Supplemental digital content is available in the text.
Key Words: Lymphatic drainage ovaries, Sentinel node detection
Abstract
Objective
In ovarian cancer, detection of sentinel nodes is an upcoming procedure. Perioperative determination of the patient’s sentinel node(s) might prevent a radical lymphadenectomy and associated morbidity. It is essential to understand the lymphatic drainage pathways of the ovaries, which are surprisingly up till now poorly investigated, to predict the anatomical regions where sentinel nodes can be found. We aimed to describe the lymphatic drainage pathways of the human ovaries including their compartmental fascia borders.
Methods
A series of 3 human female fetuses and tissues samples from 1 human cadaveric specimen were studied. Immunohistochemical analysis was performed on paraffin-embedded transverse sections (8 or 10 μm) using antibodies against Lyve-1, S100, and α-smooth muscle actin to identify the lymphatic endothelium, Schwann, and smooth muscle cells, respectively. Three-dimensional reconstructions were created.
Results
Two major and 1 minor lymphatic drainage pathways from the ovaries were detected. One pathway drained via the proper ligament of the ovaries (ovarian ligament) toward the lymph nodes in the obturator fossa and the internal iliac artery. Another pathway drained the ovaries via the suspensory ligament (infundibulopelvic ligament) toward the para-aortic and paracaval lymph nodes. A third minor pathway drained the ovaries via the round ligament to the inguinal lymph nodes. Lymph vessels draining the fallopian tube all followed the lymphatic drainage pathways of the ovaries.
Conclusions
The lymphatic drainage pathways of the ovaries invariably run via the suspensory ligament (infundibulopelvic ligament) and the proper ligament of the ovaries (ovarian ligament), as well as through the round ligament of the uterus. Because ovarian cancer might spread lymphogenously via these routes, the sentinel node can be detected in the para-aortic and paracaval regions, obturator fossa and surrounding internal iliac arteries, and inguinal regions. These findings support the strategy of injecting tracers in both ovarian ligaments to identify sentinel nodes.
Epithelial ovarian cancer has the highest mortality rate of all gynecological malignancies. This is partially because 75% of patients are diagnosed with an advanced stage of epithelial ovarian cancer. In patients with clinical early-stage epithelial ovarian cancer, a surgical staging procedure is recommended, which includes total abdominal hysterectomy with bilateral salpingo-oophorectomy, omentectomy, and numerous peritoneal biopsies. Comprehensive lymph node sampling forms an essential part in determining the clinical stage of epithelial ovarian cancer. The International Federation of Gynecology and Obstetrics recommends complete pelvic and para-aortic lymphadenectomy; however, the extent of lymph node dissection differs greatly from center to center.1–3 The more lymph nodes removed, the higher the chance of detecting metastases.4,5 Complete pelvic and para-aortic lymphadenectomy has shown to detect up to 250 lymph nodes.6 Systematic lymphadenectomy is considered the criterion standard as positive lymph nodes can be missed by lymph node sampling. However, radical lymphadenectomy is associated with serious morbidity.5,7,8 The sentinel node procedure has proven to be effective in vulvar, cervical, and endometrial cancer.9 Recently, a feasibility study has shown that detection of sentinel nodes in ovarian cancer is safe and promising by injecting tracers in the ovarian ligaments.7 To optimize the sentinel node procedure in ovarian cancer, excellent understanding of the routes for lymphatic spread is essential. Up to now, studies reporting the lymphatic drainage pathways of the ovaries on microscopic level are lacking.
Epithelial ovarian cancer can metastasize intraperitoneally (in the peritoneal cavity), lymphogenously, and hematogenously.10,11 In terms of lymphogenous spread, lymphatic metastases of epithelial ovarian cancer occur mainly in the para-aortic and paracaval lymph nodes.12 It is believed that tumor cells follow the lymph vessels accompanying the ovarian artery (OA) and ovarian vein in the suspensory ligament of the ovary (SLO; also named the infundibulopelvic ligament) up to the high para-aortic region and paracaval region. However, pelvic lymph node metastases are also frequent.4–6,13–21 These tumor cells probably follow a different route, possibly along the parauterine vessels in the broad ligament of the uterus (ligamentum latum) toward the uterine artery and vein and eventually to the iliac vessels. Some case reports have also described isolated inguinal node metastases of epithelial ovarian cancer.5,22–24 The exact mechanism of this route of metastasis is still unclear, but cells may follow the round ligament of the uterus (also named the ligamentum teres uteri) or may follow the external iliac vessels.
Immunohistochemistry is an excellent tool to study lymphatic drainage pathways on a microscopic level. The human lymphatic system develops shortly after the blood vasculature. By the end of the fifth embryonic week, lymph sacs arise from developing veins. These lymph sacs and plexuses lose their venous connection and progress to the peripheral regions as they fuse with another. By the end of the embryonic period (11th week), the formation of new plexuses has stopped, and major definitive lymphatic pathways can be identified.25
The objective of this study was to analyze immunohistochemically and to reconstruct 3-dimensionally the lymphatic drainage pathways of the ovaries in human fetal pelves. Implications of these findings for the surgical management of epithelial ovarian cancer, including detection of sentinel nodes, will also be discussed.
