Supplemental Digital Content is available in the text
Keywords: antitumor immunity, colon cancer, lymph node metastases
Abstract
Lymph node (LN) involvement in colonic carcinoma (CC) is a grave prognostic sign and mandates the addition of adjuvant treatment. However, in light of the histological variability and outcomes observed, we hypothesized that patients with LN metastases (LNM) comprise different subgroups.
We retrospectively analyzed the histological sections of 82 patients with CC and LNM. We studied various histological parameters (such as tumor grade, desmoplasia, and preservation of LN architecture) as well as the prevalence of specific peritumoral immune cells (CD8+, CD20+, T-bet+, and GATA-3+). We correlated the histological and immunological data to patient outcome.
Tumor grade was a significant prognostic factor even in patients with LNM. So was the number of LN involved (N1/N2 stage). From the morphological parameters tested (LN extracapsular invasion, desmoplasia in LN, LN architecture preservation, and mode of metastases distribution), none was found to be significantly associated with overall survival (OS). The mean OS of CD8+ low patients was 66.6 ± 6.25 versus 71.4 ± 5.1 months for CD8+ high patients (P = 0.79). However, T-helper (Th) 1 immune response skewing (measured by Th1/Th2 ratio >1) was significantly associated with improved OS. For patients with low ratio, the median OS was 35.5 ± 5 versus 83.5 months for patients with high Th1/Th2 ratio (P = 0.001).
The histological presentation of LNM does not entail specific prognostic information. However, the finding of Th1 immune response in LN signifies a protective immune response. Future studies should be carried to verify this marker and develop a strategy that augments this immune response during subsequent adjuvant treatment.
1. Introduction
Colonic carcinoma (CC) is the third most common malignancy in the United States and Europe.[1] Lymph node metastases (LNM) are major prognostic factor of this tumor, affecting both overall survival (OS) and disease-free survival (DFS). About 40% of the patients present with LNM,[2] usually mandating addition of adjuvant treatment to surgical resection. This treatment has been shown to increase OS and improve prognosis.[3] However, node-positive disease probably consists of different patient subgroups. The TNM staging system for CC, the most widely used staging system, acknowledges that the number of involved lymph node (LN) is 1 factor that defines different subpopulations with LNM, by the discrimination between N1 (up to 3 involved LN) and N2 (>3). Moreover, it has been shown that specific subpopulations with node-positive disease have the same prognosis as T3N0 patients.[4] Histological observation also reinforces the notion of heterogeneity of LN involvement. Among the varying variables are tumor location within LN, preservation of LN architecture, desmoplasia around tumor in the LN, and many more. The clinical significance of this variability is currently unknown.
Previous research on the different histological presentation of LNM dealt with some parameters. For example, extracapsular invasion (ECI), which is the extension of cancer tumor cells beyond the LN capsule into the perinodal fat tissue. The ability of tumor cells to invade the capsular tissue and degrade extracellular matrix is assumed to be a dismal sign.[5,6] However, the prognostic significance of ECI is not consistent across all studies.[7] In addition, it was shown that activation of follicles in metastatic LN is associated with improved prognosis,[8] maybe because it serves a surrogate for a protective immune response. Recently, LN size was positively correlated with patient survival,[9] another parameter that may hint on antitumor immune response.
Another unexplored area is the tumor immune interactions within the metastatic LN. In recent years, much research was performed on tumor-infiltrating lymphocytes (TILs) and their significance in various tumors. It turned out that the prognostic significance of TILs depends on the specific subtypes of immune cells present in the infiltrate. In CC, as in many other different tumors, T-helper (Th) 1 immune response, induced by cytotoxic (CD8+) T cells and their effector molecules is associated with improved prognosis. On the other hand, infiltration of tumor by regulatory T cells (CD4 + CD25 + FOXP3+) and Th2 immune cells is associated with reduced antitumor response and worse prognosis.[10–12] Each of the Th lineages is dependent on a specific transcription factor for its development, usually responsible for both chromatin remodeling and appropriate cytokine production. T-bet is the main transcription factor responsible for Th1 lineage, whereas GATA-3 is the responsible for Th2 lineage differentiation.[13,14] In the LN, tumor cells are surrounded by immune cells from various types, hence tumor survival in this hostile environment implies an evasion or escape mechanism exerted by tumor cells. Indeed, previous research done on sentinel LN from melanoma patients revealed immune suppression of these draining LNs, induced by the tumor. It is manifested in the paucity of high endothelial venules which normally afford naïve T cells transmigration into the LN, decreased proliferation of T cells from the involved LN, and reduced dendritic cells functions.[15] However, not much is known about tumor immune interactions at LNM of CC.
