Supplemental Digital Content is available in the text.
Keywords: antibiotic prophylaxis, cost-benefit analysis, endocarditis, prevention
Abstract
Background:
In March 2008, the National Institute for Health and Care Excellence recommended stopping antibiotic prophylaxis (AP) for those at risk of infective endocarditis (IE) undergoing dental procedures in the United Kingdom, citing a lack of evidence of efficacy and cost-effectiveness. We have performed a new economic evaluation of AP on the basis of contemporary estimates of efficacy, adverse events, and resource implications.
Methods:
A decision analytic cost-effectiveness model was used. Health service costs and benefits (measured as quality-adjusted life-years) were estimated. Rates of IE before and after the National Institute for Health and Care Excellence guidance were available to estimate prophylactic efficacy. AP adverse event rates were derived from recent UK data, and resource implications were based on English Hospital Episode Statistics.
Results:
AP was less costly and more effective than no AP for all patients at risk of IE. The results are sensitive to AP efficacy, but efficacy would have to be substantially lower for AP not to be cost-effective. AP was even more cost-effective in patients at high risk of IE. Only a marginal reduction in annual IE rates (1.44 cases in high-risk and 33 cases in all at-risk patients) would be required for AP to be considered cost-effective at £20 000 ($26 600) per quality-adjusted life-year. Annual cost savings of £5.5 to £8.2 million ($7.3–$10.9 million) and health gains >2600 quality-adjusted life-years could be achieved from reinstating AP in England.
Conclusions:
AP is cost-effective for preventing IE, particularly in those at high risk. These findings support the cost-effectiveness of guidelines recommending AP use in high-risk individuals.
Antibiotic prophylaxis (AP) is a widely used prevention measure for those at risk of developing infective endocarditis (IE). Following the suggestion that bacteremia secondary to invasive dental procedures might cause IE,1 the American Heart Association’s Committee on Prevention of Rheumatic Fever and Bacterial Endocarditis was the first to recommend that individuals at increased risk of IE should be given antibiotic prophylaxis (AP) before invasive dental procedures some 60 years ago.2 Over time, the American Heart Association and other international guideline committees have gradually restricted AP use, moving to single-dose AP strategies and restricting the types of patients for whom AP is recommended.3,4 In 2008, this culminated with the National Institute for Health and Care Excellence (NICE) recommending that the use of AP to prevent IE should cease in the United Kingdom.5 This recommendation was confirmed in a recent review of the NICE guidelines6 but is in contrast with current European,7 North American,4 and other international guidelines that recommend AP for high-risk individuals undergoing invasive dental procedures.
A recent interrupted time series study found that AP prescribing in England fell sharply after the 2008 NICE guidance with a significant increase in the incidence of IE. By March 2013, it was estimated that there were 34.9 (95% confidence interval, 7.9–61.9) more cases of IE per month than would have been expected from the previous trend.8 This increase was statistically significant both for those at high risk (previous history of IE, prosthetic heart valve, valve repaired with prosthetic material, unrepaired cyanotic congenital heart disease, or some repaired congenital heart defects) and moderate risk (native valve disease, unrepaired congenital heart valve anomalies, or previous rheumatic fever) of IE. This study provides unique evidence for evaluating the cost-effectiveness of AP, because the United Kingdom is the only country to have transitioned from recommending AP for those at high risk or moderate risk of IE to recommending its complete cessation. Another recent UK study demonstrated that the incidence of adverse drug reactions associated with AP was much lower than previously estimated.9
This article estimates the cost-effectiveness of AP (single-dose amoxicillin or clindamycin for those allergic to penicillin) in patients at risk of IE by using (1) recent estimates of the effect of AP on IE in the English population,8 (2) rates of AP adverse drug reactions,9 and (3) estimates for the probability of developing IE after dental procedures derived from French data10 as the foundation for analysis of cost and health benefits.
Methods
Comparators and Patient Population
The cost-effectiveness of the AP regimen that was in use in the United Kingdom before the 2008 NICE guidelines (a single 3-g oral dose of amoxicillin or a single 600-mg oral dose of clindamycin for those allergic to penicillin or who had received amoxicillin in the previous month) for all at risk individuals (ie, those at moderate risk or high risk of IE) was compared with a strategy of no AP (as per the NICE guidelines5,6). We also compared no AP with a strategy restricting AP to just those at high risk of IE (as per the European7 and North American4 guidelines). English National Health Service costs were estimated and health effects measured in quality-adjusted life-years (QALYs).
