Abstract
Background:
The aim of this meta-analysis was to compare the feasibility of en bloc transurethral resection of bladder tumor (ETURBT) versus conventional transurethral resection of bladder tumor (CTURBT).
Methods:
Relevant trials were identified in a literature search of MEDLINE, EMBASE, Cochrane Library, Web of Science, and Google Scholar using appropriate search terms. All comparative studies reporting participant demographics, tumor characteristics, study characteristics, and outcome data were included.
Results:
Seven trials with 886 participants were included, 438 underwent ETURBT and 448 underwent CTURBT. There was no significant difference in operation time between 2 groups (P = 0.38). The hospitalization time (HT) and catheterization time (CT) were shorter in ETURBT group (mean difference[MD] −1.22, 95% confidence interval [CI] −1.63 to −0.80, P < 0.01; MD −0.61, 95% CI −1.11 to −0.11, P < 0.01). There was significant difference in 24-month recurrence rate (24-month RR) (odds ratio [OR] 0.66, 95% CI 0.47–0.92, P = 0.02). The rate of complication with respect to bladder perforation (P = 0.004), bladder irritation (P < 0.01), and obturator nerve reflex (P < 0.01) was lower in ETURBT. The postoperative adjuvant intravesical chemotherapy was evaluated by subgroup analysis, and 24-month RR in CTURBT is higher than that in ETURBT in mitomycin intravesical irrigation group (P = 0.02).
Conclusion:
The first meta-analysis indicates that ETURBT might prove to be preferable alternative to CTURBT management of nonmuscle invasive bladder carcinoma. ETURBT is associated with shorter HT and CT, less complication rate, and lower recurrence-free rate. Moreover, it can provide high-qualified specimen for the pathologic diagnosis. Well designed randomized controlled trials are needed to make results comparable.
Keywords: bladder tumor, CTURBT, en bloc, ETURBT, transurethral
1. Introduction
Bladder tumor is the second most common urological malignancy and has been a growing healthcare problem all over the world.[1–4] To date, radical surgery was the most effective treatment.[5,6] For nonmuscle invasive bladder carcinoma (NMIBC), transurethral resection of bladder tumor (TURBT) remains the standard treatment.[7] Unfortunately, bladder tumor is bound up with high recurrence rates (50%–70%) after transurethral resection management, and tumor cell implantation is deemed to be a major cause of early recurrence.[8,9] Thus, it is reasonable to modify TURBT to provide en bloc transurethral resection of bladder tumor (ETURBT) of the specimen, based on the established oncological principle of dissecting through normal tissue.[10] Several studies have examined the efficacy and feasibility of en bloc TURBT.[11–16] In this study, a meta-analysis was performed to evaluate the efficacy and feasibility of ETURBT for the participants with NMIBC compared with conventional TURBT.
2. Materials and methods
2.1. Ethics statement
Ethical approval was not required for this meta-analysis. It is a meta-analysis which has not affected participants directly.
2.2. Study selection
We searched MEDLINE, EMBASE, Cochrane Library, Web of Science, and Google scholar databases for articles published before September 12, 2016. A combination of search terms was used including “en bloc resection”, “transurethral resection”, “bladder tumor”, “bladder cancer”, “TURBT”, and “cystectomy”. The search was conducted with a language restricted to English publication.
2.3. Selection criteria
The inclusion criteria were as follows: original articles in English publication; trials reporting individual demographic, survival information, and clinical follow-up data; and trials comparing the efficacy and feasibility of ETURBT versus conventional transurethral resection of bladder tumor (CTURBT) in NMIBC. Single-arm trials, case reports, and systematic reviews were excluded.
2.4. Data extraction
Two investigators (Y-PW and S-HC) extracted data, respectively, employing a predefined data extraction form. Subsequent full-text record screening was fulfilled independently by 2 investigators (Y-PW and S-HC). Disagreements were resolved by a third reviewer (NX). All of the included trials in our meta-analysis contain data as follows: first author's name, published year, surgical method, number of patients, median age, operation time (OT), hospitalization time (HT), catheterization time (CT), 24-month recurrence rate (24-month RR), recurrence-free survival (RFS), and complications. We made several attempts to contact the corresponding authors to obtain the necessary data to meet inclusion criteria, when their studies did not meet inclusion requirements. At least 3 follow-up attempts were made for queries sent; unfortunately, these attempts were unsuccessful.
