Supplemental digital content is available in the text.
Key Words: Cervical cancer, MACC1, Invasion, Angiogenesis, Akt, NF-κB
Abstract
Objective
The aim of this study is to investigate the clinicopathologic significance and potential role of metastasis-associated in colon cancer-1 (MACC1) in the progression of cervical cancer.
Methods
MACC1 expression was examined in cervical cancer cell lines, 6 matched cervical cancer tissues, and adjacent noncancerous tissues using Western blotting and real-time reverse transcriptase polymerase chain reaction. MACC1 protein expression and localization were determined in 181 paraffin-embedded archived cervical cancer samples using immunohistochemistry. Statistical analyses were applied to evaluate the clinicopathologic significance. The effects of MACC1 on cell migration, invasion, and angiogenesis were examined using migration assay, wound healing assay, 3-dimensional morphogenesis assay, and chicken chorioallantoic membrane assay. Western blotting was performed to examine the impact of MACC1 on the Akt and nuclear factor κB signaling pathways.
Results
Both protein and messenger RNA levels of MACC1 was up-regulated in cervical cancer cell lines and cervical cancer tissues, as compared with normal tissues. High MACC1 expression was detected in 96 (53%) of 181 of the cervical cancer tissues. In addition, high MACC1 expression correlated significantly with aggressiveness of cervical cancer, including International Federation of Gynecology and Obstetric stage (P = 0.001), pelvic lymph node metastasis (P = 0.004), recurrence (P = 0.037), and poor survival (P = 0.001). Moreover, enforced expression of MACC1 in cervical cancer cell lines significantly enhanced cell migration, invasion, and angiogenesis. Conversely, knockdown of MACC1 caused an inhibition of cell migration, invasion, and angiogenesis. Up-regulation of MACC1 increased, but knockdown of MACC1 decreased the expression of matrix metalloproteinase-2 and matrix metalloproteinase-9. Furthermore, enforced expression of MACC1 could enhance, but knockdown of MACC1 could reduce AKT and nuclear factor κB pathway activity.
Conclusions
Our findings suggest that MACC1 protein, as a valuable marker of cervical cancer prognosis, plays an important role in the progression of human cervical cancer cells.
Cervical cancer is one of the most commonly causes of female death worldwide. Despite the generally good prognosis for early-stage cervical cancer, the global cervical cancer incidence is increasing and the clinical outcome and prognosis of patients with late-stages cervical cancer still remain pessimistic.1 Lymph node involvement is one of the strongest prognostic factors for unfavorable prognosis, even in early-stage cervical cancer (International Federation of Gynecology and Obstetric [FIGO] stages Ib-IIa) and provides important guidance for determining the treatment strategies.2 The penetration of lymphatic or blood vessels by tumor cells is an essential step in the formation of metastasis. However, hitherto, no efficient strategy to predict lymphatic or distant metastasis preoperatively has been established. The identification and functional characterization of molecules involved in lymphatic or distant metastasis may, therefore, reveal targets for diagnostic and therapeutic techniques. Metastasis-associated in colon cancer-1 (MACC1) was first identified by differential display reverse transcriptase polymerase chain reaction (RT-PCR) analyzing colon mucosa, primary tumors, and metastatic colon cancer.3 Further studies have shown that MACC1 was an independent prognostic predictor of metastasis formation and metastasis-free survival in patients with colon cancer.4 Functional investigations demonstrated that MACC1 could promote cell proliferation, motility, tumor growth, and metastasis abilities of colon cancer cells, and acts as a key regulator of the HGF-MET pathway.4 Bioinformatic analyses indicate that MACC1 also involves in regulation of apoptosis.5 More recently, MACC1 is demonstrated to be up-regulated in several types of cancer and serves as a biomarker for prediction of invasion and metastasis, as well as prognosis in several types of human cancers, including hepatocellular cancer, nasopharyngeal cancer, gastric cancer, pancreatic cancer, ovarian cancers, and non–small cell lung cancer.6–12 These findings suggest that MACC1 plays an important role in the development and progression of human cancers. Recently, it was reported that MACC1 was up-regulated in cervical cancer tissues. High expression of MACC1 was correlated with aggressive characteristics and poor survival for patient with this disease.13 However, the potential role of MACC1 in cervical cancer progression and underlying mechanism remain unknown and thus are worth further studying. Here, we sought to investigate the expression patterns and the potential role of MACC1 in the progression of cervical cancer.
MATERIALS AND METHODS
Tissue Specimens
A total of 181 archived, formalin-fixed, paraffin-embedded human cervical cancer (FIGO stages I-III) samples were obtained from the Department of Pathology, NanFang hospital, Southern Medical University, China. All of the cases were clinically and histologically diagnosed between November 1999 and September 2005. The medical records of the patients were reviewed to collect the following clinicopathologic information: age, FIGO stage, histologic type, differentiation, pelvic lymph node involvement, and recurrence. A total of 113 patients received adjuvant chemoradiation. The latest follow-up was updated in August 2010, and the median follow-up for overall survival was 58 (range, 0.5–131) months for patients who remained alive at the time of this analysis. The 6 freshly collected cervical cancer biopsies and their matched adjacent noncancerous mucosa tissues were collected at the operation room, Nan-fang Hospital. The fresh biopsies were frozen and stored in liquid nitrogen until further use. Prior approval was obtained from the Southern Medical University Institutional Board (Guangzhou, China) for the use of clinical materials for research purposes.
