Supplemental Digital Content is available in the text
Keywords: antidepressants, meta-analysis, post-stroke depression
Abstract
Background:
Depression greatly impacts the quality of life in most stroke survivors. Therefore, effective treatment of post-stroke depression (PSD) is critically important. However, evidence supporting the effectiveness and feasibility of antidepressant treatment in this population is limited and somewhat confusing.
Methods:
A comprehensive literature search of the Cochrane, PubMed, Web of Science, and Embase databases from inception up to November 2015 was conducted. We reviewed all randomized controlled trials (RCTs) that assigned patients with a clinical diagnosis of PSD to antidepressant or placebo treatment. Reduction in depression rating scale scores and response rate to antidepressants were defined as the efficacy outcomes. Rates of dropout for any reason and for adverse effects were defined as the acceptability outcomes. We also assessed improvements in activities of daily living (ADL) as functional outcomes.
Results:
In total, 11 trials consisting of 740 participants were indentified. A significant advantage of antidepressants compared with placebo treatment in PSD was observed in overall pooled effect size analysis (SMD = −0.96; 95% CI = −1.41 to −0.51; P <0.0001). In addition, patients receiving antidepressants presented a much greater improvement in various depressive symptoms than those with placebo (RR = 1.36; 95% CI = 1.01–1.83; P = 0.04). However, antidepressants were less well tolerated than placebo because of some adverse events (RR = 2.72; 95% CI = 1.37–5.43; P = 0.04). Intriguingly, no consistent evidence was found for a positive effect of antidepressants on ADL in our analysis.
Conclusions:
This meta-analysis suggests that antidepressants treatment confers potentially positive effects in patients with PSD as compared with simple placebo treatment. However, this must be carefully considered in light of its possible adverse events in some individual patients.
1. Introduction
Post-stroke depression (PSD) occurs in approximately one-third of all stroke survivors.[1] Patients with PSD usually present with a wide range of symptoms, including apathy, weight changes, sleep disturbances, feelings of worthlessness, and fatigue.[2] It is already well documented that PSD impedes rehabilitation and reduces the quality of life in majority of stroke survivors.[3] However, up to now, the pathophysiological mechanisms of PSD are still not fully illuminated.[4] Mounting evidence indicates that PSD is a complex multifactorial process involving various biological, behavioral, and social factors.[4]
The main risky factors for development of PSD include the degree of disability and a poorly social support network.[4] PSD is also often associated with poorer cognitive activity, impaired functional rehabilitation, and poorer quality of life, as well as higher mortality, which may be up to 10 times higher than in patients without PSD.[4–6] It is therefore critical to develop effective antidepressant treatment for this specific population to alleviate neurological deficits and facilitate rehabilitation after stroke.
Limited few studies have demonstrated that antidepressants can alleviate several domains of depressive symptoms in patients with PSD.[7–10] However, evidence for the feasibility and effectiveness of antidepressants in improving mood and enhancing recovery of neurologic functional deficits in this population is lacking.
To our knowledge, only 4 meta-analyses evaluated the effects of antidepressants in PSD.[7–10] However, the validity of all of these analyses deserves to be questioned. Among these published reviews, 2 did not include recently published evidence.[7,8] Another included non-English literature and studies of aniracetam rather than antidepressants.[9] The last analysis included studies of aniracetam and deanxit, neither of which belongs to the classic type of antidepressants.[10] Therefore, further systemic analyses are necessary to resolve such concerns.
In this study, we undertook a substantial meta-analysis of all randomized controlled trials (RCTs) of antidepressants, including selective serotonin reuptake inhibitors (SSRIs), serotonin–norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and others, to further clarify the efficacy and feasibility of antidepressant treatment in patients with PSD.
In this study, we aim to systematically assess the effectiveness and feasibility of antidepressant treatment in patients with PSD based on currently available literature review.
2. Methods
2.1. Data sources and search strategy
We conducted a comprehensive search of the Cochrane, PubMed, Web of Science, and Embase databases from inception to November 2015 using search terms such as post-stroke depression (see the Supplemental Table for full search terms). Only studies in English literature were included in this investigation. Further relevant trials were obtained by manual search of conference summaries and reference lists of all available records identified in the initial search. We also contacted authors to obtain additional detailed information for relevant trials if necessary.
2.2. Selection criteria
Inclusion criteria were as follows: RCT that enrolled patients with a clinical diagnosis of PSD; RCT that enrolled patients were assigned to either an antidepressant or a placebo group; and study reported complete efficacy outcome(s). Exclusion criteria were as follows: combination therapy, such as antidepressant combined with psychotherapy; 2 or more interventions were compared with each other rather than only with a placebo; studies of preventative treatment of PSD; duplicate or secondary analyses; outcome data were unavailable or incomplete (either from the report of the trial or from the authors).
Two reviewers independently assessed the titles and abstracts of each literature, to identify suitable studies meeting the inclusion criteria. All disagreements were resolved through discussion or following arbitration by a third reviewer if necessary.