2.5. Statistical analysis
Statistical analysis was conducted utilizing RevMan5.3 (Cochrane Collaboration, Oxford, UK). Chi-square and I2 tests were employed to test the heterogeneity of different trials[17,18]; no heterogeneity existed when P > 0.1 and I2 < 50%, a fixed-effects model was applied to pool the trial results. Significant heterogeneity was identified if P < 0.1 and I2 > 50%, and a random-effects model was employed.[19] OT, HT, and CT were determined applying continuous variables. Complications and 24-month RR were determined applying dichotomous variables. Subgroup analysis of postoperative adjuvant intravesical chemotherapy was also determined applying dichotomous variables. RFS was calculated using effect variables. Hazard ratios (HRs) with 95% confidence interval (CI) were extracted from the survival curves when HRs were unavailable for RFS.[20] Sensitivity analysis and publication bias analysis were conducted utilizing Stata 12.0 (Stata-Corp, College Station, TX). Begg funnel plot and Egger test were used to identify whether publication bias existed.
3. Results
3.1. Workflow of literature research
There were 304 potential relevant studies in the primary literature search, and 22 duplicate studies existed. After removing the duplicate studies, 258 studies were further excluded by reading the title and abstract. Then, a total of 17 additional studies were removed by 2 authors (Y-PW and S-HC) independently reading the full text. Therefore, 7 studies were included in this meta-analysis.[11–16] We described study procedure's details in Fig. 1. Two authors (Y-PW and S-HC) independently completed this work, and any disagreements were dealt with by discussion.
Figure 1.
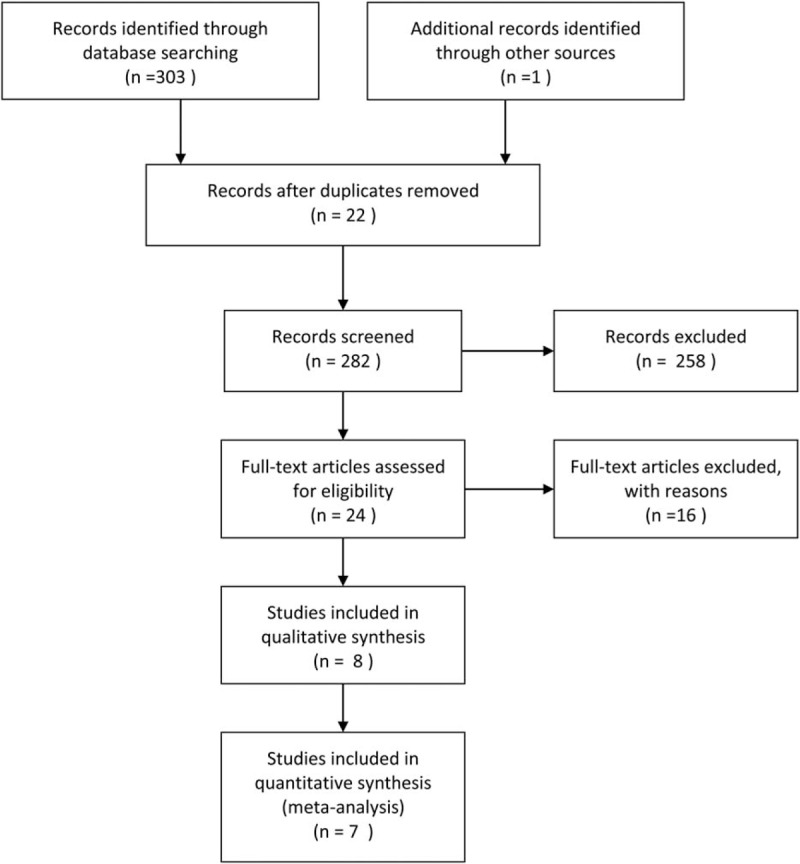
Flow diagram for selection of the included trials reviewed.