MATERIALS AND METHODS
Material
Three human female fetuses with an embryonic age of 14, 15, and 20 weeks, respectively, from the Department of Anatomy and Embryology, Leiden University Medical Center in the Netherlands and the University of Warsaw in Poland were studied.25 Fetal tissues allow accurate exploration of the 3-dimensional (3D) anatomy of the human pelvic lymphatic system. The fetus of 20 weeks had a trisomy 14 with terminal 5p depletion. The other fetuses were phenotypically normal and did not show congenital macroscopic malformations. All fetuses were obtained with informed consent after miscarriage or legal abortion. The patients were informed that the fetuses were used for medical research. In the fetuses of 14 and 15 weeks, the bony pelvis was manually removed. The fetus of 20 weeks was decalcified in a 10% ethylenediaminetetraacetic acid solution for 72 hours. An additional human cadaveric specimen was used to study the round ligament of the uterus, the SLO (infundibulopelvic ligament), and the proper ligament of the ovary (ovarian ligament). This cadaver was fixed in Fix 4 Life solution facilitating subsequent immunohistochemical analysis. This concerned a person who during life decided by means of a written consent to donate her body, after death, to medical science.
Immunohistochemistry
All tissues were embedded in paraffin blocks. Serial transverse sections of 8 and 10 μm were produced and stained with hematoxylin and eosin or with azan. In addition, series of selected sections were stained with antibodies against Lyve-1 (ReliaTech), S100 (DAKO), and α-smooth muscle actin (SMA; Sigma-Aldrich). We used Lyve-1 to visualize lymphatic endothelial cells, S100 to stain Schwann cells to reveal the peripheral neural network, and SMA to detect vascular and visceral smooth muscle fibers. Lyve-1 and SMA were incubated on consecutive sections to distinguish between blood and lymph vessels. The sections were rinsed twice with phosphate-buffered saline (PBS) and once with PBS/0.05% Tween-20 between all steps, unless otherwise indicated. After deparaffinization and rehydration in graded ethanol dilutions, antigen retrieval was performed by heating the sections (12 minutes to 98°C) in citric acid buffer (0.01 Mol/L, pH 6.0). Then, sections were incubated for 20 minutes in PBS with 0.3% H2O2 to inhibit endogenous peroxidase. Sections were incubated overnight with primary antibodies to Lyve-1 (1/200 μL), S100 (1/5.000 μL), or SMA (1/5.000 μL) diluted in a 1% PBS-bovine serum albumin/Tween-20 solution. The next day, sections were incubated for 45 minutes with a biotin-labeled secondary antibody (goat-anti-rabbit, BA-1000; Vector Labs) and additionally incubated for 45 minutes with the Vectastain ABC staining kit (PK-6100; Vector Labs). Sections stained with SMA were incubated only with a secondary antibody (rabbit-anti-mouse, P-0260; DAKO). Before visualization, all sections were rinsed twice with PBS and once with tris/maleate (pH 7.6). Visualization was performed with 3-3′diaminobenzidine tetrahydrochloride as a chromogen, and hematoxylin was used as a counterstain. All sections were mounted with Entellan (1.07961.0100; Merck).
Image Processing
Three-dimensional reconstructions were generated showing the ovarian lymphatic drainage pathways and autonomic nerves of the fetus aged 14 weeks and the ovarian lymphatic pathways of the fetus aged 15 weeks. With an Olympus AX70-microscope and Olympus D12 camera (Olympus, Tokyo, Japan), micrographs were made. Every 20th section was used for the reconstruction, comprising the entire fetal pelvis up to the upper limit of the ovaries. This created a cross-sectional interval of 160 μm. The Amira software package version 5.3.3 (Template Graphics Software; Visage Imaging, San Diego, CA) was used for the 2-dimensional labeling of anatomical structures. The DeVIDE software was used to assemble the 3D volume and to reconstruct the 3D surface meshes.26 DAZ studio version 4.6 (DAZ 3D, Salt Lake City, UT) was used to develop an interactive PDF file in which the 3D models could be explored.
RESULTS
Lymph vessels were detected by Lyve-1 positivity in the lymphatic endothelium and by the absence of smooth muscle fibers and red blood cells. In all fetuses, the fallopian tubes and ovaries were situated longitudinally in the intra-abdominal cavity rather than transversally as a consequence of the still developing female genital tract. In addition, the position of the fallopian tubes and ovaries remained relatively high in the abdomen. All results can be visualized 3-dimensionally online at https://graphics.tudelft.nl/lymphaticdrainageovaries.
The ovaries drained via 3 routes. The first identified route ran cranially from the ovaries. Lymph vessels draining the ovaries at its cranial part were seen in the SLO (infundibulopelvic ligament) following the course of the OA (Fig. 1; Supplemental Digital Content 1, http://links.lww.com/IGC/A298). This pathway will be referred to as the abdominal pathway. Lymph vessels accompanied the OA as it entered the retroperitoneal space and traveled along the common iliac artery and aorta on the left side, and the common iliac artery and caval vein on the right side (Fig. 2). Over a certain length of this route, the SLO (infundibulopelvic ligament) was attached to the retroperitoneum, but no lymph vessels from the abdominal pathway were seen to cross the retroperitoneum before the OA. As a consequence, no connections were seen between the lymph vessels draining the ovaries and the lymph plexuses surrounding the external iliac artery at this level.
FIGURE 1.