2.3. Outcome measures
Three categories of analyses were conducted in this study, including efficacy analyses, acceptability analyses, and functional disability analyses.
For efficacy analyses, the primary outcome was defined as mean change from baseline in depression rating scales. When a trial reported multiple depression rating scales, the Hamilton Depression Scale (HAMD) was preferred. A negative value indicated greater relief from depressive symptoms. The secondary outcome was defined as the proportion of patients who responded to treatment (showing a decrease of ≥50% in depression rating scale scores) or experienced remission (e.g., HAMD <13; or Montgomery–Asberg Depression Scale, MADRS <7).
For acceptability analyses, the primary acceptability outcome was represented by the proportion of patients who discontinued the study for any reason. The secondary outcome was the proportion of patients who discontinued the study because of adverse effects of antidepressants.
In addition, we also calculated the pooled outcome for functional disability in activities of daily living (ADL) at the end of treatment. Measures of functional disability included the Barthel Index (BI),[11] Functional Independence Measure (FIM),[12] and the Scandinavian Stroke Scale (SSS).[13] Where multiple scales were used to evaluate patients’ functional outcomes, we gave preference to the BI, then the FIM, and then others.
2.4. Data extraction and quality assessment
Two reviewers independently extracted the data from the included trials and any discrepancies were resolved via discussion. Extracted data included the key characteristics of studies, patients, methods, and outcomes. We also used the Risk of Bias Assessment Tool from the Cochrane Handbook for Systematic Reviews of Interventions[14] to assess the methodological quality of these studies.
2.5. Statistical analysis
All statistical analyses were conducted with RevMan 5.3 software (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Antidepressant treatment effects were described using standardized mean differences (SMD) with 95% confidence intervals (CIs) for continuous data, and using risk ratios (RR) with 95% CIs for categorical data. Statistical significance was defined as P <0.05. Where possible, meta-analyses were carried out on the full intention-to-treat (ITT) population. Heterogeneity of treatment effects across trials was assessed with the I2 statistic.[14] If substantial heterogeneity for outcome data was observed (I2 >50% or P <0.10), pooled estimates were calculated using a random-effects model. Otherwise, pooled estimates were calculated using a fixed-effect model.
Subgroup analyses were performed based on the type of antidepressant, the sample size (<50 vs >50), age (>70 vs <70), gender (male/female >1 vs male/female <1), and depression severity (mild to moderate vs moderate to severe). Sensitivity analyses were performed to explore the impact of randomization and double-blinding. The funnel plot regression method and Egger statistical test were used to assess publication bias.[15]
3. Results
3.1. Study selection and characteristics
Our database search identified 4360 potentially relevant studies (957 from PubMed, 1515 from Cochrane, 1131 from Web of Science, and 757 from Embase). After removing duplicates, there were 2822 records. Of these, 2771 were excluded based on independent screening of titles and abstracts by 2 reviewers. Fifty-one full texts that were potentially relevant were further reviewed. Finally, we identified 11 studies for further data extraction[16–26] (Fig. 1). Two of these studies included 2 active treatments and therefore we considered these as 4 separate trials.[17,22] All 13 trials included totally 740 participants, with 393 randomly assigned to antidepressants treatment and 347 randomly assigned to receive a placebo, respectively.
Figure 1.
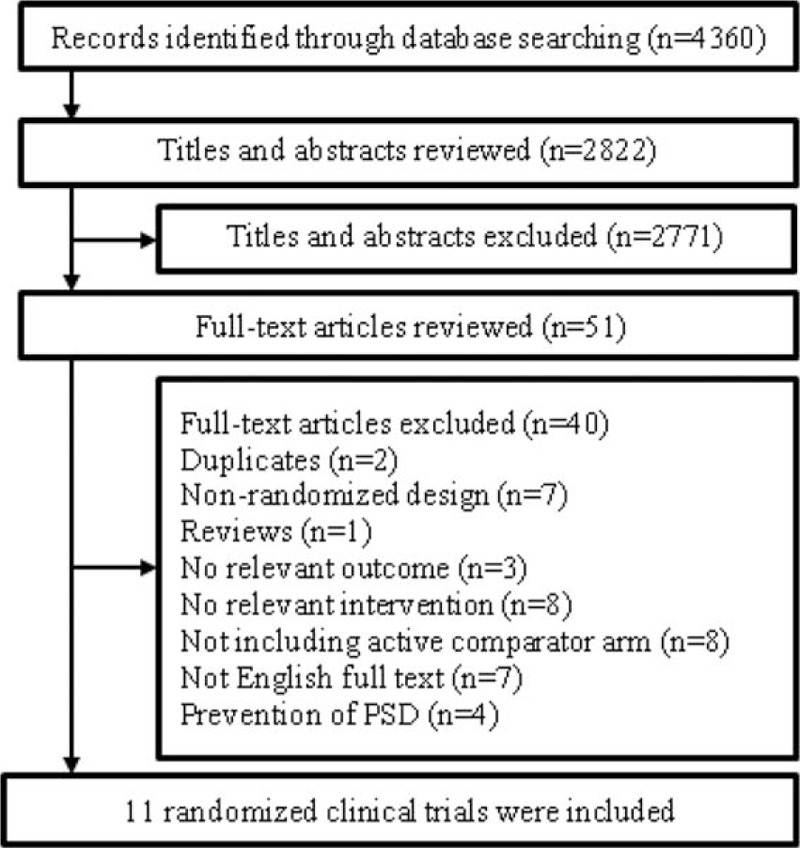
Flowchart of study selection.