Model Structure
A decision model, based on the decision model used by NICE for the health-economic analysis performed to inform the 2008 guidelines,5 was constructed to estimate differences in costs and health benefits accruing from the short-term consequences of the decision to administer AP, or not, and the longer-term sequelae of AP and IE (Figure). AP-related adverse events and IE may be fatal or lead to differences in the probability of a patient being otherwise healthy, requiring valve replacement surgery or experiencing congestive heart failure (CHF). These longer-term impacts were captured by using a state transition model, with 1-year cycle periods and a lifetime (50 years) time horizon. We used Treeage Pro software (https://www.treeage.com) and the Sheffield Accelerated Value of Information Tool (http://savi.shef.ac.uk/SAVI/).
Figure.
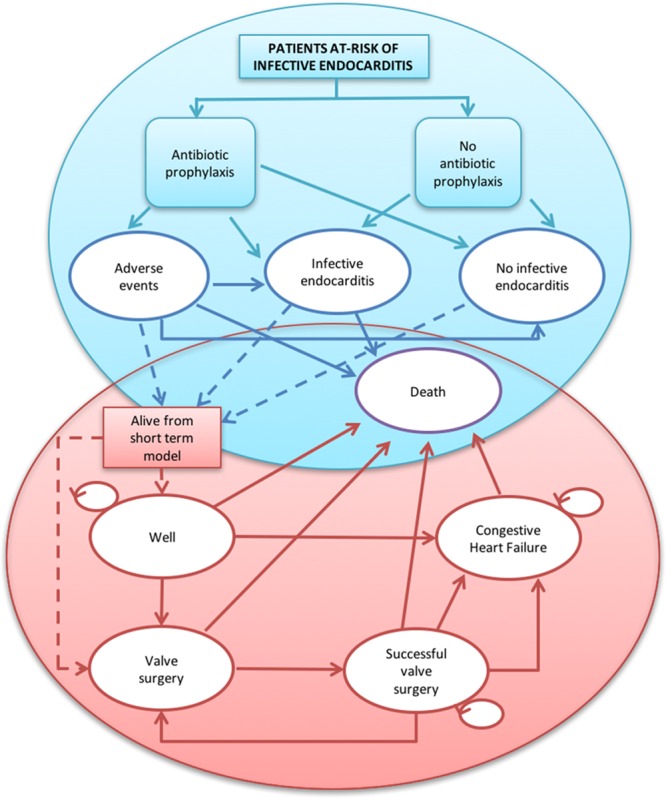
Illustration of the decision model used for the analysis. Key: Blue represents initial decision tree. Red represents subsequent health state transitions. Solid arrows are feasible pathways. Dashed arrows represent patient pathways from the decision tree to Markov models (ie, all living patients begin in either the well or valve surgery states).
Parameter Values
Data used to calculate the probability of IE after a high-risk (invasive) dental procedure were based on previous definitions and estimates of the risk of adults with predisposing cardiac conditions developing IE.10 The number of IE hospital admissions (International Classification of Diseases, 10th Revision code I33.0) was obtained from Hospital Episode statistics (HES; http://www.hscic.gov.uk/hes)11 and population data from the Office of National Statistics.12 A more detailed explanation of the calculations and values is provided in the online-only Data Supplement Methods and online-only Data Supplement Tables I and II).
Probability of IE Following a High-Risk Dental Procedure
The probability of IE after a high-risk dental procedure was based on analysis of recent English data8 that estimated that reduced use of AP was associated with 34.9 (95% confidence interval, 7.9–61.9) additional IE cases per month.
The incidence of IE with AP use was derived from 2007, the year immediately before the introduction of NICE guidance (1486 cases, 28.91 per million). Table 1 reports parameter estimates required to translate this incidence into an estimate of the probability of IE. All other probabilities are shown in Table 2. Duval et al10 provided data on the proportion of IE cases associated with a predisposing cardiac condition, the proportion associated with a high-risk dental procedure, the mean number of high-risk dental procedures per year in those with a predisposing cardiac condition, and the prevalence of a predisposing cardiac condition, resulting in an estimated probability of IE of 17.98 per million high-risk dental procedures where routine AP would be provided.
Table 1.