3.2. Study characteristics
These included studies recruited 886 participants with various stages of bladder cancer comprising 438 cases that underwent ETURBT and 448 with CTURBT. The demographics of enrolled individuals and tumor characteristics are presented in Tables 1 and 2, respectively. All of the cases applied postoperative installation therapy, mitomycin was used in 3 trials,[11,15,16] and epirubicin was used in 4 trials.[12–14]
Table 1.
Characteristics of trials included in the meta-analysis.
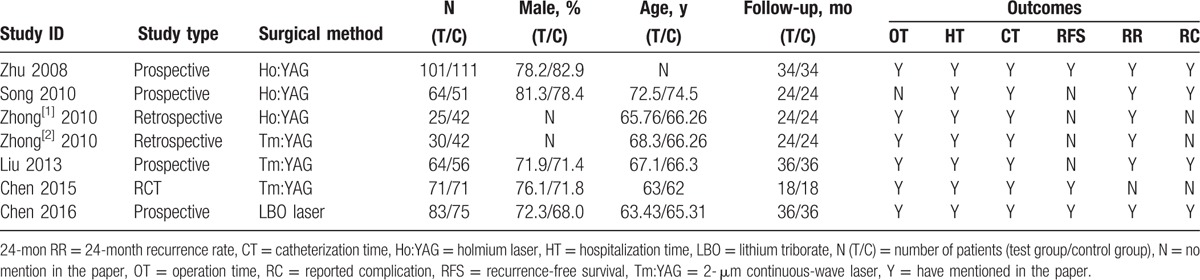
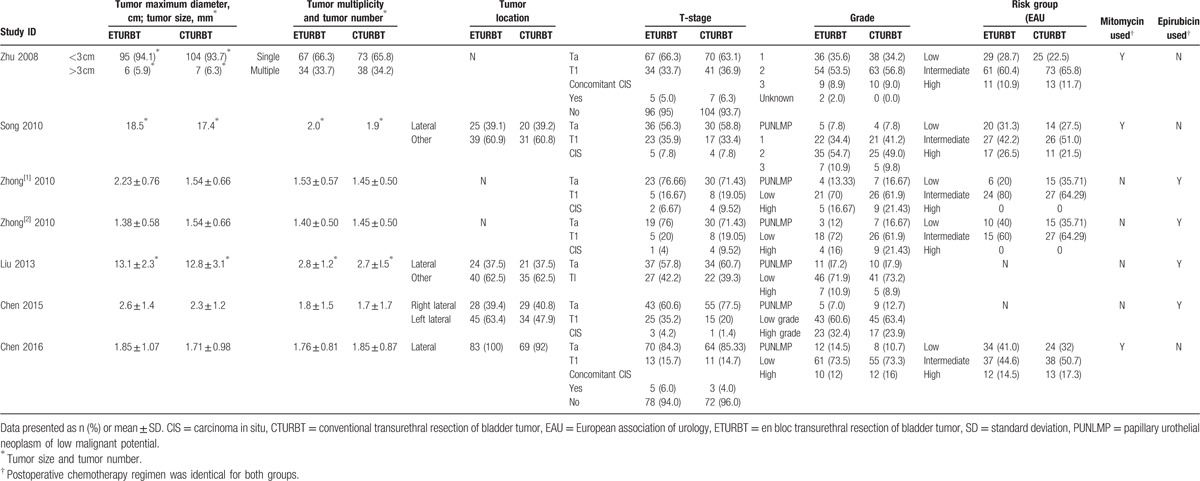
Table 2.
Tumor characteristics.