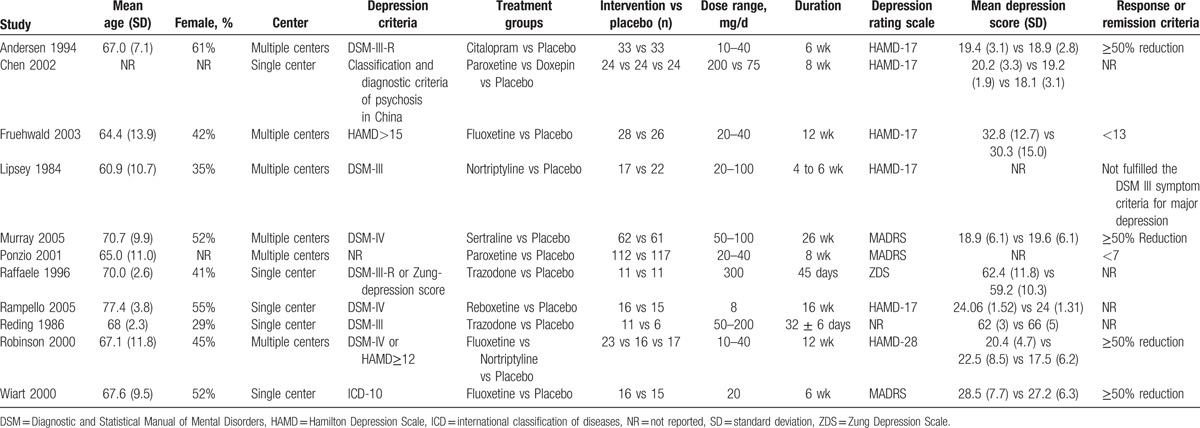
Table 1 demonstrated patients’ characteristics and design features of each trial. These trials were published between 1984 and 2005. Their sample sizes ranged from 17 to 229, with a mean sample size of 67.27. The mean age of participants was 67.1 years old (SD = 10.7) and less than half of participants (47.61%) were female. The mean total treatment duration was 10 weeks (range: 3.7–12 weeks).
Table 1.
Demographic and clinical characteristics of included randomized controlled trials.
3.2. Efficacy outcomes
Data analyses on the primary efficacy outcomes are summarized in Fig. 2. Ten studies (2 of which included 2 active treatments) presented continuous depression outcomes in the form of changes in depression scale scores from baseline to end-point.[16–18,20–26] The overall pooled effect size showed a significant advantage of antidepressants over placebo, with an SMD of −0.96 (95% CI = −1.41 to −0.51; P <0.0001) and significant heterogeneity (I2 = 84%; P <0.00001). Treatment response rates were available for 7 trials[16,20,22,23,25] (1 trial included 2 active treatments). Consistent with primary efficacy outcomes, antidepressants-treated patients showed a higher treatment response rate as compared with placebo group (RR = 1.36; 95% CI = 1.01–1.83; P = 0.04) with moderate heterogeneity (I2 = 43%; P = 0.09) (see Supplemental Figure 1).
Figure 2.
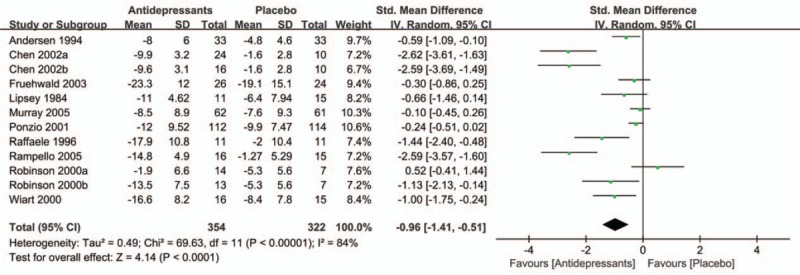
Effect of antidepressants in the treatment of PSD compared with placebo. PSD = post-stroke depression.