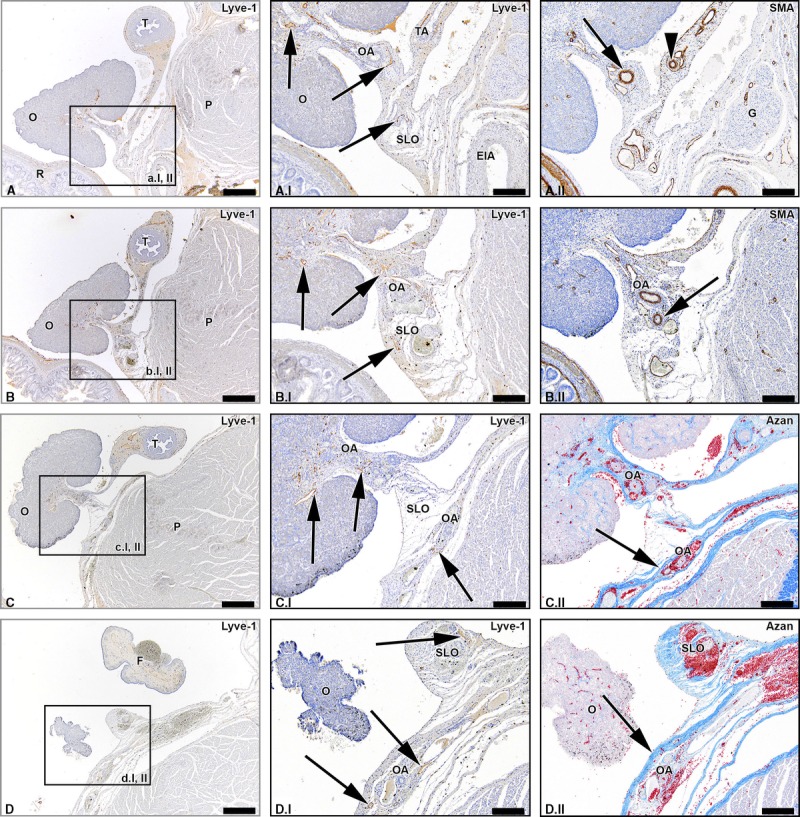
Lymph vessels draining the ovary via the SLO (infundibulopelvic ligament). Transverse sections of the fetus aged 15 weeks showing consecutive superior levels (A-D). The black arrows in the Lyve-1–stained sections point out lymph vessels draining the ovary (O) via the SLO running along the OA. The arrowhead in window A.II shows the tubal branch of the OA (TA). The superior part of the OA courses in the SLO (arrow window B.II), while the inferior part of the OA runs in the mesovarium. The superior part of the OA continues to run in the retroperitoneal compartment (arrows in azan-stained windows). Some lymph vessels remain in the distal part of the SLO (upper arrow window D.I), whereas the major lymph vessels follow the course of the OA (lower 2 arrows window D.I). The arrow in window D.II points out the retroperitoneum. T indicates uterine tube; G, genital branch of genitofemoral nerve; EIA, external iliac artery; P, psoas major muscle; F, fimbriae. Scale bar overview, 500 μm; detail, 200 μm.
FIGURE 2.
Suspensory ligament of the ovary (infundibulopelvic ligament) and retroperitoneum. Transverse azan-stained sections of the fetus aged 15 weeks showing collagen (blue) and cell nuclei (purple) at consecutive superior levels (A-C) The SLO does not form a direct continuity with the retroperitoneum as the peritoneum (arrowheads) forms a solid border. Lymph vessels draining via the SLO do not cross this border, but run along the OA and shift to the retroperitoneal compartment when the OA does (window C.I). P indicates psoas major muscle; Ut, uterus; UA, umbilical artery; IIA, internal iliac artery; R, rectum; O, ovary; U, ureter; EIA, external iliac artery; L, lymph nodes along the EIA; GFN, genitofemoral nerve; EIV, external iliac vein; ON, obturator nerve; IAV, internal iliac vein; T, uterine tube; G, genital branch of GFN. Scale bar overview, 1 mm; scale bar detail, 500 μm.
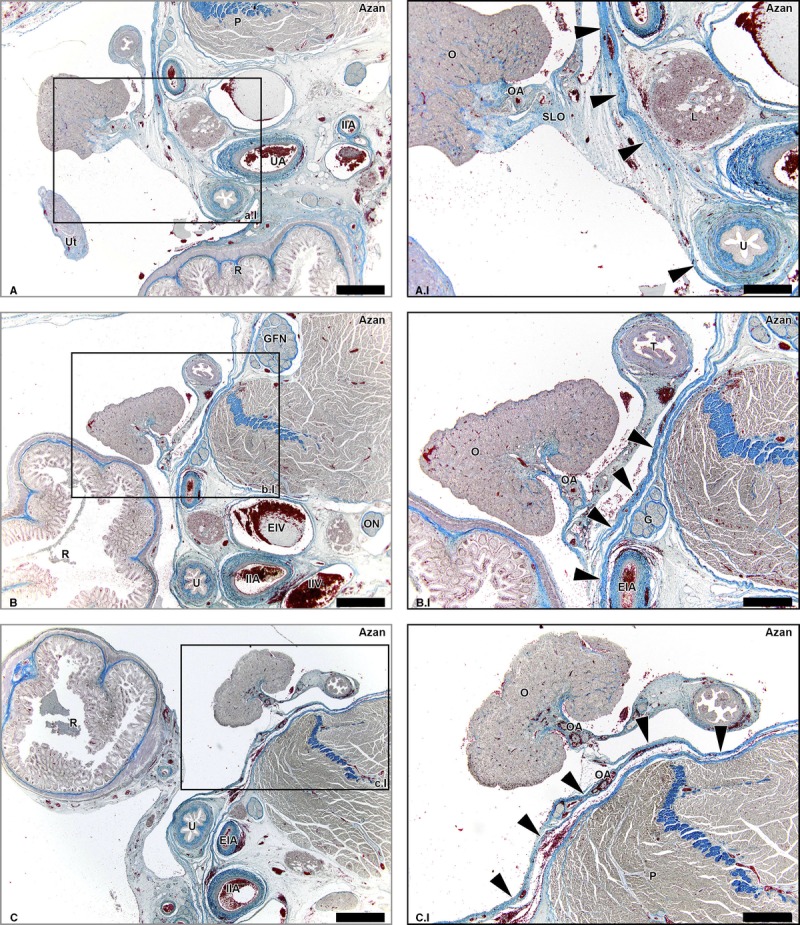
A second lymphatic drainage pathway ran from the caudal part of the ovaries downwards to the proper ligament of the ovary (ovarian ligament). These lymph vessels followed the OA that anastomosed with a branch of the uterine artery, the so-called ovarian-uterine branch (Fig. 3; Supplemental Digital Content 2, http://links.lww.com/IGC/A299). We will refer to this pathway as the pelvic pathway. Lymph vessels accompanying this branch of the uterine artery were detected along the whole corpus uterus (Supplemental Digital Content 2, http://links.lww.com/IGC/A299). At the level of the cervix of the uterus, where the branch of the uterine OA entered the uterine artery, the lymph vessels continued to follow the uterine artery. In the lateral parametrium, the lymph vessels from this pelvic pathway drained into the supraureteral pathway that ran along the course of the uterine artery, crossing the ureter superiorly. The supraureteral pathway drained into the lymph plexuses surrounding the internal iliac artery and obturator fossa.