3.3. Operation time, hospitalization time, and catheterization time
OT was available for 6 trials[11–14,16] (Fig. 2A). The pooled weight mean difference (MD) was 1.92 (95% CI = −2.33 to 6.18; I2 = 79%; P = 0.38), indicating that there was no significant difference between ETURBT and CTURBT. HT and CT was available for all 7 trials[11–16] (Fig. 2B and C). The pooled weight MD was −1.22 (95% CI = −1.63 to −0.80; I2 = 82%; P < 0.01) and MD was −0.61 (95% CI = −1.11 to −0.11; I2 = 92%; P = 0.02), indicating that ETURBT yielded a shorter HT and CT over CTURBT. However, there was a significant heterogeneity existing (I2 = 82% and 92%). Thus, a random model was applied, and further discussion was made to explain the heterogeneity.
Figure 2.
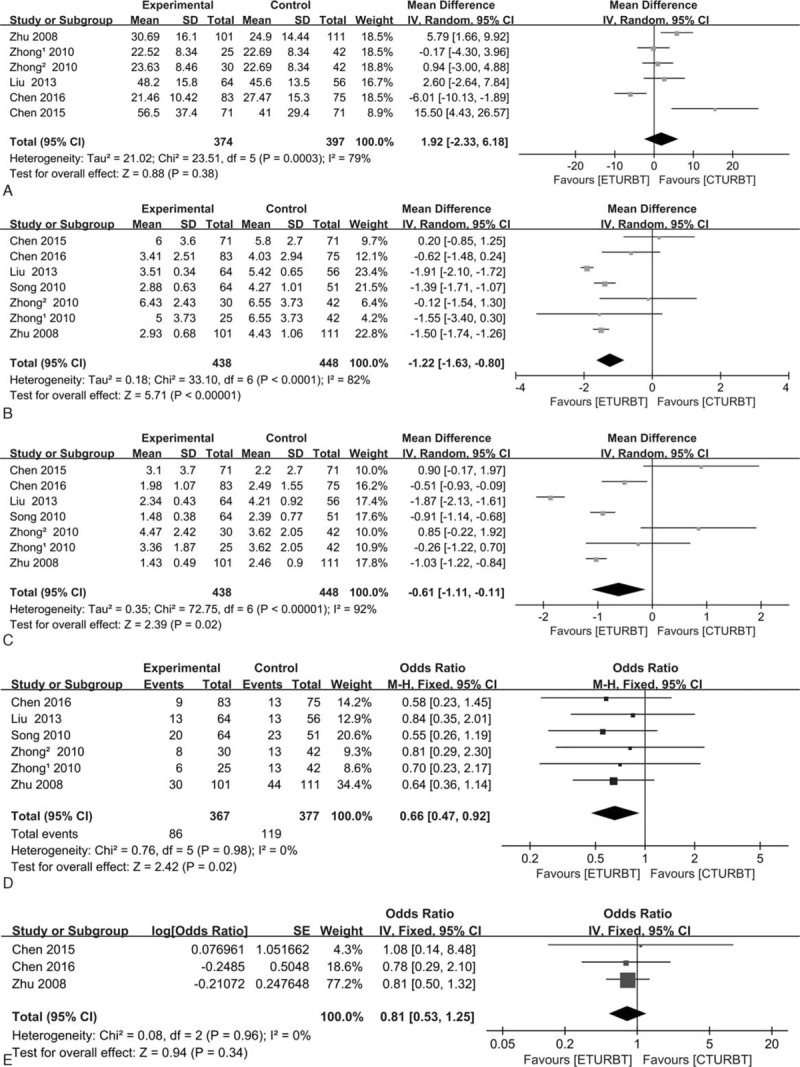
Forest plot comparing operation time (A), hospitalization time (B), catheterization time (C), 24-month recurrence rate (D), and recurrence-free survival (E).
3.4. 24-month recurrence rate and recurrence-free survival
Analysis showed that there was a significant difference in 24-month RR reported by 6 trials.[11,13–16] The pooled odds ratio (OR) was 0.66 (95% CI = 0.47–0.92; I2 = 0; P = 0.02) (Fig. 2D). Three trials[11,12,16] reported RFS, and there was no significant difference between two arms, the pooled OR was 0.81 (95% CI = 0.53–1.25; I2 = 0; P = 0.34) (Fig. 2E).