3.3. Subgroup analyses
Owing to significant heterogeneity in the primary efficacy outcome, we conducted various subgroup analyses to detect potential bias. In the subgroup analysis of antidepressant types (SSRIs, TCAs, and others), both SSRIs (SMD = −0.53; 95% CI = −0.97 to −0.09; P = 0.02) and TCAs (SMD = −1.41; 95% CI = −2.51 to −0.31; P = 0.01) had a significant advantage over placebo (Fig. 3 A). In a subgroup analysis of sample size (Fig. 3 B), antidepressants showed statistically significant treatment efficacy compared with placebo (SMD = −1.41; 95% CI = −2.16 to −0.66; P = 0.0002) in studies with groups sample size less than 50. Besides, this significant effect also persisted in studies where groups included more than 50 patients (SMD = −0.26; 95% CI = −0.44 to −0.08; P = 0.005). Figure 3 C demonstrates the beneficial effects of antidepressants are more pronounced in older individuals (SMD = −1.33; 95% CI = −2.89 to 0.24; P = 0.10) than in younger individuals (SMD = −0.45; 95% CI = −0.75 to −0.14; P = 0.004). However, this evidence was less convincing for the heterocyclic antidepressants. Of interest, we also found that antidepressants therapy had a significantly greater benefit in female patients (SMD = −0.97; 95% CI = −1.79 to −0.14; P = 0.02) than male patients (SMD = −0.57; 95% CI = −1.17 to 0.03; P = 0.06) (Fig. 3 D). We did not observe significant differences between patients with mild to moderate depression (SMD = −1.16; 95% CI = −1.90 to −0.42; P = 0.002) compared with moderate to severe depression (SMD = −0.92; 95% CI = −1.71 to −0.13; P = 0.02) in subgroup analysis of depression severity (Fig. 3 E). However, antidepressants appear to be more effective in patients with less severe depression than those with more severe depression.
Figure 3.
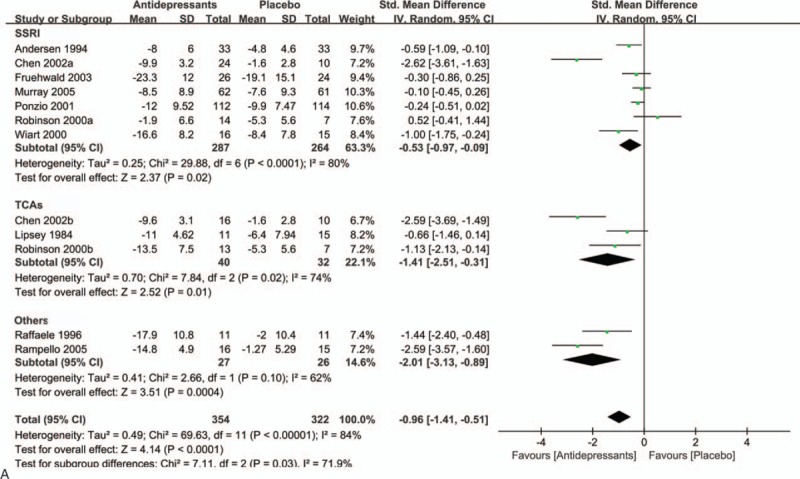
A, Subgroup analysis of continuous outcomes compared with placebo based on the type of antidepressants. B, Subgroup analysis of continuous outcomes compared with placebo based on the sample size. C, Subgroup analysis of continuous outcomes compared with placebo based on the age. D, Subgroup analysis of continuous outcomes compared with placebo based on the sex. E, Subgroup analysis of continuous outcomes compared with placebo based on the severity of PSD.
3.4. Acceptability outcomes
In this study, overall acceptability was represented by data on completion of the whole trial protocols. We found that there was no statistical difference between the antidepressant-treated and control groups in completion of trial protocols (primary acceptability outcome, Fig. 4A). The RR was 1.05 (95% CI = 0.79 to 1.38; P = 0.74), with low heterogeneity (P = 0.41; I2 = 3%). However, secondary acceptability outcome analysis showed a statistical significant difference (Fig. 4B), with an RR of 2.72 (95% CI = 1.37–5.43; P = 0.004) and no significant heterogeneity (I2 = 0%; P = 0.63). Common adverse effects in the treatment group included gastrointestinal symptoms, headache, dizziness, and increased hepatic alanine aminotransferase and aspartate aminotransferase levels.
Figure 3 (Continued).