The purpose of this study was to identify specific patient subpopulations within patients with CC and LNM (stage III CC). For this purpose, we analyzed both histological and immunological parameters in the metastatic LN.
2. Materials and methods
2.1. Patient population
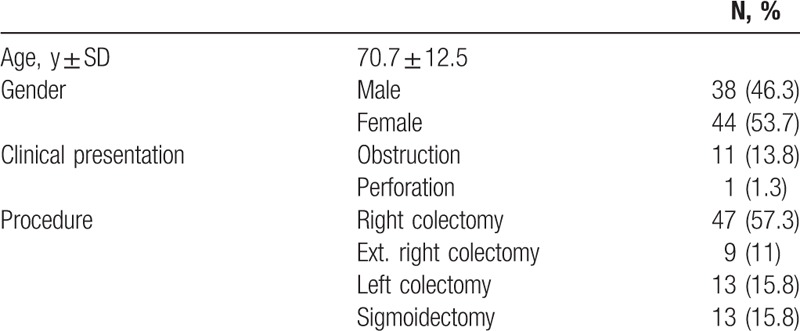
The study included 82 adult patients with pathologically proven CC. All kinds of colonic resections (right, extended right, left, and sigmoid) were included, preformed in either open or laparoscopic techniques. Rectal cancer was not included in this study due to neoadjuvant treatment and its possible effects on immunological parameters. Patients were operated between April 2008 and April 2011 at the Tel Aviv Sourasky Medical Center (TASMC). Only patients who had LN metastasis (i.e., N-positive disease) were included. The number of cases was calculated according to 30% difference in OS for Th1 predominant immune response, based on previous studies that explored the effects of a protective immune response in CC.[10,16,17] We excluded patients under the age of 18, patients with distant metastases at presentation (including synchronous liver metastases), patients who were diagnosed with any other primary tumor within 5 years before their operation, patients with any autoimmune or active infectious disease, and those who were being treated with immune-modifying medications (such as steroids, methotrexate, etc.). The study was approved by the Institutional Ethics Board of the TASMC and was conducted according to the principles of the Helsinki Declaration. Data were extracted from electronic patients’ files and pathological reports. Recurrence was defined as a new lesion found on any imaging study (CT or positron emission tomography scan). DFS was defined as the time from surgical resection until the date of recurrence. OS was defined from the time of surgery until death (determined by the national population registry, censored in January 2016). For DFS in this study, we analyzed the last time of patient follow-up in our institution, as some of the patients continued their postoperative treatment in other institutions. However, the data regarding DFS were not comprehensive due to loss to follow-up, hence we focused our analysis on OS.
2.2. Morphological analysis
Histological sections (paraffin embedded, 4–6-μm width) stained with hematoxylin and eosins of metastatic LNs were analyzed. Lymphoid tissue (usually germinal center and/or capsule) was needed to classify a structure as LN. Tumor deposits, that is, macroscopic or microscopic nests or nodules of cancer, found in the pericolic adipose tissue without histological evidence of residual LN, were excluded. Desmoplasia (in LNM) was defined as peritumoral fibrosis. ECI was defined as the extension of cancer tumor cells into the LN capsule.
2.3. Immunohistochemistry of LN sections
Immunohistochemistry was performed on the formalin fixed, paraffin embedded tissue sections. We selected the LN presumed to be with the worse features (i.e., ECI, LN with distorted architectural structure) for further staining. Briefly, tissue was rehydrated, using xylol and descending concentrations of alcohol. Antigen retrieval was done with citrate buffer. Then H2O2 (3%) was applied to neutralize the endogenous peroxidase. Staining itself consisted of blocking solution, primary antibody as indicated in Table 1, biotinylated second antibody, conjugated enzyme, and 3,3′-diaminobenzidine. We have then stained the tissue using Mayer hematoxylin. The final step was dehydrating the tissue, using ascending concentrations of alcohol. Slides were covered with coverslips and mounting media before microscopy analysis. We have used specific antibodies for various immune markers, described as follows: CD8 (MSK011-05, Zytomed, Berlin, Germany), CD20 (SIG-3324-1000, Covance-signet, Princeton, NJ), T-bet (Sa-RBK049, Zytomed, Berlin, Germany), and GATA-3 (MAB6330, R&D Systems, Minneapolis, MN).