Data Used to Estimate the Probability of IE After a High-Risk (Invasive) Dental Procedure
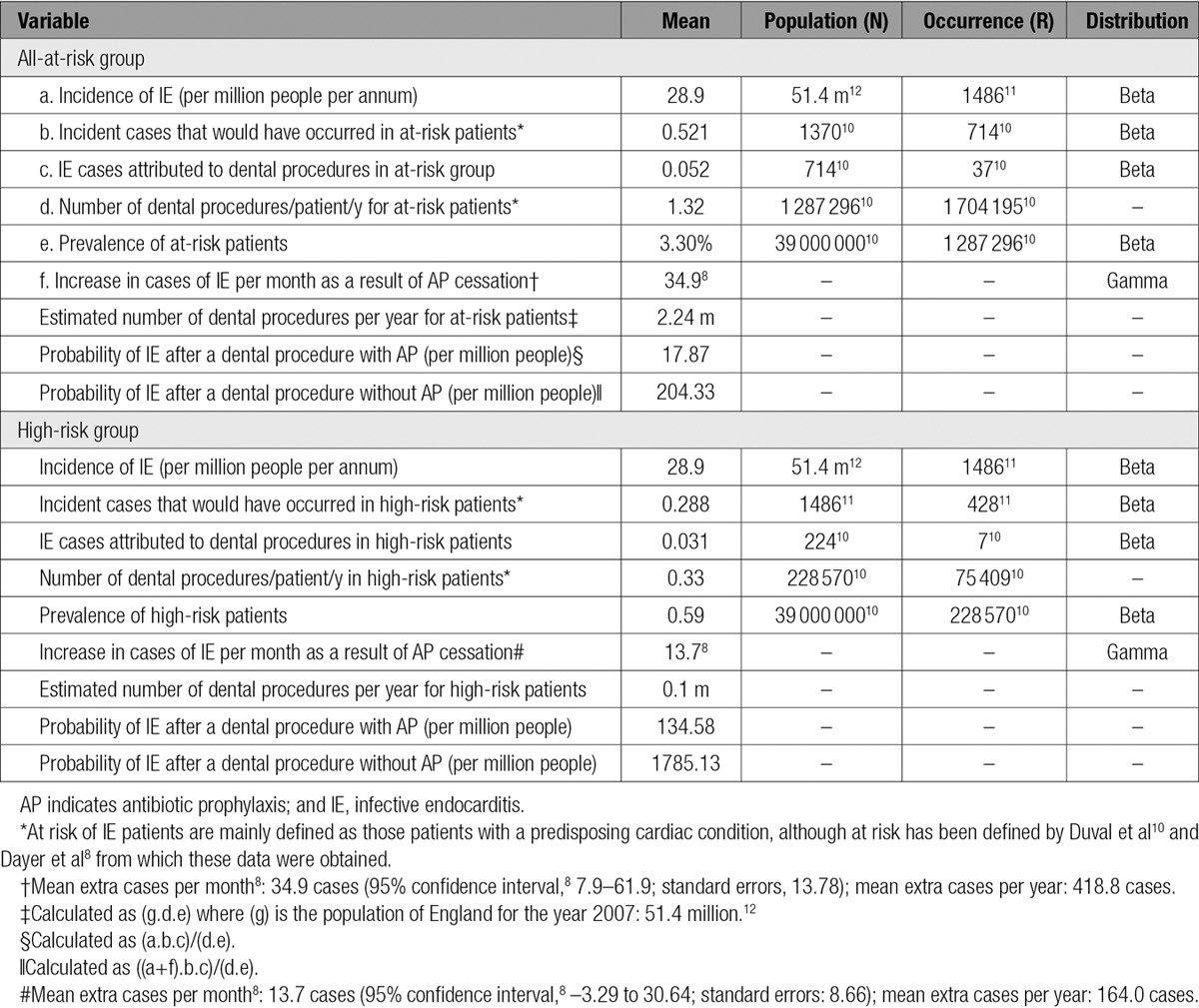
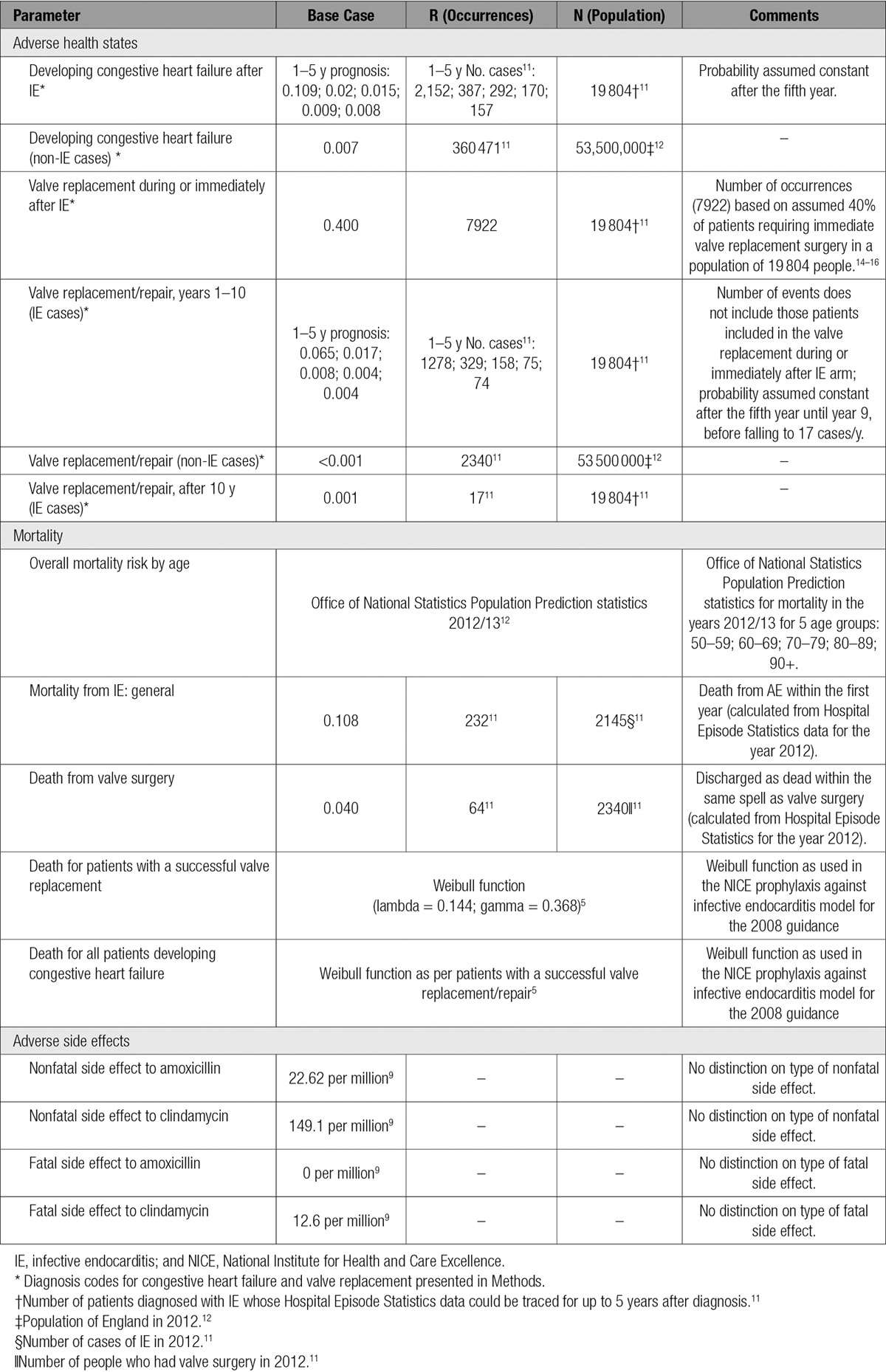
Table 2.
Summary of Transitional Probabilities for Adverse Health States, Mortality, and Adverse Side Effects
The higher estimated annual incidence of IE in the absence of AP leads to an estimated probability of IE of 1785.13 cases per million in the high-risk group and 204.33 cases per million dental procedures in the all-at-risk group.
Mortality From IE
All patients were tracked for mortality within 1 year of IE diagnosis (International Classification of Diseases, 10th Revision code I33.0) using HES.11 Deaths in the community secondary to IE were not included because HES only records in-hospital mortality, resulting in a small underestimate of IE-related mortality, a limitation of this data set.
Fatal and Nonfatal Reactions to AP
NHS Business Service Authority prescribing data were cross-referenced with adverse drug reaction data for prescriptions of standard AP (single oral dose amoxicillin 3 g or clindamycin 600 mg) from the Medicine and Health Products Regulatory Agency Yellow Card reporting scheme.9 The fatal and nonfatal adverse drug reaction rates per million prescriptions were 0 and 22.6, and 12.6 and 149.1 for amoxicillin and clindamycin, respectively.9
Long-Term Survival and Outcomes
Age-adjusted, all-cause mortality was estimated by using Office of National Statistics Population Prediction statistics for 2012 to 2013.12 Mortality risk for patients that survive valve surgery or develop CHF (International Classification of Diseases, 10th Revision code I50) was estimated by using published prosthetic valve endocarditis registry data.13 One, 5, and 10 year survival in this cohort was 67.1%, 55%, and 37.6%, respectively, and used to estimate a Weibull survival function.5 Mortality after valve replacement surgery was estimated using HES data for patients admitted for valve replacement surgery (OPCS-4 Classification of Interventions and Procedures version 4 codes: K25.1–K25.4, K26.1–K26.4, K27.1–K27.4, K28.1–K28.4, or K29.1–K29.4) and discharged as dead within the same spell for the year 2012.