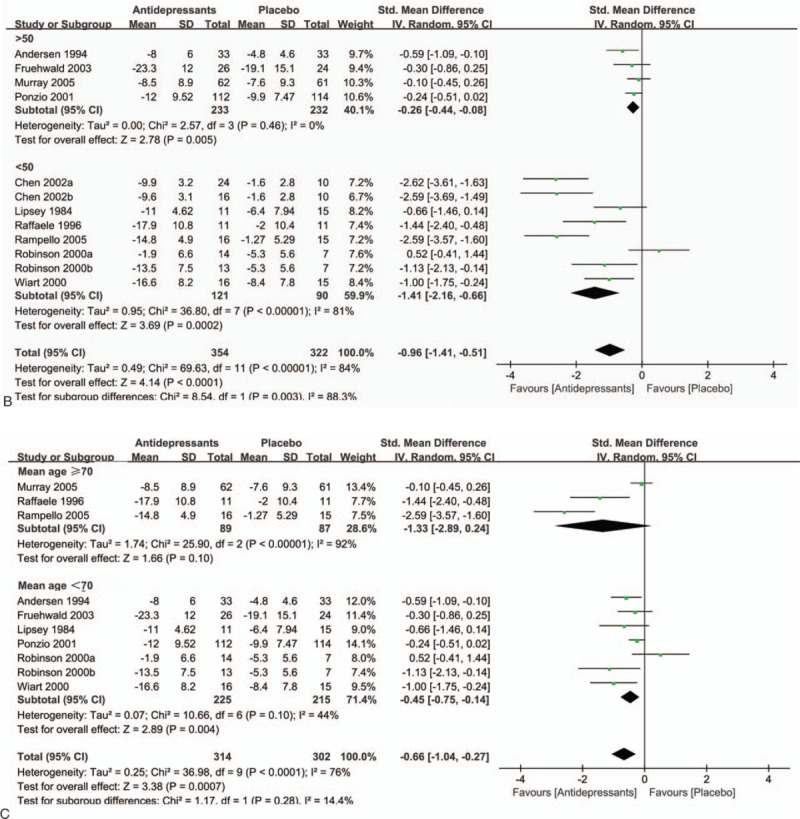
A, Subgroup analysis of continuous outcomes compared with placebo based on the type of antidepressants. B, Subgroup analysis of continuous outcomes compared with placebo based on the sample size. C, Subgroup analysis of continuous outcomes compared with placebo based on the age. D, Subgroup analysis of continuous outcomes compared with placebo based on the sex. E, Subgroup analysis of continuous outcomes compared with placebo based on the severity of PSD.
Figure 4.
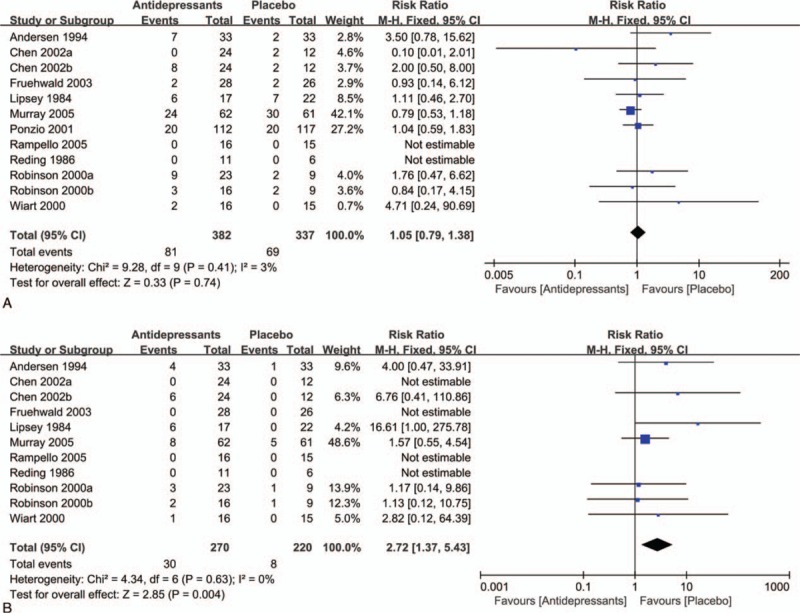
Acceptability of antidepressants in the treatment of PSD. A, Withdrawals for any reason between antidepressants and control groups. B, Withdrawals for adverse events between antidepressants and control groups.
3.5. Treatment effects on activities of daily living
Treatment effects on ADL of patients with PSD were reported in 7 trials,[16,17,19,21–24] with a total of 425 participants (Fig. 5). Patients treated with antidepressants did not show a significantly bigger improvement in disability scores compared with placebo (SMD = 0.35; 95% CI = −0.22 to 0.92; P = 0.23) with moderate heterogeneity (I2 = 74%; P = 0.0004).
Figure 3 (Continued).