Immunohistochemistry
Immunochemistry (IHC) staining and data analyses were carried out as previously described.14 Briefly, tissue sections were incubated with anti-MACC1 antibody (1:500; Sigma, ST Louis, MI) overnight at 4°C. The sections were reviewed and scored independently by 2 observers, based on both the proportion of positively stained tumor cells and the intensity of staining.14 The proportion of tumor cells was scored as follows: 0 (no positive tumor cells), 1 (<10% positive tumor cells), 2 (10%–50% positive tumor cells), and 3 (>50% positive tumor cells). The intensity of staining was graded according to the following criteria: 0 (no staining), 1 (weak staining = light yellow), 2 (moderate staining = yellow brown), and 3 (strong staining = brown). The staining index (SI) was calculated (staining intensity score × proportion of positive tumor cells). Using this method of assessment, we evaluated the expression of MACC1 in cervical cancer lesions by determining the SI, which scores as 0, 1, 2, 3, 4, 6, and 9. Cutoff values for MACC1 were chosen on the basis of a measure of heterogeneity with the log-rank test statistical analysis with respect to overall survival. An optimal cutoff value was identified: the SI score of at least 4 was used to define tumors as having high MACC1 expression and at most 3 as having low expression of MACC1. To account for the inconsistencies in IHC staining intensities, the mean optical density (MOD) method, which was used for the scoring of the staining intensity, was applied in the current study. In brief, the stained slides were evaluated at ×200 magnification using the SAMBA 4000 computerized image analysis system with Immuno 4.0 quantitative program (Image Products International, Chantilly, VA). Ten representative staining fields of each tumor sample were analyzed to determine the MOD, which represented the concentration of the stain or proportion of positive pixels within the whole tissue. A negative control for each staining batch was used for background subtraction in the quantitative analysis. The data were statistically analyzed using t test to determine the differences in average MOD values between different groups of tissues. P < 005 was considered significant.
Cell Lines and Plasmids
The cervical cancer cell lines HeLa, SiHa, and C33A were cultured in Eagle minimum essential medium (GIBCO BRL, Rockville, MD) and ME180, HeLa229, Caski, and MS7501 were cultured in RPMI-1640 medium (GIBCO BRL), all supplemented with 10% fetal bovine serum (HyClone Laboratories, Logan, UT). The MACC1 construct was generated by subcloning PCR amplified full-length human MACC1 complementary DNA into pMSCV. For deletion of MACC1, 2 small interfering RNA (siRNA) oligonucleotides (RNAi #1: AAGATTGGACTTGTACACTGC and RNAi #2: AAGCTTGGAAAAGGCTGGAGG) were cloned into pSuper-retro puro. Retroviral production and infection were performed as described previously.15 Stable cell lines expressing MACC1 or MACC1 RNAi were selected for 10 days with 0.5 μg/mL puromycin.
Western Blotting
Protein lysates were prepared, subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred onto polyvinylidene fluoride, membranes, and blotted according to standard methods, using rabbit anti-MACC1 (Abcam, Cambridge, MA), anti–phospho-AKT, anti-AKT, anti–p-IKKa/b, anti-IKKa/b (Cell Signaling Technology), rabbit anti–matrix metalloproteinase (MMP)-2, and anti–MMP-9 (Santa Cruz Biotechnology). Mouse anti–α-tubulin (Sigma) monoclonal antibody was used as a loading control.
RNA Extraction and Real-Rime RT-PCR
Total RNA from cultured cells and fresh tissues was extracted using the Trizol reagent (Invitrogen) according to the manufacturer’s instruction. Two micrograms of RNA from each sample was used for complementary DNA synthesis. Real-rime RT-PCR was used to quantify the relative messenger RNA (mRNA) expression levels of MACC1 by Bio-RadCFX96 sequence detection system. The qPCR primers were listed in supplemental Table S1, available online as Supplemental Digital Content at http://links.lww.com/IGC/A302. All genes were tested in triplicate. Expression data were normalized to the geometric mean of the housekeeping gene GAPDH and calculated as 2−[(Ct of MACC1)−(Ct of GAPDH)], where Ct represents the threshold cycle for each transcript.
Migration Assay
The migration assay was done by using Matrigel-coated Boyden chamber of 24-well transwell plate consisting of 8-mm membrane filter inserts (Corning). Briefly, cells were trypsinized and suspended in serum-free medium. Then 1.5 × 105 cells added to the upper chamber were coated with 50% Matrigel (BD Biosciences), whereas lower chamber was filled with medium with 10% fetal bovine serum. After incubation for 36 hours, cells invaded through the coated membrane to the lower surface were fixed with 4% paraformaldehyde and stained with hematoxylin. The cell count was done under the microscope (×20). All experiments were performed in triplicate.
Wound Healing Assay
Cells were trypsinized and seeded equally into 6-well tissue culture plates and grew to reach almost total confluence in 24 hours. The confluent cells were serum starved (0.5% serum) for 24 hours. Wounds were created in the confluent cells using a 100-μL pipette tip, and the cells were then cultured in complete media (10% serum). Images of cells migrating into the wound were captured at time points of 0, 12, and 24 hours by inverted microscope (×20). All experiments were performed in triplicate.
Chicken Chorioallantoic Membrane Assay
A 1-cm diameter window was opened in the shell of each egg with 8-day-old chicken embryo (Yueqin Breeding Co Ltd, Guangdong, China). The surface of the dermic sheet on the floor of the air sac was removed to expose the chorioallantoic Membrane (CAM). A 0.5-cm diameter filter paper was first placed on top the CAM, and 100-μL conditioned media harvested from cervical cancer cells were added onto the center of the paper. Then the eggs were incubated at 37°C under 80% to 90% relative humidity for 48 hours. After fixation with stationary solution (methanol/acetone, 1:1) for 15 minutes, CAMs were cut and harvested, and gross photos of CAMs were taken under a digital camera (Panasonic, Osaka, Japan). The effect of conditioned media harvested from different cells was evaluated by the number of second- and third-order vessels in comparison with that treated with the medium harvested from control group. The formation of vessels was quantified by comparing the vascular area to the CAM area (corresponding to the area of the 0.5-cm diameter filter paper) using Image-Pro Plus 6.0 software.