Table 1.
Clinical characteristics of patients.
For quantification of peritumoral immune cells in LN, each slide was converted to digital image using Olympus Ch30 (Tokyo, Japan) microscope. From each slide, 3 representative fields (×200 magnification) were photographed. As immune cells commonly reside in the LN, we focused only on peritumoral cells (limited to 100 μm from the metastases border). Quantification was done automatically on a constant area determined around the tumor metastasis using Image Pro software (Rockville, MD, USA).
2.4. Statistical analysis
Comparison of quantitative variables between 2 independent groups was carried out using Student t test. The association between 2 qualitative variables was assessed using the chi-square test. Survival analysis was performed according to the Kaplan–Meier method, and survival curves were compared using the log-rank test. Univariate and multivariate analysis by the Cox proportional hazard model was performed to estimate the independent potential risk factors, quantitative and qualitative, that influence OS. Statistical analysis was done using SPSS (v22) software (Chicago, IL).
3. Results
3.1. Patients’ clinical and pathological characteristics
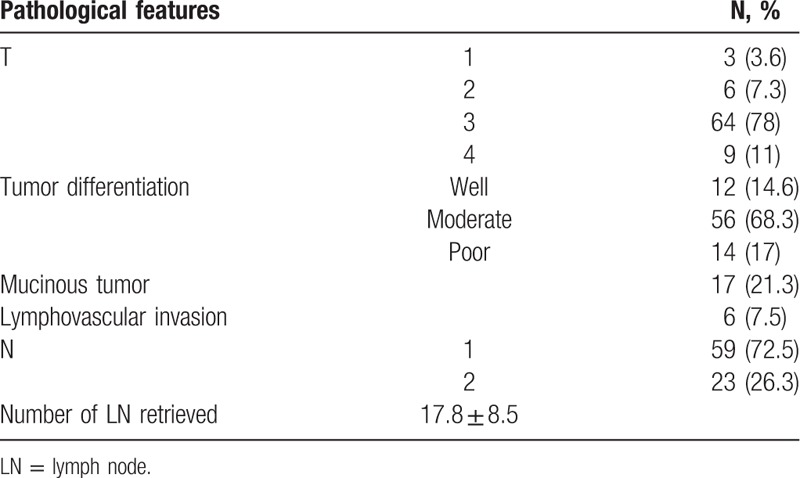
Tables 1 and 2 describe clinical and pathological characteristics, respectively, of participating patients. Age and sex distribution is concordant with the presentation of CC. The majority of the tumors arose in the right colon. A total of 17 tumors were defined as mucinous tumors, among them 14 (82%) arose in the right colon. As expected from the LNM inclusion criteria, most of the patients (89%) had advanced T stage (T3–T4) tumor.
Table 2.
Pathological characteristics of primary tumor.
3.2. Effects of pathological parameters on clinical outcome
We found a significant correlation between the tumor differentiation and patients’ OS. The mean OS was 42.5 ± 9.2 months for patients with poorly differentiated tumors, versus 74.5 ± 3.9 or 69.5 ± 11.7 months for patients with moderate or well differentiated tumors, respectively (P = 0.002) (Fig. 1A). N stage was also significantly correlated with the OS. The mean OS for patients with N1 stage was 76.7 ± 3.9 versus 45.7 ± 6.2 months for patients with N2 stage (P < 0.001) (Fig. 1B).
Figure 1.
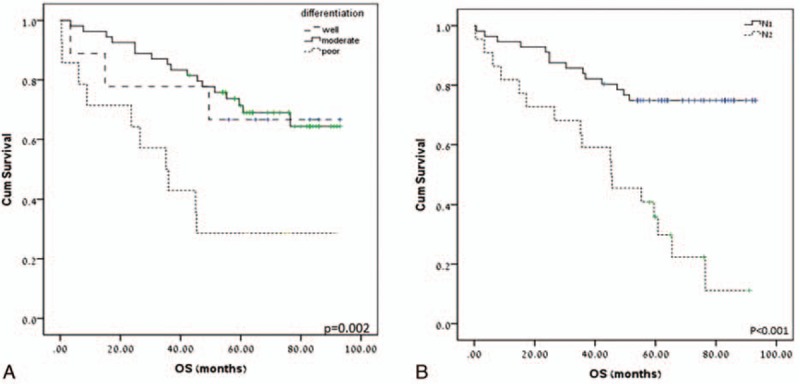
Effect of tumor differentiation (A) and N stage (B) on patients’ overall survival, see text for details.