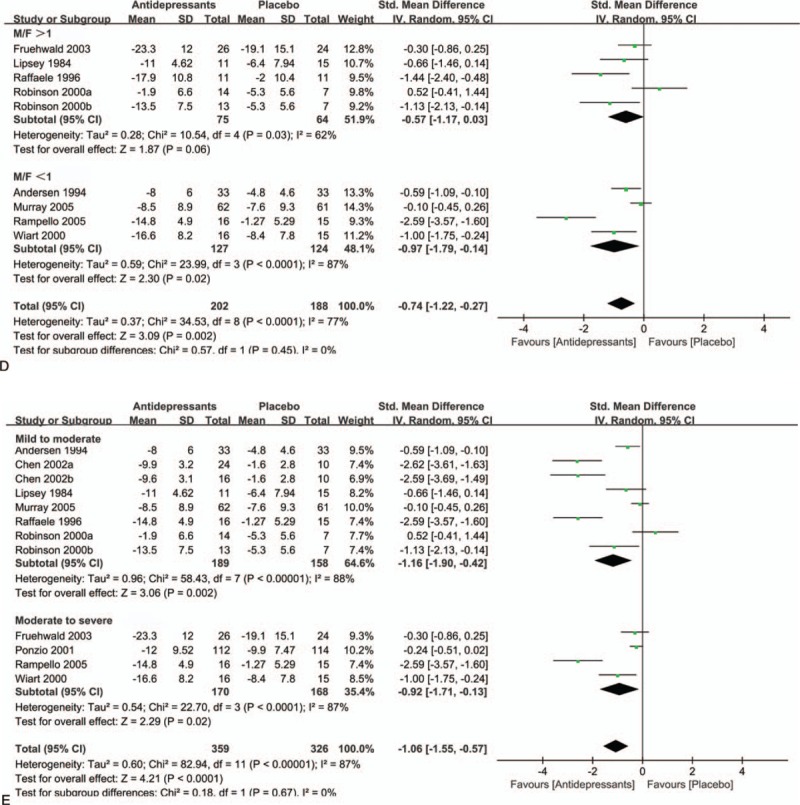
A, Subgroup analysis of continuous outcomes compared with placebo based on the type of antidepressants. B, Subgroup analysis of continuous outcomes compared with placebo based on the sample size. C, Subgroup analysis of continuous outcomes compared with placebo based on the age. D, Subgroup analysis of continuous outcomes compared with placebo based on the sex. E, Subgroup analysis of continuous outcomes compared with placebo based on the severity of PSD.
Figure 5.
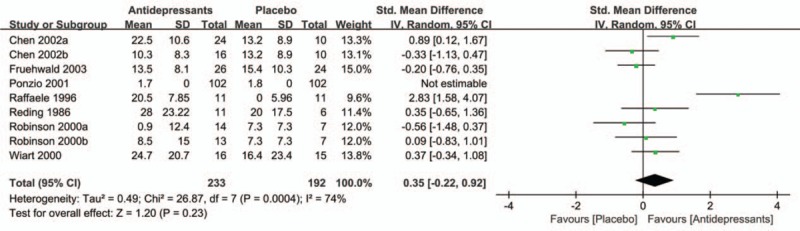
Treatment effects of antidepressant on ADL of patients with PSD. ADL = activities of daily living, PSD = post-stroke depression.
3.6. Sensitivity analysis
In the sensitivity analyses, exclusion of trials without a double-blind design, the pooled estimate of effect size was −0.58 (95% CI = −0.97 to −0.20; P = 0.003), with significant heterogeneity (P <0.0001; I2 = 75%) (Supplemental Figure 2). Moreover, for trials at low risk of bias for randomization, the pooled effect size was −0.74 (95% CI = −1.35 to −0.12; P = 0.02), with significant heterogeneity (P = 0.0001; I2 = 82%) (Supplemental Figure 3).
3.7. Quality assessment and publication bias
Supplemental Figures 4 and 5 showed the quality of all studies based on the Cochrane risk-of-bias method. The overall quality of studies was rated as moderate, although some reports did not provide details about randomization, allocation concealment, or blinding. Few question-based entries in the included trials met the criteria for high risk of bias. Visual inspection of the inverted funnel plots of these studies showed them to be asymmetrical(Supplemental Figures 6). And the inverted funnel plots of acceptability were symmetrical (Supplemental Figures 7). An Egger test was performed and the results showed that the depression outcomes were not influenced by publication bias (t = 1.18; P = 0.273).
4. Discussion
This meta-analysis confers us a valuable and relatively complete picture of the efficacy, acceptability, and tolerability of antidepressants treatment for patients with PSD, which contributes to resolution of some confusing questions and serious concerns presented in previously published literature. In this meta-analysis, we identified 11 trials comparing antidepressants treatment with placebo, involving 740 patients with PSD. The pooled analyses suggested that antidepressants were significantly more effective than placebo at reducing depressive symptoms of patients with PSD, although there was significant heterogeneity existed in continuous depression outcomes which might be sample size dependent. Further analysis of response rates also revealed a relatively consistent positive effect for antidepressants treatment in patients with PSD. We observed a significant difference in secondary acceptability analysis because of the common side effects of antidepressants. However, there were no significant changes in primary acceptability analysis between both groups. Intriguingly, we did not observe the beneficial effects of antidepressant treatment in improvement of neurological functional scores (ADL) in patients with PSD.
From this study, we found that both SSRIs and TCAs had a significant benefit in relieving patients’ major depressive symptoms. These beneficial effects are more pronounced in older females with less severe depression. Additionally, our results suggest that the effect size in smaller samples seems to be higher than those with larger samples. This is consistent with interpretations by some statisticians that smaller sample size may increase the variability of results by producing a larger standard deviation.[27] This may affect the reliability of a survey's results, with a higher risk of bias and overestimation of medication effects.[28] Thus, future studies could employ a multicenter design, recruiting larger samples and more evaluating parameters, such as antidepressant types, administration protocols, ethnic groups, and gender differences.