Statistical Analysis
All statistical analyses were performed using the SPSS 13.0 statistical software package. The 2-tailed Student t test was used to evaluate the significance of the differences between 2 groups of data in all pertinent experiments. The χ2 test was used to analyze the relationship between MACC1 expression (high or low) and the clinicopathologic features of cervical cancer. Survival curves were plotted by the Kaplan-Meier method and compared by the log-rank test. Multivariate survival analysis was carried out for all of the parameters that were significant in the univariate analysis using the Cox regression model. A 2-sided probability value less than 0.05 was considered statistically significant.
RESULTS
MACC1 Was Up-Regulated in Cervical Cancer
Western blotting and real-time RT-PCR analyses revealed an evidently higher level of MACC1 protein and mRNA in all 7 cervical cancer cell lines than in 1 case of normal cervical epithelial tissue (Fig. 1A). To determine whether the up-regulation of MACC1 in cervical cancer cell lines is clinically correlated with cervical cancer progression, we did Western blotting analysis on 6 pairs of matched normal cervical epithelium and cervical cancer samples. As shown in Figure 1B (left), MACC1 was found to be differentially overexpressed in all 6 examined human primary cervical cancer samples paired with normal tissues from the same patients. By real-time RT-PCR analysis, the tumor/adjacent noncancerous ratio of MACC1 mRNA expression was greater than 2-fold in all these samples (Fig. 1B, right). These findings are consistent with the results obtained in our immunohistochemical analysis (Fig. 1C).
FIGURE 1.
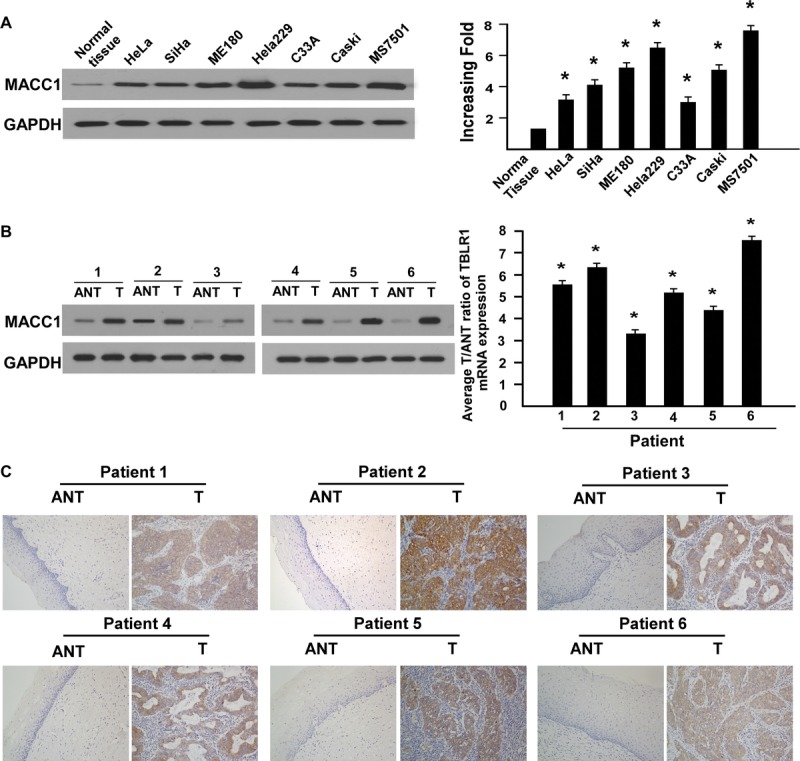
Up-regulation of MACC1 in cervical cancer cell lines and tissues. A, Expression of MACC1 proteins and mRNA in normal cervical tissue and cervical cancer cell lines (HeLa, SiHa, ME180, HeLa229, C33A, Caski, MS7501) analyzed by Western blotting (left; repeated 3 times; *P < 0.05) and real-time RT-PCR (right; each bars represent mean ± SD from 3 independent experiments; *P < 005). B, Expression of MACC1 proteins and mRNA in 6 pairs of cervical cancer tissues and matched adjacent noncancerous tissues by Western blotting (left; repeated 3 times; *P < 0.05) and real-time RT-PCR (right; error bars represent mean ± SD from 3 independent experiments; *P < 005). C, Expression of MACC1 in each paired cervical cancer tissues and adjacent normal tissues by IHC.
MACC1 Overexpression Was Associated With Progression and Poor Survival in Cervical Cancer
Expression of MACC1 protein was determined by IHC in 181 paraffin-embedded, archived cervical cancer tissues. Two histologic types were included in these cervical cancer tissues: squamous cell carcinoma and adenocarcinoma (Fig. 2A). MACC1 protein was detected in 165 (91.2%) of 181 cases of cervical cancer tissues, whereas there was no or weak signal in adjacent noncancerous areas in all sections detected. Positive staining of MACC1 protein was mainly observed in the cytoplasm (Fig. 2B). Quantitative IHC analysis revealed that the MOD values of MACC1 staining in all primary cervical cancer were higher than that in normal tissues. In addition, the MOD values of MACC1 staining were significantly increased along with the progression of stage I to III (P < 0.001; Fig. 2C).
FIGURE 2.