The T stage did not correlate with the OS of the patients. The mean OS for T1 stage was 65.4 ± 0 months, for T2 stage 59.3 ± 15.3 months, for T3 stage 68.7 ± 4.1 months, and for T4 stage 55.4 ± 9.2 months (P = 0.94). Mucinous subtype of CC also did not confer any implication on OS (data not shown). Similarly, lymphovascular invasion in the primary tumor had no effect on OS (67.3 ± 3.9 for patients with lymphovascular invasion versus 66.6 ± 6.1 months for those without, P = 0.65).
3.3. Effects of morphological parameters on patient outcome
ECI was not associated with shorter OS in our patient cohort. Patients without ECI had OS of 67.7 ± 4.8 versus 67.7 ± 5.3 months for those who had not (P = 0.48).
Desmoplasia was previously described on primary tumor of CC[18]; however, it is also present around LNM (Fig. 2A). There are many contradicting reports about the role of desmoplasia in pancreatic adenocarcinoma and in CC.[18,19] We examined the effect of desmoplasia when present in LNM. OS of patient without desmoplasia was 54.6 ± 10 versus 71.3 ± 3.7 for those with desmoplasia; however, the results did not reach statistical significance (P = 0.13).
Figure 2.
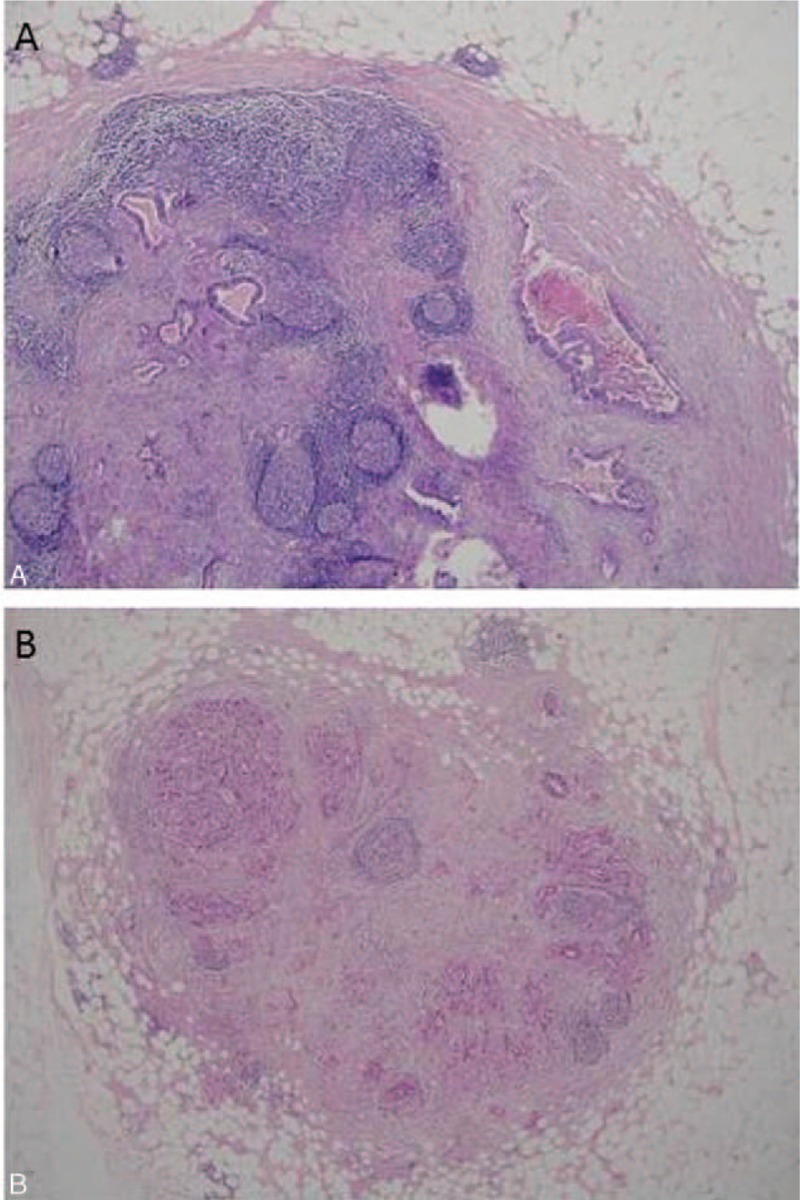
Analysis of histological parameters as prognostic markers in colonic carcinoma. (A) Desmoplasia, defined as peritumoral fibrosis, around metastatic tumor in the lymph node (LN). (B) Tumor metastases almost erase native LN structure. A few germinal centers and capsule are remnants of the LN structure.