In our study, there was insufficient evidence to support the notion that antidepressants led to significant improvements in ADL, which is consistent with a previous meta-analysis.[9] However, further confirmation is required because of the relatively small sample size available in our analysis. In terms of acceptability outcomes, no statistically significant differences were detected between the medicated and placebo group. With regard to the tolerability measures, we found antidepressants were significantly less well tolerated for some adverse events. This suggests that we must carefully take care of the common adverse effects of antidepressants when treating patients with PSD or we may employ safer types of antidepressants in those sensitive individuals. However, given a relatively limited sample size in our analysis, such negative conclusions might be interpreted cautiously.
In current clinical practice stroke survivors with depression have been routinely treated with antidepressants. Our results broadly agree with other systematic reviews[7–10] that had provided evidence of the benefits of antidepressants in patients with PSD. If the clinical effects are robust, while the risk of adverse events is relatively low and acceptable, antidepressants could be favored as one of the first-line treatments for stroke survivors with depression.
One of the strengths of our meta-analysis is that we performed substantially extensive searches in a wide range of currently available data sources to include most recently updated studies. We also conducted sufficient subgroup and sensitivity analyses to ensure the robustness of our analyses and conclusions. In addition, we included data analyses on adverse effects and quality of life outcomes, which are closely clinic correlated parameters and are important for clinicians and patients to make an appropriate treatment option. Additionally, most of trials recruited in our analyses reported adequate concealment of the randomization sequence, blinding of investigators and outcome assessors, which further strengthened the validity of the conclusions.
Duo to the heterogeneity among trials, our findings should be interpreted with several concerns. First, it may be owing to a relatively small number of PSD subjects in the included studies that our review could not be sufficiently powered to reveal the potential treatment effects of antidepressants on mood. Second, the existing variations in the clinical features of recruited participants (e.g., age, socioeconomic status, time since stroke, other unmeasured baseline variables, types of antidepressant, and different doses of antidepressants) can strengthen the generalizability of our findings, but also may introduce potential bias. Third, given the frequent presence of neurological impairments and aphasia in stroke survivors, a proportion of survivors were excluded from included trials, which may weaken the clinical application of our findings. Finally, as the guidelines from the American College of Physicians suggested, antidepressants should be continued for at least 4 months beyond initial recovery, and treatment should be changed if no response has been shown for 6 weeks.[29] The duration of interventions in included trials was not adequate to show the maximal response or the long-term effects of antidepressant therapy, which might hinder us to further reveal the potentially positive roles of antidepressant treatment in PSD.
5. Conclusions
Our present investigation further reveals the beneficial roles of antidepressant treatment in patients with PSD as well as the possible concerns in clinical practice. Given limitations of currently available clinical studies and the promising positive impacts of antidepressants on stroke survivors, further robust RCTs should be warranted to more precisely evaluate its complex effects in PSD, which should at least contain such key features as larger samples, multiple medical centers and ethnic groups, as well as rational therapeutic dosages and sufficient intervention durations.
Supplementary Material
Footnotes
Abbreviations: ADL = activities of daily living, BI = Barthel Index, CIs = confidence intervals, FIM = Functional Independence Measure, HAMD = Hamilton Depression Scale, ITT = intention-to-treat, MADRS = Montgomery–Asberg Depression Scale, PSD = post-stroke depression, RCTs = randomized controlled trials, RR = risk ratios, SMD = standardized mean differences, SNRIs = serotonin–norepinephrine reuptake inhibitors, SSRIs = selective serotonin reuptake inhibitors, SSS = Scandinavian Stroke Scale, TCAs = tricyclic antidepressants.
X-mX and D-zZ contributed equally to this work.