FIGURE 3.
Lymph vessels draining the ovary via the ovarian ligament and the broad ligament of the uterus. Transverse sections of the fetus aged 14 weeks showing the lymphatic drainage pathway of the ovary (O) via the proper ligament of the ovary (PLO) and broad ligament of the ovary (BLO) at consecutive inferior levels (A-D). The arrows in the Lyve-1–stained sections show the lymph vessels running along the OA, merging eventually with the supraureteral pathway that follows the course of the uterine artery (UA). Ut indicates uterus; T, uterine tube; EIA, external iliac artery; L, lymph nodes along the EIA; TA, tubal branches of OA; OA-UA, anastomosis of OA and UA; R, rectum; U, ureter; D, Douglas’ pouch. Scale bar overview, 500 μm; scale bar detail, 200 μm.
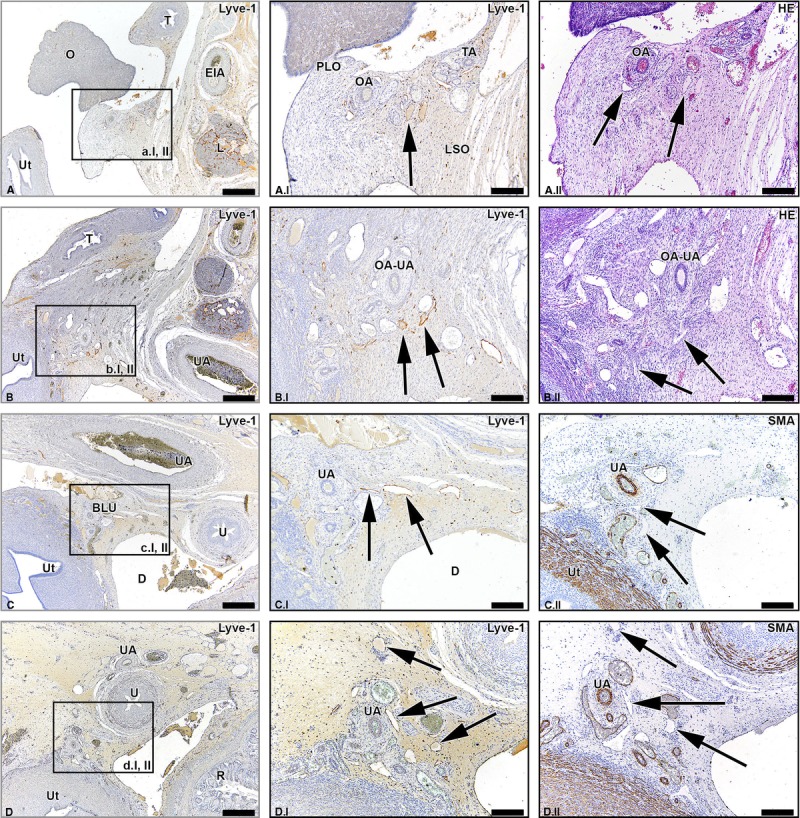
Besides lymphatic drainage via the abdominal and pelvic pathways, a third minor lymphatic drainage pathway was detected to run in the round ligament of the uterus (Fig. 4). This pathway is termed the inguinal pathway. The number of lymph vessels seen in the round ligament of the uterus was small in comparison to the number of lymph vessels in the proper ligament of the ovary (ovarian ligament) and SLO (infundibulopelvic ligament). This pathway was not bilaterally present in all fetal specimens and appeared more prominently in some specimens than in others. In the fetus of 20 weeks, this pathway was less prominent than in the fetuses of 14 and 15 weeks. In the fetus of 15 weeks, the lymph vessels were seen on both sides, although the vessels abruptly disappeared on the left side when traveling more caudally. The vessels that did not disappear became thinner toward the inguinal region and eventually drained into inguinal lymph nodes.
FIGURE 4.
Lymph vessels draining the ovary via the round ligament of the uterus. Transverse sections of the fetus aged 14 weeks showing lymph vessels (arrows) that drain the ovary (O) via the round ligament of the uterus (RLU, ligament teres uteri) at consecutive inferior levels (A-C). Note the presence of smooth muscle fibers in the RLU (brown stain in the SMA-stained sections). At an inferior level, the RLU passes under the umbilical artery (UA) as can be seen in window B. The RLU runs to the inguinal region and its lymph vessels eventually fuse with inguinal lymph vessels. Ut indicates uterus; O, ovary; EIA, external iliac artery; L, lymph nodes along the EIA. Scale bar overview, 500 μm; scale bar detail, 200 μm.
A considerable number of lymph vessels were seen in the mesovarium along the entire length of the ovary, as well as surrounding the fallopian tube in the mesosalpinx (Fig. 5). The lymph vessels in the mesosalpinx drained into all 3 lymphatic drainage pathways of the ovaries. Over the entire length of the fallopian tube up until the fimbriae, prominent lymphatic drainage was visible. There was no connection detected between the left and the right ovarian pathways.