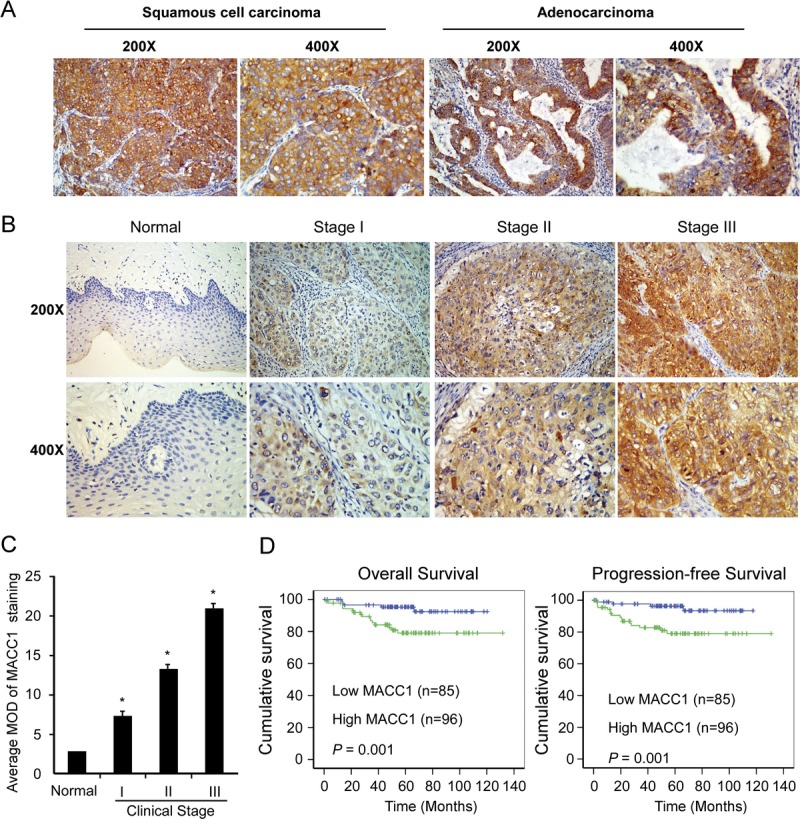
MACC1 protein overexpression in archived paraffin-embedded cervical cancer tissue sections as examined by IHC. A, Representative images from IHC analyses of MACC1 expression in squamous cell carcinoma and adenocarcinoma. B, Representative images from IHC analyses of MACC1 expression in normal human cervical epithelial tissue and primary cervical cancer tissues of different clinical stages. C, Statistical analyses of the average MOD of MACC1 staining between cervical epithelial tissues (10 cases) and cervical cancer tissues of different clinical stages. *P < 0.05. D, Kaplan-Meier survival curves of patients with cervical cancer according to MACC1 expression (left, overall survival; right, progression-free survival).
The χ2 tests in 181 primary human cervical cancer samples indicated that expression level of MACC1 was strongly correlated with FIGO stage (P = 0.001), pelvic lymph node metastasis (P = 0.004), and recurrence (P = 0.037). However, our analyses did not show a significant association between MACC1 expression and other clinicopathologic features including age, histologic type, and differentiation grade (Table S2, available online as Supplemental Digital Content at http://links.lww.com/IGC/A302). Kaplan-Meier analysis and the log-rank test revealed that both the overall survival time and progression-free survival time of patients with high-level MACC1 were shorter than those with low level (Fig. 2D). Univariate and multivariate analyses indicated that MACC1 expression level, FIGO stage, pelvic lymph node metastasis, and histology were independent prognostic markers for cervical cancer (Table S3, available online as Supplemental Digital Content at http://links.lww.com/IGC/A302).
Because there were strong associations between the MACC1 status and clinicopathologic parameters, the overall survival might be further distinguished based on MACC1 expression and adjusting the status based on the clinicopathologic parameters. As shown in Supplementary Figure 1, available online as Supplemental Digital Content at http://links.lww.com/IGC/A302, significantly different outcomes based on MACC1 expression were compared in patient subgroups with stages I, II, and III as well as pelvic lymph nodes and no pelvic lymph nodes. We found that MACC1 expression was related to a worse survival in different stratifications, including stages I, II, and III as well as pelvic lymph nodes and no pelvic lymph nodes. In addition, there were 113 patients received adjuvant chemoradiation. MACC1 expression was related to a worse survival in patients receiving adjuvant chemoradiation. However, no obvious difference was observed when MACC1 expression was compared in the subgroup with no adjuvant chemoradiation. Overexpression of MACC1 promotes the aggressiveness of cervical cancer cells.
To investigate whether MACC1 plays a role in aggressiveness of cervical cancer, SiHa and HeLa cervical cancer cells stably overexpressing MACC1 were established (Fig. 3A). Matrigel-coated Boyden chamber invasion assays demonstrated that overexpression of MACC1 significantly increased the number of invading cervical cancer cells (Fig. 3B). Wound healing assays demonstrated that overexpression of MACC1 dramatically improved the migration of cervical cancer cells (Fig. 3C). Furthermore, overexpression of MACC1 strongly promoted the formation of second- and third-order vessels in the CAM assay, indicating the significant role of MACC1 in promoting angiogenesis (Fig. 3D).
FIGURE 3.
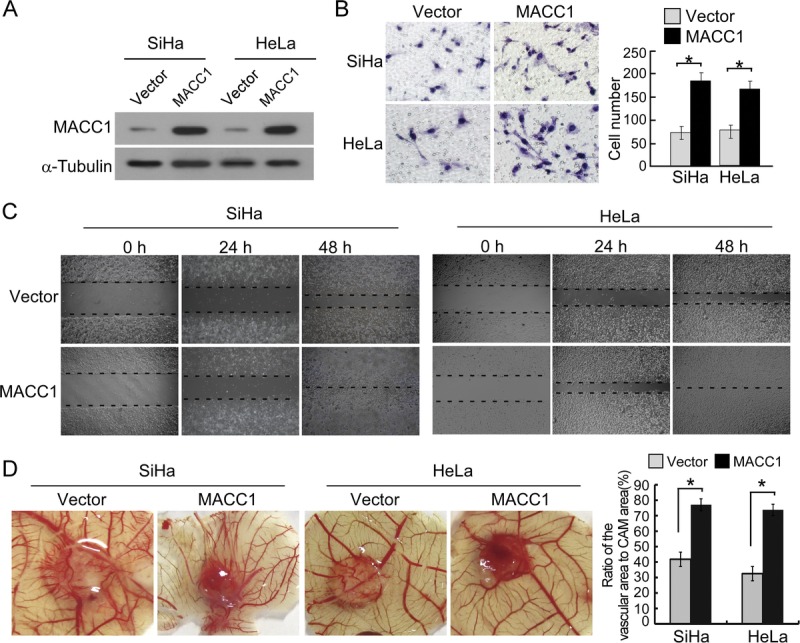
Overexpression of MACC1 promotes the aggressiveness of SiHa and HeLa cervical cancer cells. A, Expression of MACC1 in SiHa and HeLa cell lines overexpressed with vector or MACC1 was analyzed by immunoblotting. α-Tubulin was used as a loading control. B, The representative pictures (left panel) and quantification (right panel) of invaded cells were analyzed using Matrigel-coated Boyden chamber assay. Migrated cells were plotted as the average number of cells per field of view from 3 different experiments, as described in “Materials and Methods.” Original magnification ×200. *P < 005. C, The mobility of cells overexpressed with vector or MACC1 was measured by testing the rate of wound closure at 0, 24, and 48 hours. Original magnification ×200. D, Representative images of the chicken CAM blood vessels stimulated with conditioned medium from indicated cells (left). The formation of vessels was quantified by comparing the vascular area to the CAM area using Image-Pro Plus 6.0 software (right). *P < 005.