We hypothesized that LN structure replacement by tumor attests to the invasiveness and aggressiveness of the tumor (Fig. 2B). When we compared patients with LN replaced by the tumor to those in which the tumor only partially erases the LN architecture, we found no difference in OS (data not shown). The same is true for the pattern of tumor distribution in the LN (focal or multifocal, data not shown).
We examined the effect of active follicles on survival. For this purpose, we divided patients into 3 groups according to the fraction of active follicles: more than third (active), between third and 2/3 (intermediate), and more than 2/3 (nonactive). There was no effect of follicle activation on OS (78.8 ± 5.6, 63.3 ± 10.5, and 66.2 ± 4.4 months for active, intermediate, and nonactive, respectively, P = 0.81).
3.4. Effect of peritumoral immune infiltration on patient prognosis
We next examined tumor immune interactions in the LN using immunohistochemistry. Figure 3 shows the staining for the various markers used. We first examined the ability of single markers to predict patient OS. We tested whether increased peritumoral CD8+ predicts improved OS. We divided patients into CD8+ high and CD8+ low groups according to the median CD8+ count. The mean OS of CD8+ low patients was 66.6 ± 6.25 versus 71.4 ± 5.1 months for CD8+ high patients (P = 0.79). Similarly, CD20+ peritumoral infiltration was also not associated with improved OS. The mean OS of CD20+ low patients (divided according to median CD20+ count) was 70.7 ± 5.8 versus 64.3 ± 4.9 months for CD20+ high patients (P = 0.6).
Figure 3.
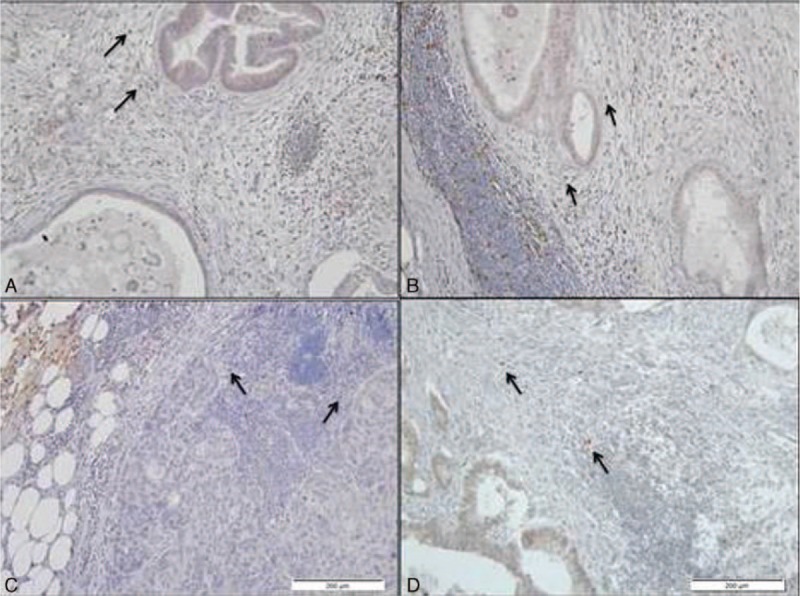
Staining of various immune cells markers in the tumor vicinity. (A) CD8, (B) CD20, (C) T-bet, and (D) GATA-3.
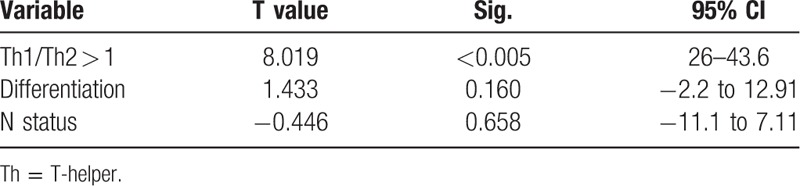
As Th1 immune response is associated with protective antitumoral immunity, we examined whether the Th1 skewing is associated with improved OS. However, the absolute number of T-bet+ is not a reliable indicator of Th1 immune response as it is also dependent on the Th2 immune response (number of GATA-3+ cells). Hence, Th1 skewing was defined as Th1/Th2 ratio >1. Indeed, Th1/Th2 > 1 was associated with improved OS. For patients with low ratio, the median OS was 35.5 ± 5 versus 83.5 months for patients with high Th1/Th2 ratio (P = 0.001) (Fig. 4). As can be seen in Table 3, Th1 skewing was the only prognostic predictor of OS statistically significant in multivariate analysis.