FIGURE 5.
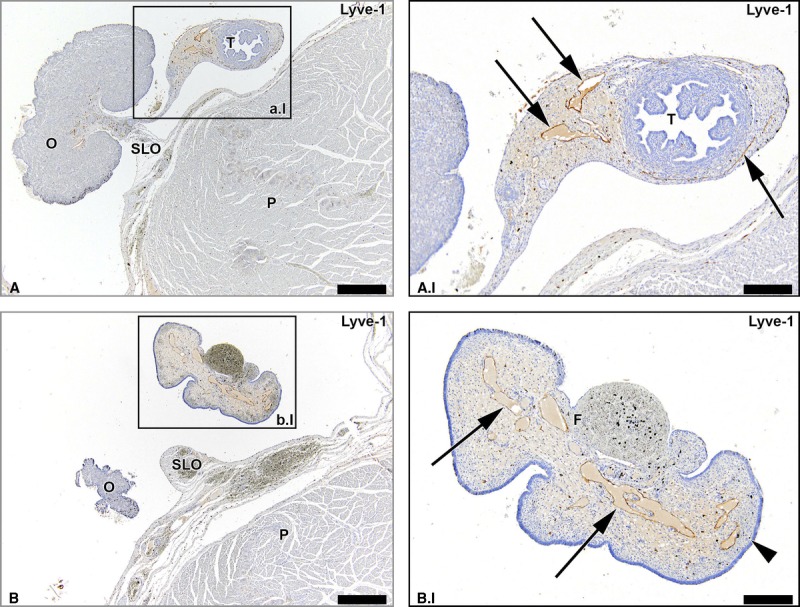
Lymph vessels draining the uterine tubes. Transverse Lyve-1–stained sections of the fetus aged 15 weeks showing that lymph vessels draining the uterine tubes (arrows) run in the mesosalpinx. Note the substantial amount of lymph vessels along the entire length of the uterine tubes until its fimbriae (F). O indicates ovary; T, uterine tube; P, psoas major muscle. Scale bar overview, 500 μm; scale bar detail, 200 μm.
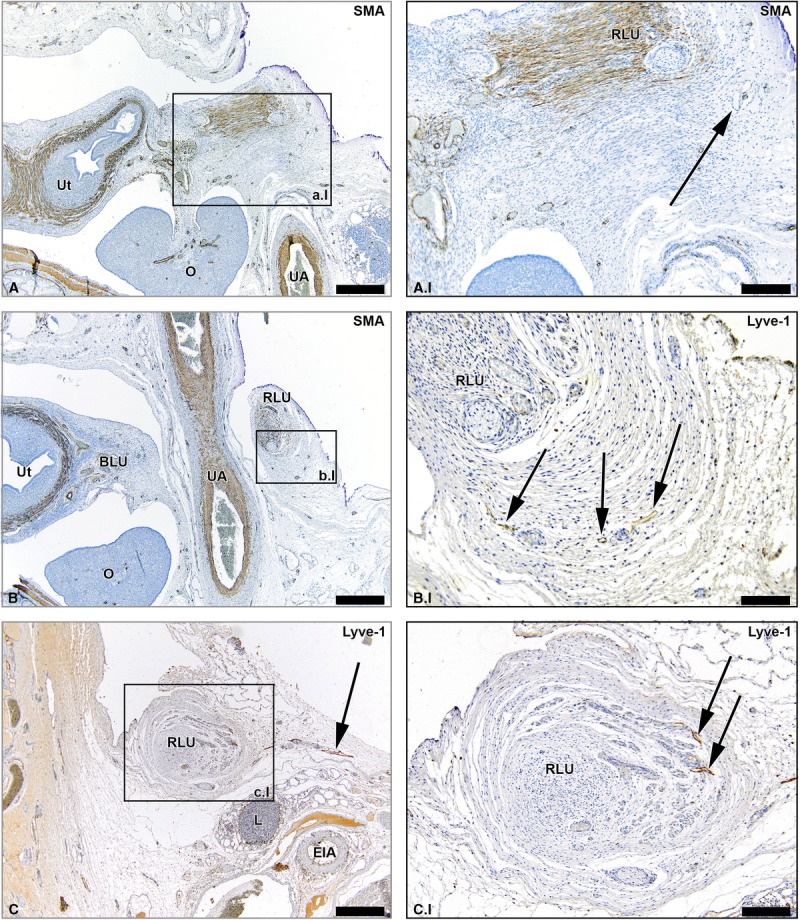
We confirmed the presence of lymph vessels in the SLO (infundibulopelvic ligament), proper ligament of the ovary (ovarian ligament), and round ligament of the uterus of the human adult cadaveric tissues samples (Fig. 6).
FIGURE 6.