3.5. Complications
Four trials[13–15] reported obturator nerve reflex, and 4 trials[11,13–15] reported bladder perforation, respectively. The pooled OR was 0.04 (95% CI = 0.01–0.15; I2 = 0; P < 0.01) and 0.14 (95% CI = 0.04–0.54; I2 = 0; P = 0.004) (Fig. 3A and B), respectively, which indicated that ETURBT may cause less bladder perforation than CTURBT. Four trials[13–15] reported bladder irritation and 3 trials[11,13,15] reported urethral stricture, respectively. The pooled OR was 0.22 (95% CI = 0.11–0.43; I2 = 55%; P < 0.01) and 0.57 (95% CI = 0.16–2.06; I2 = 0; P = 0.39) (Fig. 3C and D), respectively, which indicated that ETURBT may cause less obturator nerve reflex and bladder irritation than CTURBT.
Figure 3.
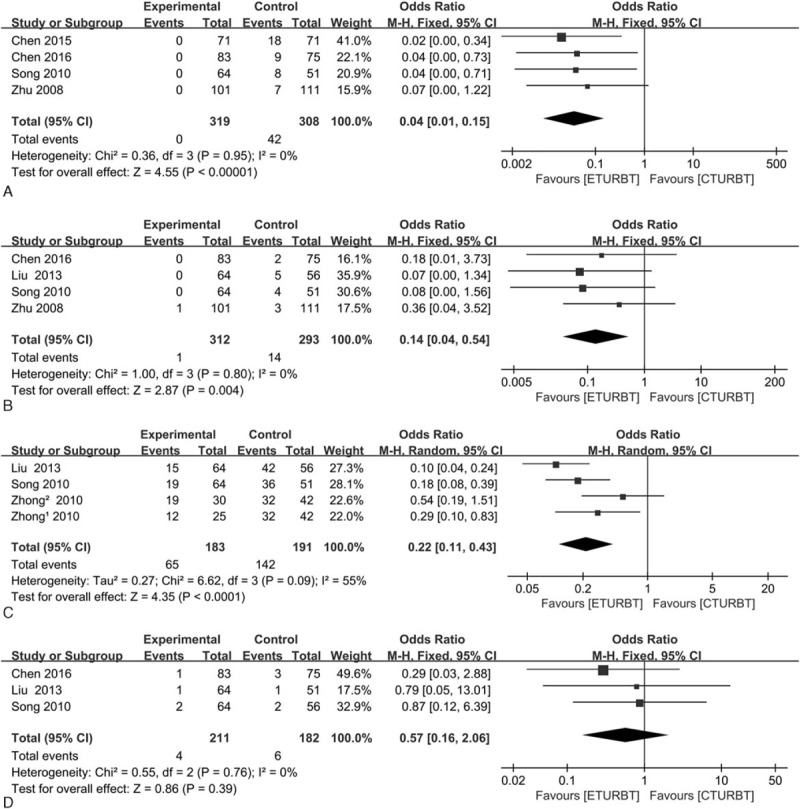
Forest plot comparing complications in terms of obturator nerve reflex (A), bladder perforation (B), bladder irritation (C), and urethral stricture (D).
3.6. Sensitivity analysis and publication bias
A sensitivity analysis was performed by deleting a study at a time to assess the influence of an individual study on synthetic statistics. In terms of HT, when Liu trial[13] was removed, I2 altered from 82% to 68%. In addition, when Xu trial[12] was removed, I2 altered from 82% to 77%. When both trials[12,13] were removed, the overall results altered obviously, I2 altered from 82% to 43%, which indicated that those 2 studies should be responsible for the heterogeneity of our eligible statistics. The reason could be explained as follows: the study type of Xu trial[12] was a randomized controlled trial, it is different from other eligible studies. Moreover, when Chen trail[11] was removed with respect to OT, I2 altered from79% to 60%, this heterogeneity could be explained by the laser type. Chen trail performed ETURBT using green-light laser, and other trails included performed ETURBT using holmium laser or 2-μm continuous-wave laser. In addition, publication bias was evaluated by Begg funnel plot and Egger test, and no obvious biases were identified.
