH and FHB intrinsic plantar muscles are reduced, whereas the thickness of the anterior, middle, and posterior PF portions are increased in subjects with HV compared with subjects without HV.
Footnotes
Abbreviations: AbH = abductor hallucis, CSA = cross-sectional area, FDB = flexor digitorum brevis, FHB = flexor hallucis brevis, HV = hallux valgus.
Author contributions: All authors contributed to the study concept and design. DRS and CCL did the main statistical analysis and interpretation of data. CCL and AGM contributed to draft the report. DLL, PPL, CRM, and ISC supervised the study. All authors revised the text for intellectual content and have read and approved the final version of the manuscript.
Disclosures: The authors certify that there are not competing interests, source of funding, or financial benefits.
References
- 1.Menz HB, Jordan KP, Roddy E, et al. Characteristics of primary care consultations for musculoskeletal foot and ankle problems in the UK. Rheumatology (Oxford) 2010; 49:1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nix S, Smith M, Vicenzino B. Prevalence of hallux valgus in the general population: a systematic review and meta-analysis. J Foot Ankle Res 2010; 3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.López DL, Callejo González L, Losa Iglesias ME, et al. Quality of life impact related to foot health in a sample of older people with hallux valgus. Aging Dis 2016; 7:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.López DL, Fernández JM, Iglesias ME, et al. Influence of depression in a sample of people with hallux valgus. Int J Ment Health Nurs 2016; Feb 19. doi: 10.1111/inm.12196. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 5.Hagedorn TJ, Dufour AB, Riskowski JL, et al. Foot disorders, foot posture, and foot function: the Framingham foot study. PLoS One 2013; 8:e74364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galica AM, Hagedorn TJ, Dufour AB, et al. Hallux valgus and plantar pressure loading: the Framingham foot study. J Foot Ankle Res 2013; 6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menz HB, Roddy E, Marshall M, et al. Demographic and clinical factors associated with radiographic severity of first metatarsophalangeal joint osteoarthritis: cross-sectional findings from the Clinical Assessment Study of the Foot. Osteoarthritis Cartilage 2015; 23:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart S, Ellis R, Heath M, et al. Ultrasonic evaluation of the abductor hallucis muscle in hallux valgus: a cross-sectional observational study. BMC Musculoskelet Disord 2013; 14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim MH, Yi CH, Weon JH, et al. Effect of toe-spread-out exercise on hallux valgus angle and cross-sectional area of abductor hallucis muscle in subjects with hallux valgus. J Phys Ther Sci 2015; 27:1019–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potter CL, Cairns MC, Stokes M. Use of ultrasound imaging by physiotherapists: a pilot study to survey use, skills and training. Man Ther 2012; 17:39–46. [DOI] [PubMed] [Google Scholar]
- 11.Crofts G, Angin S, Mickle KJ, et al. Reliability of ultrasound for measurement of selected foot structures. Gait Posture 2014; 39:35–39. [DOI] [PubMed] [Google Scholar]
- 12.Angin S, Crofts G, Mickle KJ, et al. Ultrasound evaluation of foot muscles and plantar fascia in pes planus. Gait Posture 2014; 40:48–52. [DOI] [PubMed] [Google Scholar]
- 13.Becerro-de-Bengoa-Vallejo R, Losa-Iglesias ME, Rodriguez-Sanz D. Static and dynamic plantar pressures in children with and without sever disease: a case-control study. Phys Ther 2014; 94:818–826. [DOI] [PubMed] [Google Scholar]
- 14.Cartwright MS, Walker FO. Neuromuscular ultrasound in common entrapment neuropathies. Muscle Nerve 2013; 48:696–704. [DOI] [PubMed] [Google Scholar]
- 15.Relph N, Herrington L, Tyson S. The effects of ACL injury on knee proprioception: a meta-analysis. Physiother 2014; 100:187–195. [DOI] [PubMed] [Google Scholar]
- 16.Haviv B, Bronak S, Thein R. Correlation between body mass index and chondral lesions in isolated medial meniscus tears. Indian J Orthop 2015; 49:176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meadows JR, Finnoff JT. Lower extremity nerve entrapments in athletes. Curr Sports Med Rep 2014; 13:299–306. [DOI] [PubMed] [Google Scholar]
- 18.Lee AJ, Armour P, Thind D, et al. The prevalence of acetabular labral tears and associated pathology in a young asymptomatic population. Bone Joint J 2015; 97-B:623–627. [DOI] [PubMed] [Google Scholar]
- 19.Cass SP. Piriformis syndrome: a cause of nondiscogenic sciatica. Curr Sports Med Rep 2015; 14:41–44. [DOI] [PubMed] [Google Scholar]
- 20.Kurosawa D, Murakami E, Aizawa T. Referred pain location depends on the affected section of the sacroiliac joint. Eur Spine J 2015; 24:521–527. [DOI] [PubMed] [Google Scholar]
- 21.Belavý DL, Miokovic T, Rittweger J, et al. Estimation of changes in volume of individual lower-limb muscles using magnetic resonance imaging (during bed-rest). Physiol Meas 2011; 32:35–50. [DOI] [PubMed] [Google Scholar]
- 22.Holt GR. Declaration of Helsinki-the world's document of conscience and responsibility. South Med J 2014; 107:407. [DOI] [PubMed] [Google Scholar]
- 23.White RG, Hakim AJ, Salganik MJ, et al. Strengthening the reporting of observational studies in epidemiology for respondent-driven sampling studies: “STROBE-RDS” statement. J Clin Epidemiol 2015; 68:1463–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menz HB, Fotoohabadi MR, Wee E, et al. Validity of self-assessment of hallux valgus using the Manchester scale. BMC Musculoskelet Disord 2010; 11:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nix S, Russell T, Vicenzino B, et al. Validity and reliability of hallux valgus angle measured on digital photographs. J Orthop Sports Phys Ther 2012; 42:642–648. [DOI] [PubMed] [Google Scholar]
- 26.Hurn SE, Vicenzino BT, Smith MD. Non-surgical treatment of hallux valgus: a current practice survey of Australian podiatrists. J Foot Ankle Res 2016; 9:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coşkun G, Talu B, Bek N, et al. Effects of hallux valgus deformity on rear foot position, pain, function, and quality of life of women. J Phys Ther Sci 2016; 28:781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray CJ, Barber RM, Foreman KJ, et al. GBD 2013 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet 2015; 386:2145–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]