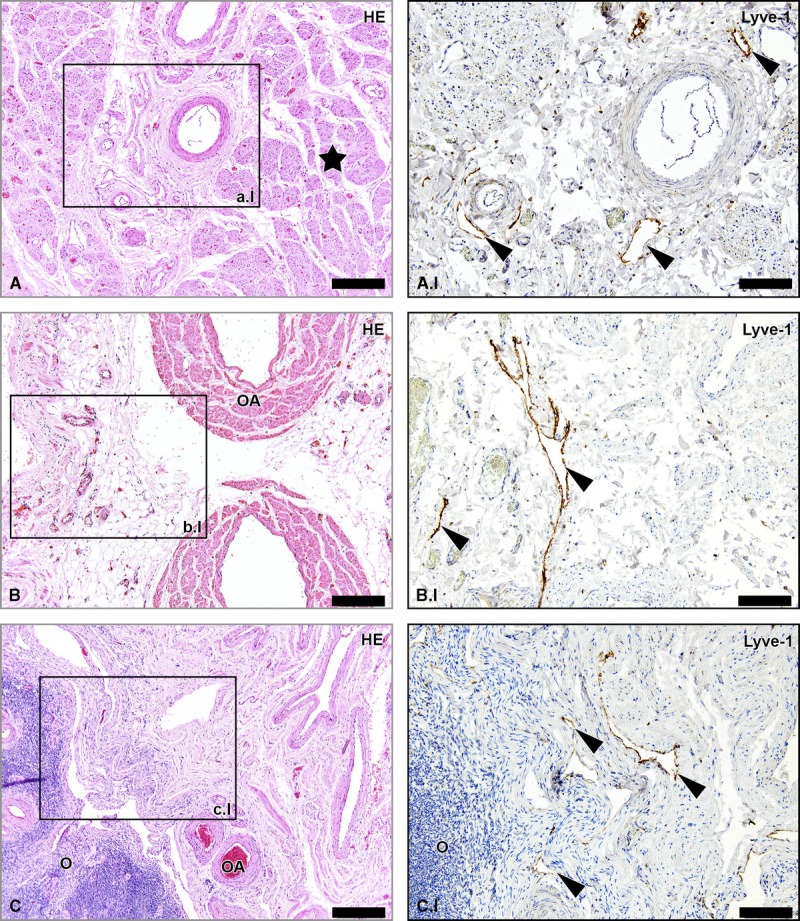
Lymph vessels in the human adult RLU, SLO, and PLO. Hematoxylin-eosin–stained sections of tissue samples from human adult round ligament of the uterus (RLU, window A), SLO (window B), and proper ligament of the ovary (PLO, window C). Detail windows A.I, B.I, and C.I are Lyve-1–stained adjacent sections revealing the presence of lymph vessels in the 3 ligaments (arrowheads). The star shows the presence of smooth muscle fibers in the RLU. O indicates ovary. Scale bar overview, 200 μm; scale bar detail, 100 μm.
DISCUSSION
So far, few in vivo studies focussed on revealing the lymphatic drainage pathways of the ovaries, but histological studies are lacking. To our knowledge, we are the first to have studied the lymphatic drainage pathways of the ovaries on microscopic level. The sentinel node procedure is an upcoming technique to preoperatively determine the nodal status in patients experiencing epithelial ovarian cancer. Excellent knowledge of the lymphatic drainage pathways of the ovaries is essential to predict the routes for lymphatic spread and, consequently, the sites where sentinel nodes can be detected. Unlike in other sentinel node procedures, such as in breast cancer and malignant melanoma, injection of tracers cannot be done in the skin near the tumor. In addition, injection in the tumor itself should be avoided because this can cause spillage of tumor cells. The ovarian ligaments are therefore a good alternative site for injection of the tracers.7
We identified 2 major and 1 minor lymphatic drainage pathways of the ovaries; these are as follows: (1) the abdominal pathway, running via the SLO (infundibulopelvic ligament) to the para-aortic (left side)/paracaval (right side) regions; (2) the pelvic pathway, draining via the lateral parametrium and supraureteral pathway25 to the internal iliac artery and obturator fossa; and (3) the inguinal pathway, draining via the round ligament of the uterus to the inguinal regions. Sentinel nodes can be found in any of these regions. Besides, the findings reflect the most common locations of lymph node metastases in patients with ovarian cancer; 50% of patients have metastasis solely in the high para-aortic/paracaval area, whereas 20% have metastasis solely in the pelvic area and 30% in both the para-aortic/paracaval and pelvic area.2–6,10–24,27 Some rare cases of isolated inguinal metastases have been described in patients with ovarian cancer.5,22–24 The inguinal pathway as studied in the 3 fetal specimens significantly diminished when traveling further away from the uterus. This pathway was also less prominent in the fetus of 20 weeks. A potential explanation for this finding is that this pathway disappears during embryologic development in the majority of female fetuses, but in a small percentage of women, some lymph vessels may persist forming a route for lymphatic spread of ovarian cancer. Remarkably, the lymphatic drainage pathways of the ovaries via the proper ligament of the ovary (ovarian ligament), SLO (infundibulopelvic ligament), and round ligament of the uterus all arise from the same embryologic structure called the gubernaculum.
Accurate knowledge of the anatomy and embryology of the human ovaries is essential to understand the patterns of lymphatic metastases in ovarian cancer. In embryos, the mesonephric (wolffian) and paramesonephric (müllerian) ducts are discrete primordia that coexist during the ambisexual period of development (until 8 weeks). Thereafter, 1 duct system persists, while the other regresses, leaving only nonfunctional vestiges. The wolffian duct develops first, differentiates into the epididymis, vas deferens, and seminal vesicles in men and regresses in women (in the sixth or seventh week). The müllerian duct develops later and differentiates into the fallopian tubes, uterus, and upper portion of the vagina.28 The ovaries develop at the level of the thoracic vertebra 12 and descend toward the sacral region. Two bands secure the gonads at the lower and upper poles, forming a structure called the gubernaculum. After atrophy of the müllerian ducts, the upper gubernaculum disappears in men, whereas the SLO (infundibulopelvic ligament) is formed in women, extending from the upper pole of the ovary upwards toward the lumbar region. The lower part of the gubernaculum inserts into the lower part of the gonad. In women, the proper ligament of the ovary (ovarian ligament) and the round ligament of the uterus are formed.29 The minor inguinal pathway cannot be explained by embryological development. However, the presence of lymph vessels in human cadaveric tissue samples suggests that the inguinal pathway is a definitive drainage pathway and may act as a route for lymphatic spread of epithelial ovarian cancer.