4. Discussion
To the best of our knowledge, this study is the first meta-analysis with a focus on comparing the efficacy and feasibility between ETURBT and CTURBT. It remains controversial whether the actual potential of ETURBT resides more in the therapeutic or diagnostic sector.[21,22] On one hand, CTURBT is dependent on in situ tumor fragmentation for tumor removal and specimen retrieval, and basic oncological surgical tenet was violated by CTURBT. This practice will promote tumor cell dispersal, and the pathological integrity of the specimen will be jeopardized.[21] On the other hand, well controlled resection of the whole tumor was yield, and the detrusor muscle present in the specimen was significantly better with ETURBT due to better visualization.[10] Therefore, this meta-analysis was performed to systematically evaluate these 2 techniques, providing evidence for the optimal treatment of NMIBC.
After combining results from 6 studies consisting of 7 trials, no significant trend of decreased OT in patients treated with ETURBT than CTURBT was observed. There were statistically significant differences in HT and CT between ETURBT and CTURBT. Moreover, it appeared that patients who underwent ETURBT experienced significant reductions in 24-month RR. But in terms of RFS, there was no statistically significant difference between the 2 arms. The complications including bladder perforation, obturator nerve reflex, and bladder irritation were less in ETURBT. No statistically significant difference in urethral stricture rate was noted. The CT and HT in ETURBT were less than that in CTURBT. However, the heterogeneity in pooled HT and CT should not be ignored (I2 = 82%, P < 0.001 and I2 = 92%, P < 0.001). There are several reasons for those differences: first of all, the high heterogeneity indicated that these differences might be unrelated to the technique itself, there are plenty of confounding factors that should be taken into account[11]; second, in personal comprehension, patient demographics and tumor characteristics are different, further researches should be done to ascertain the affect factors; third, previous studies[11–13] demonstrated that ETURBT may have some advantages in terms of perioperative complication rates. Our meta-analysis indicated that ETURBT may cause less bladder perforation, obturator nerve reflex, and shorter durations of postoperative bladder irritation than CTURBT. Moreover, 2 kinds of study type were pooled (retrospective study and prospective study), some bias may exist in this procedure, which would increase the heterogeneity of outcome and diminish the validity of our study. Finally, patients underwent surgery in different hospitals, different hospitals possessed different medical levels.
Staging quality was determined by the condition of detrusor muscle presented in specimens and demonstrated complete resection.[11] Complete resection of the pathologic specimen plays an important role in bladder tumor transurethral resection, which determines postoperative pathologic diagnosis and prognosis.[23] Unfortunately, the trials enrolled in our study had not provided sufficient information about the data of detrusor muscle-positive specimens. To the best of our knowledge, detrusor muscle-positive specimens in 78% and 100% were reported in 2 small case series,[24,25] and the presence of the detrusor muscle layer in 97.3% of 221 samples was demonstrated by Kramer et al.[26] All of enrolled trials possessed the view that ETURBT can obtain adequate complete tumor specimens, containing the mucosa, lamina propria, and muscle layer for determining pathological diagnosis and treatment procedure.[12]
The postoperative adjuvant intravesical chemotherapy was performed for each patient in our meta-analysis. The characteristics of regimen of every trial were demonstrated in Table 3. The application of postoperative adjuvant intravesical chemotherapy was quite inconsistent in each trial. Three trials[11,15,16] received mitomycin and 4 trials[12–14] received epirubicin. However, the duration of administration and the drugs administered varied. We performed a subgroup analysis to evaluate the definitive relationship between 24-month RRs and postoperative adjuvant intravesical chemotherapy (Fig. 4). In mitomycin group, 3 trials[11,15,16] were enrolled. The results showed a statistical significant difference between 2 arms when mitomycin was postoperatively used (P = 0.02). In eprirubicin group, 3 trials[13,14] were included, and there was a statistical significant difference between the 2 arms when eprirubicin was postoperatively used (P = 0.43). The subgroup analysis demonstrated that postoperatively used mitomycin might be an important factor which affected the 24-month RRs between ETURBT and CTURBT.