Figure 4.
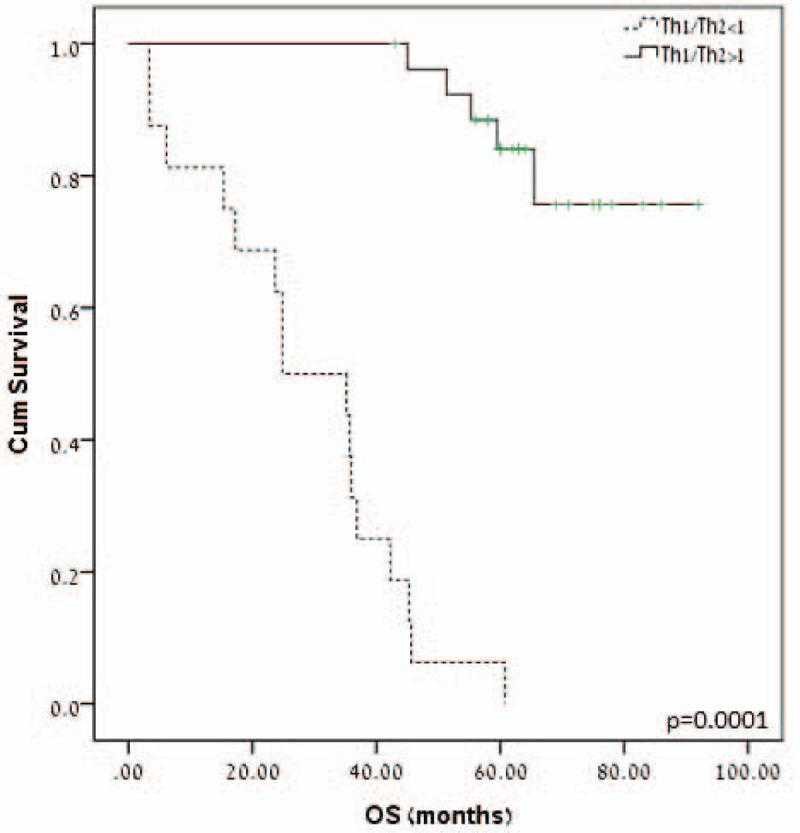
T-helper (Th)1/Th2 ratio predicts patients’ overall survival (OS). Patients with Th1 immune response skewing in lymph node metastases had significantly longer OS (P = 0.001).
Table 3.
Multivariable analysis of prognostic factors for OS in patients with LNM.
We further analyzed several parameters of the primary tumor which could attest to its biology (T stage, grade, and mucinous subtype) and correlated with immune parameters in the LN. We found no significant correlations (Supplementary table 1).
4. Discussion
The aim of this study was to examine whether specific parameters of LN involvement can identify patient subgroups within stage III CC.
As could be predicted from its mere inclusion in the TNM system, the N stage (N1/N2) could predict OS. Interestingly, tumor grade was also a prognostic factor, attesting to the importance of tumor biology in addition to disease extent, in overall outcome. Other studies also demonstrated tumor grade to be a stage-independent prognostic factor.[20,21]
We found no correlation between T stage and lymphovascular invasion to patient OS. A possible explanation could be that neither tumor local extent (T) nor preliminary signs of metastasis (lymphovascular invasion) factors has significant influence in the presence of LNM.
Here, we report on the presence of desmoplasia around the tumor metastasis in the LN. The creation of the desmoplastic niche around the metastasis may hint at the importance of the desmoplastic reaction to tumor survival. Desmoplasia is considered a negative prognostic sign when present in primary colonic tumor.[18,22] Indeed, in pancreatic cancer, the presence of the desmoplastic reaction is associated with vascular paucity and increased tissue pressure, all preventing the efficient delivery of chemotherapeutic agents.[19] However, desmoplasia may also have a protective role as treatment with antidesmoplastic agents was also shown to increase tumor development in experimental models and in humans.[19] We found that desmoplasia was associated with improved OS, although this association was not statistically significant. This again may point to the protective role of desmoplasia also in CC.