Knockdown of MACC1 Inhibits Aggressive Behaviors in Cervical Cancer
We further silenced endogenous MACC1 expression in SiHa and HeLa cervical cancer cells using specific siRNAs (Fig. 4A). Silencing MACC1 in cervical cancer cells significantly reduced the invasiveness and migration of cervical cancer cells (Fig. 4B, C). Furthermore, knocking down of MACC1 strongly inhibited the formation of second- and third-order vessels in the CAM assay (Fig. 4D).
FIGURE 4.
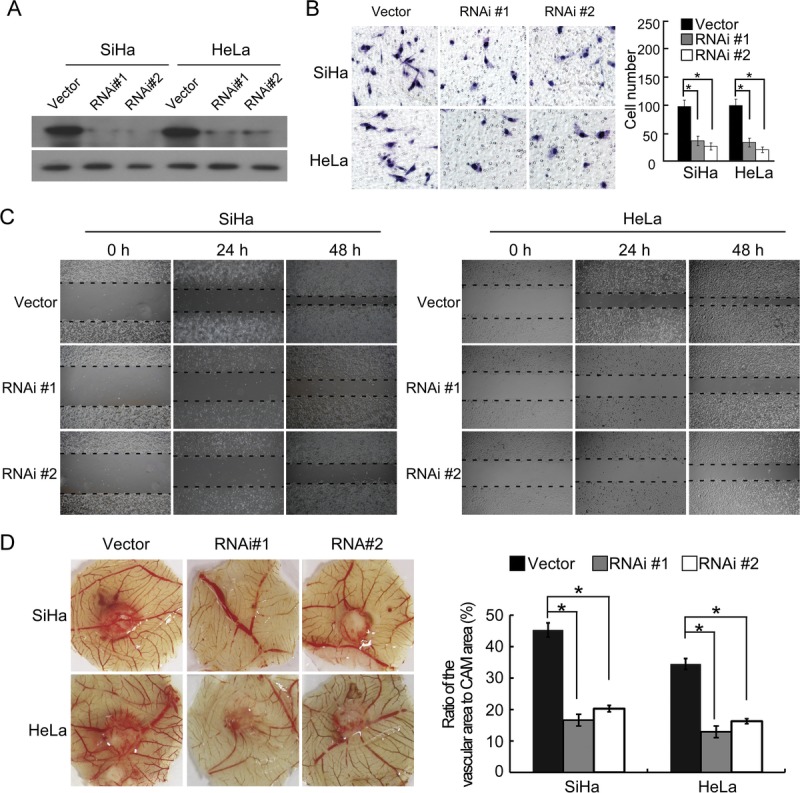
Silencing MACC1 inhibits the malignant properties of SiHa and HeLa cells. A, Expression of MACC1 in SiHa and HeLa cell lines expressed with vector or MACC1 siRNAs was analyzed by immunoblotting. α-Tubulin was used as a loading control. B, The representative pictures (left panel) and quantification (right panel) of invaded cells were analyzed using Matrigel-coated Boyden chamber assay. Migrated cells were plotted as the average number of cells per field of view from 3 different experiments, as described in “Materials and Methods.” Original magnification ×200. *P < 005. C, The mobility of cells expressed with vector or MACC1 siRNAs was measured by testing the rate of wound closure at 0, 24, and 48 hours. Original magnification ×200. D, Representative images of the chicken CAM blood vessels stimulated with conditioned medium from indicated cells (left). The formation of vessels was quantified by comparing the vascular area to the CAM area using Image-Pro Plus 6.0 software (right). *P < 005.
MACC1 Up-Regulated MMP-2 and MMP-9: Activation of AKT and Nuclear Factor κB Signaling Pathways in Cervical Cancer Cells Was Involved
To understand the mechanisms that facilitate the enhanced aggressiveness and angiogenesis mediated by MACC1, the expression levels of MMP-2 and MMP-9 were detected. As shown in Figure 5A, overexpression of MACC1 led to dramatic up-regulation of MMP-2 and MMP-9. Conversely, silencing MACC1 in cervical cancer cells led to decreased expression of MMP-2 and MMP-9. Moreover, the modulation of MMP-2 and MMP-9 by MACC1 was regulated at the transcriptional level (Fig. 5B). Furthermore, phosphorylated levels of AKT, p-IKKα/β, and p-P65 were increased in MACC1-overexpressed cervical cancer cells, whereas they were significantly decreased in MACC1-knockdown cervical cancer cells compared with control cells (Fig. 5A).
FIGURE 5.