Table 3.
Postoperative adjuvant intravesical chemotherapy regimen.
Figure 4.
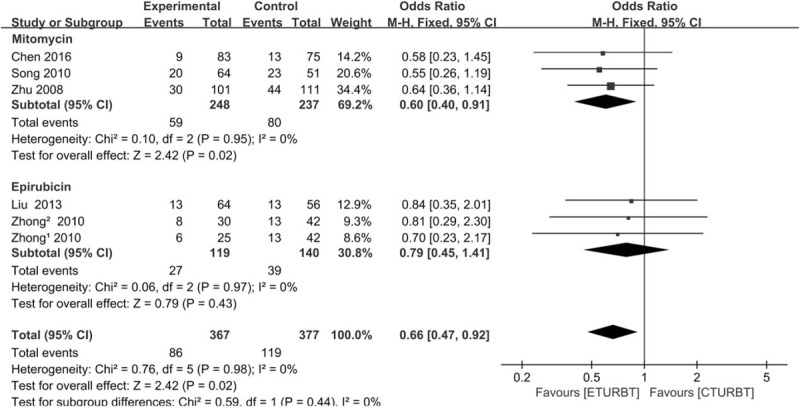
Subgroup analysis of the 2 groups according to the mitomycin and eprirubicin used for postoperative adjuvant intravesical chemotherapy.
There are several limitations of our study. First of all, included trails are consisted of 2 retrospective studies and 4 prospective nonrandomized studies and 1 randomized controlled trial. This certainly attenuated the value of our meta-analysis. Further studies need to be done in the near future. Second, in terms of the small sample size and limited number of studies enrolled, the results may lack statistical power.
5. Conclusion
ETURBT is superior to CTURBT in terms of shorter HT and CT, less complication rate, and lower recurrence-free rate. Moreover, it can provide a better tumor specimen for pathological evaluation. ETURBT is potentially useful alternative to CTURBT. However, this result needs to be validated in further prospective, randomized, controlled studies.
Footnotes
Abbreviations: 24-month RR = 24-month recurrence rate, CI = confidence interval, CT = catheterization time, CTURBT = conventional transurethral resection of bladder tumor, EAU = European association of urology, ETURBT = en bloc transurethral resection of bladder tumor, HRs = hazard ratios, HT = hospitalization time, MD = mean difference, NMIBC = nonmuscle invasive bladder carcinoma, OR = odds ratio, OT = operation time, RFS = recurrence-free survival, SD = standard deviation, TURBT = transurethral resection of bladder tumor.
Yu-Peng Wu and Ting-Ting Lin have contributed equally to the article.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Xu C, Zeng S, Zhang Z, et al. Deceptive muscle invasive bladder cancer recurrence with benign biopsy foci after bladder sparing treatment. Medicine 2015; 94:e898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun L, Lu J, Niu Z, et al. A potent chemotherapeutic strategy with Eg5 inhibitor against gemcitabine resistant bladder cancer. PLoS One 2015; 10:e0144484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao Y, Wu K, Chen Y, et al. Beyond proliferation: KLF5 promotes angiogenesis of bladder cancer through directly regulating VEGFA transcription. Oncotarget 2015; 6:43791–43805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63:11–30. [DOI] [PubMed] [Google Scholar]
- 5.Stenzl A, Cowan NC, De Santis M, et al. The updated EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol 2009; 55:815–825. [DOI] [PubMed] [Google Scholar]
- 6.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1054 patients. J Clin Oncol 2001; 19:666–675. [DOI] [PubMed] [Google Scholar]
- 7.Zeng S, Yu X, Ma C, et al. Low-dose versus standard dose of bacillus Calmette–Guerin in the treatment of nonmuscle invasive bladder cancer: a systematic review and meta-analysis. Medicine 2015; 94:e2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho CH, Sung KC, Lim SW, et al. Chronic indwelling urinary catheter increase the risk of bladder cancer, even in patients without spinal cord injury. Medicine 2015; 94:e1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramer MW, Abdelkawi IF, Wolters M, et al. Current evidence for transurethral en bloc resection of non-muscle-invasive bladder cancer. Minim Invasive Ther Allied Technol 2014; 23:206–213. [DOI] [PubMed] [Google Scholar]