It was tempting to hypothesize that the structural preservation of the LN upon tumor infiltration is associated with tumor aggressiveness and overall prognosis. However, we found no significant correlation. It is possible that the distinction between partial and total replacement of LN architecture is temporal: tumors that erase native LN architecture will finally replace all LN architecture and are not essentially different from those that totally replace the LN.
Our hypothesis was that tumor may induce immune response in the metastatic LN and that this response may influence prognosis. However, this was not true for single cell type markers, even for CD8+ T cells, a well characterized prognostic marker in primary tumor. In contrast to the primary tumor, in which CD8+ presence implies specific antigen-driven migration and hence antitumoral activity, CD8+ cells are normal residents of the LN. So, a methodological challenge in our study is to determine which of the immune cells that reside in the LN is associated with tumor immunity. We assumed that distance from the metastases could serve as a marker for tumor immunity. However, it is possible that not all peritumoral CD8+ are indeed involved with antitumor immunity. This may be the reason that the only immune marker significant in our study was the Th1/Th2 ratio, which incorporates only activated T cells. Similarly, we also did not find any correlation between active follicles and CD20+ peritumoral infiltration and prognosis. Both parameters are associated with antibody-mediated immunity against CC. Interestingly, although antitumor antibodies are well described in many tumor types (such as breast, pancreas, lung, and esophagus),[23] only a minority of patients with CC were found to have an antitumor antibody response.[24]
The main finding of this study is that Th1/Th2 ratio reliably predicts patient OS. Th1 type immune response is well known for its antitumor activity, including in CC. Thus, our findings stand in line with previous data. Of note, determination of Th immune response in a sample of primary tumors from our cohort revealed that there was a correlation between Th1 skewing in the primary tumor and in LNM. We herein describe a simple immunostain for 2 markers (T-bet and GATA-3) which can serve as robust prognostic marker.
The finding of Th1 predominant immune response can have several clinical implications regarding adjuvant treatment. First, the presence of a protective immune response should drive the clinician toward protocols that preserve this function. Present adjuvant therapy protocols use maximal dose therapy strategy. In this mode of treatment, chemotherapy dosage is selected to be the highest tolerated and administration intervals are widely spaced in order to prevent treatment toxicity. This mode of treatment was shown to depress antitumor immunity, causing both antigen presentation impairment and inhibition of cellular effectors. On the other hand, metronomic treatment uses lower drug doses with more frequent intervals of administration. It was shown that metronomic treatment augments antigen presentation and may induce long-lasting antitumor immune response.[25,26] Thus, metronomic adjuvant therapy could be a way to boost the protective immune response discovered histologically.
Second, the finding of protective immune response may prompt the use of immune blockade inhibitors. Although CC is considered resistant to immune blockade inhibitors, recent study showed that a significant portion of patients with mismatch repair (MMR) deficiency respond to these agents. MMR-associated tumors contain higher number of mutations and induce an endogenic immune response.[27] Along the same lines, we suggest, at least theoretically, that Th1 type immune response may also serve as a marker for responsiveness to immune blockers inhibitors.
This retrospective study has several limitations. First, its relatively small sample size may hamper the ability to identify some prognostic factors. In addition, only 1 representative LN was analyzed from each patient. Of note, the morphological and architectural pattern of LN involvement by metastasis was generally similar across several involved LN from the same patient (data not shown).
In conclusion, tumor grade and the N stage predict OS in stage III CC OS. Th1 immune response skewing, as calculated by the Th1/Th2 ratio, can also predict patient prognosis. The finding of a protective antitumor immune response even in advanced stage CC may serve as a modifier of the subsequent adjuvant treatment in the future.
Supplementary Material
Footnotes
Abbreviations: CC = colonic carcinoma, DFS = disease-free survival, ECI = extracapsular invasion, LN = lymph node, LNM = lymph node metastases, MMR = mismatch repair, OS = overall survival, TASMC = Tel Aviv Sourasky Medical Center, Th = T-helper, TILs = tumor infiltrating lymphocytes.