Although very rare, contralateral metastases have been found in patients with ovarian cancer.27 Therefore, guidelines advise that lymphadenectomy should always be performed on both sides, even in cases of a unilateral tumor.27 In this study, we did not detect connections between the right and left lymphatic ovarian drainage pathways in the analyzed fetuses. A possible explanation for contralateral metastases could be retrograde metastasis from 1 side to the other at the level of the cervix.30,31 However, we cannot support this theory based on our findings.
In addition, a large number of lymph vessels were seen in the ovaries as well as in the mesosalpinx of the fallopian tubes. The fact that all of these lymph vessels drain via 2 major pathways located in the ovarian ligaments supports the strategy of identifying the sentinel node in ovarian cancer by injecting tracers into the ovarian ligaments.7
The theory on the origin of epithelial ovarian cancer has changed in recent years. Traditionally, epithelial ovarian cancer was thought to arise from damaged ovarian epithelium. However, recently, studies suggest the fallopian tube as the source of epithelial ovarian cancer because a precursor of high-grade carcinoma can be found in the uterine tube (serous tubal intraepithelial carcinoma).32 The fimbriae, which are in close contact with the ovaries, are the preferred site of serous tubal intraepithelial carcinoma. Because the lymph vessels detected in the mesosalpinx eventually drain into ovarian pathways, irrespective of the exact origin of epithelial ovarian cancer (uterine tubes or ovaries), the locations where the lymphogenous metastases can be found are the same.
The study of the lymphatic system in fetuses has some limitations.25 Lyve-1 also recognizes venous endothelial cells in very early stages. Therefore, comparative immunohistochemical studies on adult specimens using Lyve-1 are needed to understand its staining patterns in developed tissues. In this study, we used human adult cadaveric tissue samples as control tissue to assess the presence of lymph vessels in the SLO (infundibulopelvic ligament), proper ligament of the ovary (ovarian ligament), and round ligament of the uterus. Although it was not feasible to study complete lymphatic drainage pathways in these tissues, we confirmed the presence of lymph vessels in all ligaments. This supported our findings in the human fetal specimens. Despite the relatively low number of fetal specimens studied, we found 3 consistent pathways of lymphatic drainage. Definitive patterns from the human lymphatic network can be recognized from embryonic 12 weeks onwards. However, significant changes may occur in later developmental stages or under pathological circumstances. Therefore, future studies that focus on the lymphatic drainage pathways of the ovaries in the human adult are needed. Finally, fetal ovaries are still located relatively high within the abdomen compared with adult ovaries. The ovaries descend slightly during embryologic development, but not as much as the testis in men. Nevertheless, although these organs may change position during embryological development, the vasculature and lymphatic drainage remains the same.
CONCLUSIONS
Epithelial ovarian cancer can metastasize via 3 lymphatic drainage pathways. The abdominal pathway travels through the SLO (infundibulopelvic ligament) along with the OA toward the aorta (right side) and caval vein (left side), whereas the pelvic pathway runs via the proper ligament of the ovary (ovarian ligament) along with the uterine artery toward the iliac artery and obturator fossa. Metastasis can also occur in the inguinal region as a result of a third minor lymphatic drainage pathway running via the round ligament of the uterus. Sentinel nodes can be detected in any of these regions.
ACKNOWLEDGMENT
The authors thank Andrev Kolesnik from the University of Warsaw in Poland for providing us with 2 human fetal pelves.
Footnotes
M.K. and A.C.K. contributed equally to this article.
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.ijgc.net).
REFERENCES
- 1. Benedet JL, Bender H, Jones H, 3rd, et al. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2000; 70: 209– 262. [PubMed] [Google Scholar]
- 2. Angioli R, Plotti F, Palaia I, et al. Update on lymphadenectomy in early and advanced ovarian cancer. Curr Opin Obstet Gynecol. 2008; 20: 34– 39. [DOI] [PubMed] [Google Scholar]
- 3. Di Re F, Baiocchi G. Value of lymph node assessment in ovarian cancer: status of the art at the end of the second millennium. Int J Gynecol Cancer. 2000; 10: 435– 442. [DOI] [PubMed] [Google Scholar]
- 4. Maggioni A, Benedetti Panici P, Dell’Anna T, et al. Randomised study of systematic lymphadenectomy in patients with epithelial ovarian cancer macroscopically confined to the pelvis. Br J Cancer. 2006; 95: 699– 704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carnino F, Fuda G, Ciccone G, et al. Significance of lymph node sampling in epithelial carcinoma of the ovary. Gynecol Oncol. 1997; 65: 467– 472. [DOI] [PubMed] [Google Scholar]
- 6. Harter P, Gnauert K, Hils R, et al. Pattern and clinical predictors of lymph node metastases in epithelial ovarian cancer. Int J Gynecol Cancer. 2007; 17: 1238– 1244. [DOI] [PubMed] [Google Scholar]