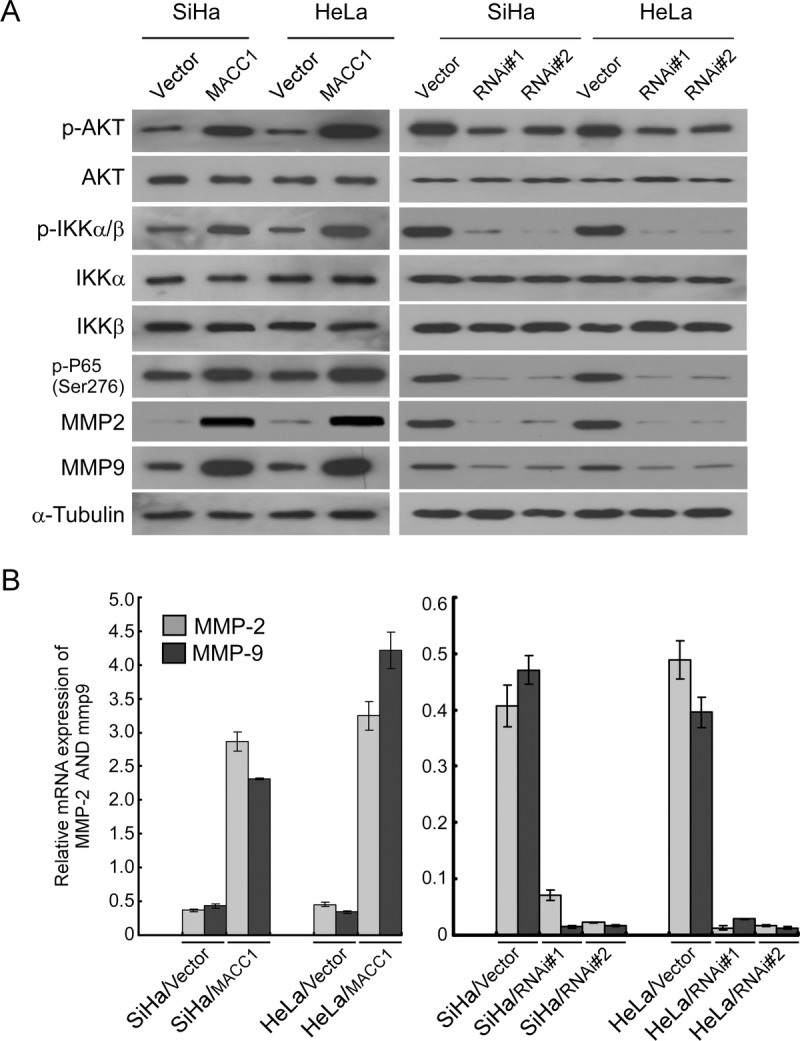
MACC1 regulates MMP-2 and MMP-9 and activates the AKT and NF-κB signaling pathways. A, Western blotting analysis of the expression of p-AKT, total AKT, p-IKKa/b, total IKKa/b, p-P65, MMP-2, and MMP-9 proteins in indicated vector-infected and MACC1 infected cervical cancer cell lines, and vector-infected and MACC1 siRNA-infected cervical cancer cell lines. B, Relative mRNA expression levels of MMP-2 and MMP-9 in indicated cervical cancer cell lines were determined by real-time RT-PCR. Error bars represent mean ± SD from 3 independent experiments.
DISCUSSION
In this study, we demonstrate that MACC1 is up-regulated and is significantly correlated with aggressive features in cervical cancer, including FIGO stage, pelvic lymph node metastasis, recurrence, and poor prognosis. Our results are consistent with previous reports on some other cancer types. Similar to our results, MACC1 overexpression has been reported in association with the clinical progression and poor outcome of patients in several human cancers, including nasopharyngeal carcinoma, lung cancer, breast cancer, epithelial ovarian cancer, hepatocellular cancer, pancreatic cancer, colorectal cancer, and gastric cancer.6–8,11,12,16–21 Recently, a study in 104 paraffin-embedded cervical cancer samples by IHC revealed that high expression of MACC1 was correlated with advanced FIGO stage, lymph nodes metastasis, and poor survival of this disease.12 Our results were consistent with this study. Taken together, these findings reflect the ability of MACC1 to play a role in cervical cancer progression. Overexpression of MACC1 protein may be a common feature in human cancer and might be served as an independent prognostic marker to identify patients with poor clinical outcomes. However, whether MACC1 can be used as a general biomarker or as a potential molecular therapeutic target for tumor progression needs further investigation.
MACC1 has been implicated in the promotion of cell migration, invasion, and metastasis in several human cancers, including colorectal cancer, gastric cancer, hepatocellular carcinoma cells, and pancreatic cancer.4,11,22,23 Mechanistically, it has been reported that MACC1 directly binds to the promoter of receptor tyrosine kinase MET and induces MET transcription, which in turn leads to the activation of the HGF-MET signaling pathway.4 MET is activated after binding of the HGF ligand and transmits intracellular signaling cascades via the RAS-MAPK and PI3K-Akt pathways.24–26 Consistent with studies in other cancer types, the present study shows that aberrant expression of MACC1 is associated with changes in aggressive properties of cervical cancer cells, including migration and invasion. Importantly, the present study reveals that overexpression of MACC1 enhances, but knockdown of MACC1 represses the angiogenesis capacity of cervical cancer cells. In addition, MACC1 promotes AKT and nuclear factor (NF) κB signaling activities in cervical cancer cells. Therefore, the enhanced migration, invasion, and angiogenesis induced by MACC1 probably resulted from enhanced AKT and NF-κB activity.