This study was supported by a “Kidum Metzuyanut” grant from the Tel Aviv Sourasky Medical Center (EN) and by The Nikolas and Elizabeth Shlezak Fund for Experimental Surgery.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63:11–30. [DOI] [PubMed] [Google Scholar]
- 2.Markl B. Stage migration vs immunology: the lymph node count story in colon cancer. World J Gastroenterol 2015; 21:12218–12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009; 27:3109–3116. [DOI] [PubMed] [Google Scholar]
- 4.Roth AD, Delorenzi M, Tejpar S, et al. Integrated analysis of molecular and clinical prognostic factors in stage II/III colon cancer. J Natl Cancer Inst 2012; 104:1635–1646. [DOI] [PubMed] [Google Scholar]
- 5.Veronese N, Nottegar A, Pea A, et al. Prognostic impact and implications of extracapsular lymph node involvement in colorectal cancer: a systematic review with meta-analysis. Ann Oncol 2016; 27:42–48. [DOI] [PubMed] [Google Scholar]
- 6.Wind J, Lagarde SM, Ten Kate FJ, et al. A systematic review on the significance of extracapsular lymph node involvement in gastrointestinal malignancies. Eur J Surg Oncol 2007; 33:401–408. [DOI] [PubMed] [Google Scholar]
- 7.Yamano T, Semba S, Noda M, et al. Prognostic significance of classified extramural tumor deposits and extracapsular lymph node invasion in T3–4 colorectal cancer: a retrospective single-center study. BMC Cancer 2015; 15:859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pihl E, Nairn RC, Milne BJ, et al. Lymphoid hyperplasia: a major prognostic feature in 519 cases of colorectal carcinoma. Am J Pathol 1980; 100:469–480. [PMC free article] [PubMed] [Google Scholar]
- 9.Markl B, Rossle J, Arnholdt HM, et al. The clinical significance of lymph node size in colon cancer. Mod Pathol 2012; 25:1413–1422. [DOI] [PubMed] [Google Scholar]
- 10.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960–1964. [DOI] [PubMed] [Google Scholar]
- 11.Pages F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol 2009; 27:5944–5951. [DOI] [PubMed] [Google Scholar]
- 12.Tosolini M, Kirilovsky A, Mlecnik B, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, Th2, treg, Th17) in patients with colorectal cancer. Cancer Res 2011; 71:1263–1271. [DOI] [PubMed] [Google Scholar]
- 13.Kanno Y, Vahedi G, Hirahara K, et al. Transcriptional and epigenetic control of T helper cell specification: molecular mechanisms underlying commitment and plasticity. Annu Rev Immunol 2012; 30:707–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tripathi SK, Lahesmaa R. Transcriptional and epigenetic regulation of T-helper lineage specification. Immunol Rev 2014; 261:62–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cochran AJ, Huang RR, Lee J, et al. Tumour-induced immune modulation of sentinel lymph nodes. Nat Rev Immunol 2006; 6:659–670. [DOI] [PubMed] [Google Scholar]
- 16.Mlecnik B, Tosolini M, Kirilovsky A, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol 2011; 29:610–618. [DOI] [PubMed] [Google Scholar]
- 17.Naito Y, Saito K, Shiiba K, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 1998; 58:3491–3494. [PubMed] [Google Scholar]
- 18.Halvorsen TB, Seim E. Association between invasiveness, inflammatory reaction, desmoplasia and survival in colorectal cancer. J Clin Pathol 1989; 42:162–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feig C, Gopinathan A, Neesse A, et al. The pancreas cancer microenvironment. Clin Cancer Res 2012; 18:4266–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Compton CC, Fielding LP, Burgart LJ, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000; 124:979–994. [DOI] [PubMed] [Google Scholar]
- 21.Erstad DJ, Tumusiime G, Cusack JC., Jr Prognostic and predictive biomarkers in colorectal cancer: implications for the clinical surgeon. Ann Surg Oncol 2015; 22:3433–3450. [DOI] [PubMed] [Google Scholar]
- 22.Torres S, Garcia-Palmero I, Herrera M, et al. LOXL2 is highly expressed in cancer-associated fibroblasts and associates to poor colon cancer survival. Clin Cancer Res 2015; 21:4892–4902. [DOI] [PubMed] [Google Scholar]
- 23.Heo CK, Bahk YY, Cho EW. Tumor-associated autoantibodies as diagnostic and prognostic biomarkers. BMB Rep 2012; 45:677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benvenuto M, Sileri P, Rossi P, et al. Natural humoral immune response to ribosomal P0 protein in colorectal cancer patients. J Transl Med 2015; 13:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galluzzi L, Senovilla L, Zitvogel L, et al. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov 2012; 11:215–233. [DOI] [PubMed] [Google Scholar]
- 26.Kareva I, Waxman DJ, Lakka Klement G. Metronomic chemotherapy: an attractive alternative to maximum tolerated dose therapy that can activate anti-tumor immunity and minimize therapeutic resistance. Cancer Lett 2015; 358:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015; 372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