This study was supported by the National Key Clinical Specialties Construction Program of China and natural science foundation project of Chongqing science and technology commission(ID:cstc 2016jcy jA0423).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Hackett ML, Yapa C, Parag V, et al. Frequency of depression after stroke: a systematic review of observational studies. Stroke 2005; 36:1330–1340. [DOI] [PubMed] [Google Scholar]
- 2.Feng C, Fang M, Liu XY. The neurobiological pathogenesis of poststroke depression. Scientific World J 2014; 2014:521349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaete JM, Bogousslavsky J. Post-stroke depression. Expert Rev Neurother 2008; 8:75–92. [DOI] [PubMed] [Google Scholar]
- 4.Esparrago Llorca G, Castilla-Guerra L, Fernandez Moreno MC, et al. Post-stroke depression: an update. Neurologia 2015; 30:23–31. [DOI] [PubMed] [Google Scholar]
- 5.Angelelli P, Paolucci S, Bivona U, et al. Development of neuropsychiatric symptoms in poststroke patients: a cross-sectional study. Acta Psychiatr Scand 2004; 110:55–63. [DOI] [PubMed] [Google Scholar]
- 6.Pohjasvaara T, Vataja R, Leppävuori A, et al. Depression is an independent predictor of poor long-term functional outcome post-stroke. Eur J Neurol 2001; 8:315–319. [DOI] [PubMed] [Google Scholar]
- 7.Cole MG, Elie LM, McCusker J, et al. Feasibility and effectiveness of treatments for post-stroke depression in elderly inpatients: systematic review. J Geriatr Psychiatry Neurol 2001; 14:37–41. [DOI] [PubMed] [Google Scholar]
- 8.Hackett ML, Anderson CS, House AO. Management of depression after stroke: a systematic review of pharmacological therapies. Stroke 2005; 36:1098–1103. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Guo JJ, Zhan S, et al. Treatment effects of antidepressants in patients with post-stroke depression: a meta-analysis. Ann Pharmacother 2006; 40:2115–2122. [DOI] [PubMed] [Google Scholar]
- 10.Hackett ML, Anderson CS, House A, et al. Interventions for treating depression after stroke. Cochrane Database Syst Rev 2008; 4:CD003437.DOI: 10.1002/14651858.CD003437.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Maryland State Med J 1965; 14:61–65. [PubMed] [Google Scholar]
- 12.Ottenbacher KJ, Mann WC, Granger CV, et al. Inter-rater agreement and stability of functional assessment in the community-based elderly. Arch Phys Med Rehabil 1994; 75:1297–1301. [PubMed] [Google Scholar]
- 13.Aberg E, Adielsson G, Almqvist A. Multicenter trial of hemodilution in ischemic stroke–background and study protocol. Scandinavian Stroke Study Group. Stroke 1985; 16:885–890. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. London, UK:The Cochrane Collaboration; 2011. [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ponzio F, Marini G, Riva E. The efficacy of paroxetine in some kinds of “critical” patients. European Neuropsychopharmacology 2001;11 Suppl 2:S49-S50 Abstract P.1.29. [Google Scholar]
- 17.Chen W, Wang G-F, Chen X-H, et al. Effects of paroxetine on function recovery in patients with poststroke depression. Chinese J Clin Rehabil 2002; 6:2014–2015. [Google Scholar]
- 18.Lipsey JR, Robinson RG, Pearlson GD, et al. Nortriptyline treatment of post-stroke depression: a double-blind study. Lancet 1984; 1:297–300. [DOI] [PubMed] [Google Scholar]
- 19.Reding MJ, Orto LA, Winter SW, et al. Antidepressant therapy after stroke. A double-blind trial. Arch Neurol 1986; 43:763–765. [DOI] [PubMed] [Google Scholar]
- 20.Andersen G, Vestergaard K, Lauritzen L. Effective treatment of poststroke depression with the selective serotonin reuptake inhibitor citalopram. Stroke 1994; 25:1099–1104. [DOI] [PubMed] [Google Scholar]
- 21.Raffaele R, Rampello L, Vecchio I, et al. Trazodone therapy of the post-stroke depression. Arch Gerontol Geriatr 1996; 22 (suppl 1):217–220. [DOI] [PubMed] [Google Scholar]
- 22.Robinson RG, Schultz SK, Castillo C, et al. Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled, double-blind study. Am J Psychiatry 2000; 157:351–359. [DOI] [PubMed] [Google Scholar]
- 23.Wiart L, Petit H, Joseph PA, et al. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke 2000; 31:1829–1832. [DOI] [PubMed] [Google Scholar]
- 24.Fruehwald S, Gatterbauer E, Rehak P, et al. Early fluoxetine treatment of post-stroke depression—a three-month double-blind placebo-controlled study with an open-label long-term follow up. J Neurol 2003; 250:347–351. [DOI] [PubMed] [Google Scholar]
- 25.Murray V, von Arbin M, Bartfai A, et al. Double-blind comparison of sertraline and placebo in stroke patients with minor depression and less severe major depression. J Clin Psychiatry 2005; 66:708–716. [DOI] [PubMed] [Google Scholar]
- 26.Rampello L, Alvano A, Chiechio S, et al. An evaluation of efficacy and safety of reboxetine in elderly patients affected by “retarded” post-stroke depression. A random, placebo-controlled study. Arch Gerontol Geriatr 2005; 40:275–285. [DOI] [PubMed] [Google Scholar]
- 27.Baer L, Ahern DK. Statistical problems with small sample size. Am J Psychiatry 1993; 150:356–357. [DOI] [PubMed] [Google Scholar]
- 28.Wickenberg-Bolin U, Goransson H, Fryknas M, et al. Improved variance estimation of classification performance via reduction of bias caused by small sample size. BMC Bioinformatics 2006; 7:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snow V, Lascher S, Mottur-Pilson C. Pharmacologic treatment of acute major depression and dysthymia. American College of Physicians-American Society of Internal Medicine. Ann Intern Med 2000; 132:738–742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