Angiogenesis is central to cervical cancer growth and metastasis.27 It has been documented that angiogenesis plays essential roles in both primary tumor growth and spreading of tumor cells to distant organs.28 High levels of vascular endothelial growth factor in tumor microenvironment promote angiogenesis.29 In addition, MMP-2 and MMP-9, 2 major MMPs secreted from HeLa cells,30 are involved in angiogenesis, tumor growth, and metastasis through degradation of extracellular matrix and release and/or activation of growth factors, which finally results in cancer dissemination.31 Increased MMP activation has been found in cervical cancer, and multiple MMPs, including MMP-2 and MMP-9, are reported to be independent prognostic factors in cervical cancer and correlate with progression.32–34 Nuclear factor κB signal transduction cascade, required for angiogenesis, was frequently involved in tumor progression and dissemination.35 Compelling evidence has indicated that activated NF-κB is associated with the initiation and progression of cervical cancer.36–38 Nuclear factor κB is constitutively activated in high-grade and more invasive histologic grades of cervical cancer.39 Multiple downstream targets of NF-κB, such as vascular endothelial growth factor, MMPs, and cyclooxygenase-2 were shown to be significantly associated with worse prognosis or aggressive characteristics of cervical cancer.40 These studies demonstrate that NF-κB activation plays essential roles in cervical cancer progression. Nuclear factor κB signaling involves in angiogenesis and metastasis in human cancers probably through regulating vascular endothelial growth factors and MMPs.41,42 The MMP promoter is subject to a tight regulatory network involving NF-κB, which can in turn be activated by the AKT pathway.43,44 In the present study, we found that both MMP-2 and MMP-9 expressions were up-regulated by MACC1 in cervical cancer cells. We also showed that the AKT and NF-κB pathways were activated because p-AKT, p-IKKα/β, and p-P65 were up-regulated by MACC1. Therefore, the regulation of MMP-2 and MMP-9 by MACC1 probably resulted from enhanced AKT and NF-κB activity. This thus explains the enhanced migration, invasion, and angiogenesis induced by MACC1. However, whether MACC1 can directly regulate MMP-2 and MMP-9 expression by associated with their promoters needs further investigation. In conclusion, our findings suggest that up-regulation of MACC1 might be a valuable prognostic marker of cervical cancer progression. Altered expression of MACC1 could be important for progression of cervical cancer. Modulation of the tumor migration, invasion, and angiogenesis through inhibiting AKT or NF-κB activation mediated by MACC1 overexpression might be used as a potential target for cervical cancer therapy. However, the underlying mechanism through which MACC1 might activate AKT or NF-κB pathways needs further investigation.
Footnotes
Xiang Zhou and Chang-Juan Xu are equal contributors.
This study was supported by The National Natural Science Foundation of China (81172055); Guangdong Provincial Natural Science Foundation of China (S2012010009643); Zhu Jiang Science & Technology New Star Foundation in Guangzhou City (2012J2200052).
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.ijgc.net).
REFERENCES
- 1. Forouzanfar MH, Foreman KJ, Delossantos AM, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet. 2011; 378: 1461– 1484. [DOI] [PubMed] [Google Scholar]
- 2. Gien LT, Covens A. Lymph node assessment in cervical cancer: prognostic and therapeutic implications. J Surg Oncol. 2009; 99: 242– 247. [DOI] [PubMed] [Google Scholar]
- 3. Ota T, Suzuki Y, Nishikawa T, et al. Complete sequencing and characterization of 21,243 full-length human cDNAs. Nat Genet. 2004; 36: 40– 45. [DOI] [PubMed] [Google Scholar]
- 4. Stein U, Walther W, Arlt F, et al. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med. 2009; 15: 59– 67. [DOI] [PubMed] [Google Scholar]
- 5. Kokoszynska K, Krynski J, Rychlewski L, et al. Unexpected domain composition of MACC1 links MET signaling and apoptosis. Acta Biochim Pol. 2009; 56: 317– 323. [PubMed] [Google Scholar]
- 6. Xie C, Wu J, Yun J, et al. MACC1 as a prognostic biomarker for early-stage and AFP-normal hepatocellular carcinoma. PLoS One. 2013; 8: e64235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meng F, Li H, Shi H, et al. MACC1 down-regulation inhibits proliferation and tumourigenicity of nasopharyngeal carcinoma cells through Akt/beta-catenin signaling pathway. PLoS One. 2013; 8: e60821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chundong G, Uramoto H, Onitsuka T, et al. Molecular diagnosis of MACC1 status in lung adenocarcinoma by immunohistochemical analysis. Anticancer Res. 2011; 31: 1141– 1145. [PubMed] [Google Scholar]
- 9. Guo T, Yang J, Yao J, et al. Expression of MACC1 and c-Met in human gastric cancer and its clinical significance. Cancer Cell Int. 2013; 13: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Z, Li Z, Wu C, et al. MACC1 overexpression predicts a poor prognosis for non–small cell lung cancer. Med Oncol. 2014; 31: 790. [DOI] [PubMed] [Google Scholar]
- 11. Wang G, Kang MX, Lu WJ, et al. MACC1: a potential molecule associated with pancreatic cancer metastasis and chemoresistance. Oncol Lett. 2012; 4: 783– 791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guo L, Lu W, Zhang X, et al. Metastasis-associated colon cancer-1 is a novel prognostic marker for cervical cancer. Int J Clin Exp Pathol. 2014; 7: 4150– 4155. [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang R, Shi H, Chen Z, et al. Effects of metastasis-associated in colon cancer 1 inhibition by small hairpin RNA on ovarian carcinoma OVCAR-3 cells. J Exp Clin Cancer Res. 2011; 30: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang SH, Wang CJ, Shi L, et al. High expression of FLOT1 is associated with progression and poor prognosis in hepatocellular carcinoma. PLoS One. 2013; 8: e64709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song LB, Li J, Liao WT, et al. The polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells. J Clin Invest. 2009; 119: 3626– 3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang Y, Zhang H, Cai J, et al. Overexpression of MACC1 and its significance in human breast cancer progression. Cell Biosci. 2013; 3: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kawamura M, Saigusa S, Toiyama Y, et al. Correlation of MACC1 and MET expression in rectal cancer after neoadjuvant chemoradiotherapy. Anticancer Res. 2012; 32: 1527– 1531. [PubMed] [Google Scholar]