- 7. Kleppe M, Brans B, Van Gorp T, et al. The detection of Sentinel Nodes in Ovarian Cancer: a feasibility study. J Nucl Med. 2014; 55: 1799– 1804. [DOI] [PubMed] [Google Scholar]
- 8. Ditto A, Martinelli F, Reato C, et al. Systematic para-aortic and pelvic lymphadenectomy in early stage epithelial ovarian cancer: a prospective study. Ann Surg Oncol. 2012; 19: 3849– 3855. [DOI] [PubMed] [Google Scholar]
- 9. Handgraaf HJ, Verbeek FPR, Tummers QR, et al. Real-time near-infrared fluorescence guided surgery in gynecologic oncology: A review of the current state of the art. Gynecol Oncol. 2014; 135: 606– 613. [DOI] [PubMed] [Google Scholar]
- 10. Panici PB, Angioli R. Role of lymphadenectomy in ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2002; 16: 529– 551. [DOI] [PubMed] [Google Scholar]
- 11. Tan DS, Agarwal R, Kaye SB. Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncol. 2006; 7: 925– 934. [DOI] [PubMed] [Google Scholar]
- 12. Burghardt E, Girardi F, Lahousen M, et al. Patterns of pelvic and paraaortic lymph node involvement in ovarian cancer. Gynecol Oncol. 1991; 40: 103– 106. [DOI] [PubMed] [Google Scholar]
- 13. Benedetti-Panici P, Greggi S, Maneschi F, et al. Anatomical and pathological study of retroperitoneal nodes in epithelial ovarian cancer. Gynecol Oncol. 1993; 51: 150– 154. [DOI] [PubMed] [Google Scholar]
- 14. Onda T, Yoshikawa H, Yokota H, et al. Assessment of metastases to aortic and pelvic lymph nodes in epithelial ovarian carcinoma. A proposal for essential sites for lymph node biopsy. Cancer. 1996; 78: 803– 808. [DOI] [PubMed] [Google Scholar]
- 15. Tsumura N, Sakuragi N, Hareyama H, et al. Distribution pattern and risk factors of pelvic and para-aortic lymph node metastasis in epithelial ovarian carcinoma. Int J Cancer. 1998; 79: 526– 530. [DOI] [PubMed] [Google Scholar]
- 16. Sakuragi N, Yamada H, Oikawa M, et al. Prognostic significance of lymph node metastasis and clear cell histology in ovarian carcinoma limited to the pelvis (pT1M0 and pT2M0). Gynecol Oncol. 2000; 79: 251– 255. [DOI] [PubMed] [Google Scholar]
- 17. Suzuki M, Ohwada M, Yamada T, et al. Lymph node metastasis in stage I epithelial ovarian cancer. Gynecol Oncol. 2000; 79: 305– 308. [DOI] [PubMed] [Google Scholar]
- 18. Morice P, Joulie F, Camatte S, et al. Lymph node involvement in epithelial ovarian cancer: analysis of 276 pelvic and paraaortic lymphadenectomies and surgical implications. J Am Coll Surg. 2003; 197: 198– 205. [DOI] [PubMed] [Google Scholar]
- 19. Negishi H, Takeda M, Fujimoto T, et al. Lymphatic mapping and sentinel node identification as related to the primary sites of lymph node metastasis in early stage ovarian cancer. Gynecol Oncol. 2004; 94: 161– 166. [DOI] [PubMed] [Google Scholar]
- 20. Takeshima N, Hirai Y, Umayahara K, et al. Lymph node metastasis in ovarian cancer: difference between serous and non-serous primary tumors. Gynecol Oncol. 2005; 99: 427– 431. [DOI] [PubMed] [Google Scholar]
- 21. Nomura H, Tsuda H, Susumu N, et al. Lymph node metastasis in grossly apparent stages I and II epithelial ovarian cancer. Int J Gynecol Cancer. 2010; 20: 341– 345. [DOI] [PubMed] [Google Scholar]
- 22. Ang D, Ng KY, Tan HK, et al. Ovarian carcinoma presenting with isolated contralateral inguinal lymph node metastasis: a case report. Ann Acad Med Singapore. 2007; 36: 427– 430. [PubMed] [Google Scholar]
- 23. Oei AL, de Hullu JA, Grefte JM, et al. An enlarged groin node as first manifestation of a malignancy: don’t forget the ovaries. Eur J Obstet Gynecol Reprod Biol. 2008; 138: 240– 242. [DOI] [PubMed] [Google Scholar]
- 24. Fournier M, Stoeckle E, Guyon F, et al. Lymph node involvement in epithelial ovarian cancer: sites and risk factors in a series of 355 patients. Int J Gynecol Cancer. 2009; 19: 1307– 1313. [DOI] [PubMed] [Google Scholar]
- 25. Kraima AC, Derks M, Smit NN, et al. Lymphatic drainage pathways from the cervix uteri: implications for radical hysterectomy? Gynecol Oncol. 2014; 132: 107– 113. [DOI] [PubMed] [Google Scholar]
- 26. Botha CP, Post FH. Hybrid scheduling in the DeVIDE dataflow visualisation environment. SCS Publishing House Erlangen. 2008: 309– 322. [Google Scholar]
- 27. Kleppe M, Wang T, Van Gorp T, et al. Lymph node metastasis in stages I and II ovarian cancer: a review. Gynecol Oncol. 2011; 123: 610– 614. [DOI] [PubMed] [Google Scholar]
- 28. Fritz MA, Speroff L. Clinical Gynecologic, Endocrinology and Infertility. 8th ed Philadelphia, PA: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 29. Van der Schoot P, Emmen JM. Development, structure and function of the cranial suspensory ligaments of the mammalian glands in a cross-species perspective; their possible role in effecting disturbed testicular descent. Human Reprod Update. 1996; 2: 399– 418. [DOI] [PubMed] [Google Scholar]
- 30. Eicher E, Bove ER. In vivo studies on the lymphatic drainage of the human ovary. Obstet Gynecol. 1954; 3: 287– 297. [PubMed] [Google Scholar]
- 31. Eicher E, Goldberg I, Bove ER. In vivo studies with direct sky blue of the lymphatic drainage of the internal genitals of women. Am J Obstet Gynecol. 1954; 67: 1277– 1287. [DOI] [PubMed] [Google Scholar]
- 32. Vang R, Shih IM, Kurman RJ. Fallopian tube precursors of ovarian low- and high-grade serous neoplasms. Histopathology. 2013; 62: 44– 58. [DOI] [PubMed] [Google Scholar]