- 10.Sureka SK, Agarwal V, Agnihotri S, et al. Is en-bloc transurethral resection of bladder tumor for non-muscle invasive bladder carcinoma better than conventional technique in terms of recurrence and progression?: A prospective study. Indian J Urol 2014; 30:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Zhao Y, Wang S, et al. Green-light laser en bloc resection for primary non-muscle-invasive bladder tumor versus transurethral electroresection: A prospective, nonrandomized two-center trial with 36-month follow-up. Lasers Surg Med 2016; 48:859–865. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Liao J, Chen L, et al. En bloc transurethral resection with 2-micron continuous-wave laser for primary non-muscle-invasive bladder cancer: a randomized controlled trial. World J Urol 2015; 33:989–995. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Wu J, Xue S, et al. Comparison of the safety and efficacy of conventional monopolar and 2-micron laser transurethral resection in the management of multiple nonmuscle-invasive bladder cancer. J Int Med Res 2013; 41:1081–1107. [DOI] [PubMed] [Google Scholar]
- 14.Zhong C, Guo S, Tang Y, et al. Clinical observation on 2 micron laser for non-muscle-invasive bladder tumor treatment: single-center experience. World J Urol 2010; 28:157–161. [DOI] [PubMed] [Google Scholar]
- 15.Xishuang S, Deyong Y, Xiangyu C, et al. Comparing the safety and efficiency of conventional monopolar, plasmakinetic, and holmium laser transurethral resection of primary non-muscle invasive bladder cancer. J Endourol 2010; 24:69–73. [DOI] [PubMed] [Google Scholar]
- 16.Zhu Y, Jiang X, Zhang J, et al. Safety and efficacy of holmium laser resection for primary nonmuscle-invasive bladder cancer versus transurethral electroresection: single-center experience. Urology 2008; 72:608–612. [DOI] [PubMed] [Google Scholar]
- 17.Luo S, Chen L, Chen X, et al. Evaluation on efficacy and safety of tyrosine kinase inhibitors plus radiotherapy in NSCLC patients with brain metastases. Oncotarget 2015; 6:16725–16734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ford AC, Forman D, Hunt RH, et al. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ 2014; 348:739–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Z, Zhang T, Li B, et al. Extracting and transforming of appropriate data of meta-analysis in survival curve. Chin J Evid Based Cardiovasc Med 2014; 6:243–247. [Google Scholar]
- 21.Kramer MW, Wolters M, Herrmann TRW. En bloc resection of bladder tumors: ready for prime time? Eur Urol 2016; 69:967–968. [DOI] [PubMed] [Google Scholar]
- 22.Karl A, Thomas R, Herrmann W. En bloc resection of urothelial cancer within the urinary bladder: the upcoming gold standard? World J Urol 2015; 33:581. [DOI] [PubMed] [Google Scholar]
- 23.Wang DS, Bird VG, Leonard VY, et al. Use of bipolar energy for transurethral resection of bladder tumors: pathologic considerations. J Endourol 2004; 18:578–582. [DOI] [PubMed] [Google Scholar]
- 24.Wolters M, Kramer MW, Becker JU, et al. Tm: YAG laser en bloc mucosectomy for accurate staging of primary bladder cancer: early experience. World J Urol 2011; 29:429–432. [DOI] [PubMed] [Google Scholar]
- 25.Das A, Gilling P, Fraundorfer M. Holmium laser resection of bladder tumors (HoLRBT). Tech Urol 1998; 4:12–14. [PubMed] [Google Scholar]
- 26.Kramer MW, Rassweiler JJ, Klein J, et al. En bloc resection of urothelium carcinoma of the bladder (EBRUC): a European multicenter study to compare safety, efficacy, and outcome of laser and electrical en bloc transurethral resection of bladder tumor. World J Urol 2015; 33:1937–1943. [DOI] [PubMed] [Google Scholar]