- 18. Qiu J, Huang P, Liu Q, et al. Identification of MACC1 as a novel prognostic marker in hepatocellular carcinoma. J Transl Med. 2011; 9: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang L, Wu Y, Lin L, et al. Metastasis-associated in colon cancer-1 upregulation predicts a poor prognosis of gastric cancer, and promotes tumor cell proliferation and invasion. Int J Cancer. 2013; 133: 1419– 1430. [DOI] [PubMed] [Google Scholar]
- 20. Stein U, Burock S, Herrmann P, et al. Circulating MACC1 transcripts in colorectal cancer patient plasma predict metastasis and prognosis. PLoS One. 2012; 7: e49249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ren B, Zakharov V, Yang Q, et al. MACC1 is related to colorectal cancer initiation and early-stage invasive growth. Am J Clin Pathol. 2013; 140: 701– 707. [DOI] [PubMed] [Google Scholar]
- 22. Lin L, Huang H, Liao W, et al. MACC1 supports human gastric cancer growth under metabolic stress by enhancing the Warburg effect. Oncogene. 2015; 34: 2700– 2710. [DOI] [PubMed] [Google Scholar]
- 23. Gao J, Ding F, Liu Q, et al. Knockdown of MACC1 expression suppressed hepatocellular carcinoma cell migration and invasion and inhibited expression of MMP2 and MMP9. Mol Cell Biochem. 2013; 376: 21– 32. [DOI] [PubMed] [Google Scholar]
- 24. Birchmeier C, Birchmeier W, Gherardi E, et al. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003; 4: 915– 925. [DOI] [PubMed] [Google Scholar]
- 25. Sattler M, Salgia R. c-Met and hepatocyte growth factor: potential as novel targets in cancer therapy. Curr Oncol Rep. 2007; 9: 102– 108. [DOI] [PubMed] [Google Scholar]
- 26. Mazzone M, Comoglio PM. The Met pathway: master switch and drug target in cancer progression. FASEB J. 2006; 20: 1611– 1621. [DOI] [PubMed] [Google Scholar]
- 27. Phoophitphong T, Hanprasertpong J, Dechsukhum C, et al. Correlation of angiogenesis and recurrence-free survival of early stage cervical cancer patients undergoing radical hysterectomy with pelvic lymph node dissection. J Obstet Gynaecol Res. 2007; 33: 840– 848. [DOI] [PubMed] [Google Scholar]
- 28. Albini A, Tosetti F, Li VW, et al. Cancer prevention by targeting angiogenesis. Nat Rev Clin Oncol. 2012; 9: 498– 509. [DOI] [PubMed] [Google Scholar]
- 29. Chien MH, Ku CC, Johansson G, et al. Vascular endothelial growth factor-C (VEGF-C) promotes angiogenesis by induction of COX-2 in leukemic cells via the VEGF-R3/JNK/AP-1 pathway. Carcinogenesis. 2009; 30: 2005– 2013. [DOI] [PubMed] [Google Scholar]
- 30. Roomi MW, Monterrey JC, Kalinovsky T, et al. In vitro modulation of MMP-2 and MMP-9 in human cervical and ovarian cancer cell lines by cytokines, inducers and inhibitors. Oncol Rep. 2010; 23: 605– 614. [DOI] [PubMed] [Google Scholar]
- 31. John A, Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol Oncol Res. 2001; 7: 14– 23. [DOI] [PubMed] [Google Scholar]
- 32. Branca M, Ciotti M, Giorgi C, et al. Matrix metalloproteinase-2 (MMP-2) and its tissue inhibitor (TIMP-2) are prognostic factors in cervical cancer, related to invasive disease but not to high-risk human papillomavirus (HPV) or virus persistence after treatment of CIN. Anticancer Res. 2006; 26: 1543– 1556. [PubMed] [Google Scholar]
- 33. Li Y, Wu T, Zhang B, et al. Matrix metalloproteinase-9 is a prognostic marker for patients with cervical cancer. Med Oncol. 2012; 29: 3394– 3399. [DOI] [PubMed] [Google Scholar]
- 34. Sheu BC, Lien HC, Ho HN, et al. Increased expression and activation of gelatinolytic matrix metalloproteinases is associated with the progression and recurrence of human cervical cancer. Cancer Res. 2003; 63: 6537– 6542. [PubMed] [Google Scholar]
- 35. Wu Y, Zhou BP. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br J Cancer. 2010; 102: 639– 644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bodelon C, Madeleine MM, Johnson LG, et al. Genetic variation in the TLR and NF-kappaB pathways and cervical and vulvar cancer risk: a population-based case-control study. Int J Cancer. 2014; 134: 437– 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shehata MF. Rel/Nuclear factor-kappa B apoptosis pathways in human cervical cancer cells. Cancer Cell Int. 2005; 5: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li J, Jia H, Xie L, et al. Association of constitutive nuclear factor-kappaB activation with aggressive aspects and poor prognosis in cervical cancer. Int J Gynecol Cancer. 2009; 19: 1421– 1426. [DOI] [PubMed] [Google Scholar]
- 39. Nair A, Venkatraman M, Maliekal TT, et al. NF-kappaB is constitutively activated in high-grade squamous intraepithelial lesions and squamous cell carcinomas of the human uterine cervix. Oncogene. 2003; 22: 50– 58. [DOI] [PubMed] [Google Scholar]
- 40. Rauvala M, Aglund K, Puistola U, et al. Matrix metalloproteinases-2 and -9 in cervical cancer: different roles in tumor progression. Int J Gynecol Cancer. 2006; 16: 1297– 1302. [DOI] [PubMed] [Google Scholar]
- 41. Basseres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006; 25: 6817– 6830. [DOI] [PubMed] [Google Scholar]
- 42. Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest. 2001; 107: 241– 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cheng JC, Chou CH, Kuo ML, et al. Radiation-enhanced hepatocellular carcinoma cell invasion with MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction pathway. Oncogene. 2006; 25: 7009– 7018. [DOI] [PubMed] [Google Scholar]
- 44. Lee SJ, Bae SS, Kim KH, et al. High glucose enhances MMP-2 production in adventitial fibroblasts via Akt1-dependent NF-kappaB pathway. FEBS Lett. 2007; 581: 4189– 4194. [DOI] [PubMed] [Google Scholar]