The annual probability of IE survivors developing CHF over 5-year follow-up was estimated from HES. Of the 19 804 patients with IE and reliable 5-year follow-up data, the numbers diagnosed with CHF were 2152, 387, 292, 170, and 157 in years 1 to 5, respectively. The subsequent probability of developing CHF until the end of the model’s time horizon was assumed to be constant after year 5. Recent studies show that 40% to 50% of patients now undergo valve replacement surgery as part of their initial IE treatment,14–16 and, for this transition probability, we adopted a conservative 40% estimate. The annual probability of IE survivors needing valve surgery after the initial admission was estimated by using HES. Of the 19 804 patients diagnosed with IE and followed for 5 years, 1278 required valve replacement surgery during the first year after their initial IE treatment, and 329, 158, 75, and 74 required valve replacement surgery in years 2 to 5, respectively. The subsequent probability of needing valve surgery is assumed to remain at 74 cases per year through to year 9, before falling to 17 cases per year until the end of the model’s time horizon.
Health-Related Quality of Life (Utility) Weights
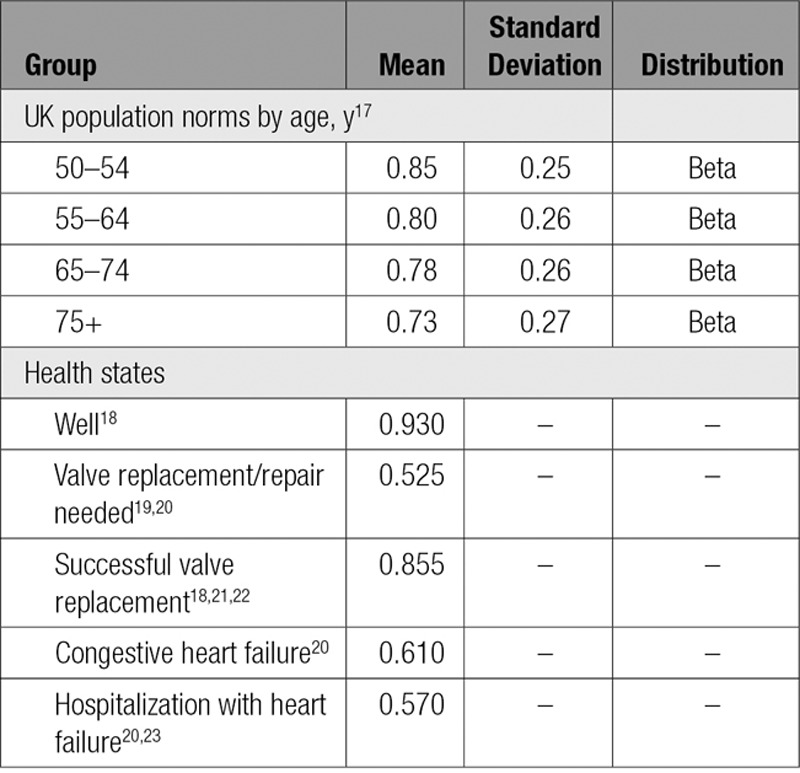
Mean health state utility values, required for estimating QALYs, and associated standard deviations were taken from different sources that are described in this section. UK population age norms17 were used to adjust and parameterize all utility values for the 5 health states in the model.
Adults in the well state were considered to be equivalent to patients with a New York Heart Association class 1 (ie, people with cardiac disease but no symptoms or limitations in function).18 Patients in the needing-valve-surgery state were assumed to correspond to New York Heart Association class III/IV.19,20 We assumed the utility value of 0.525 would be relevant only for 6 months before the same value as the Successful-valve-surgery state utility value would be relevant. Successful valve surgery was estimated by using data for New York Heart Association class I/II patients after valve replacement, with a utility value of 0·855.18,21,22 CHF was estimated as the weighted average of those not hospitalized20 and those hospitalized (New York Heart Association class III)20,23 based on 0.53 hospitalizations per year24 of 7.53 days duration.25 The health state and age utility values used are shown in Table 3.
Table 3.
Utility Values, by Health State and Age Group
Costs
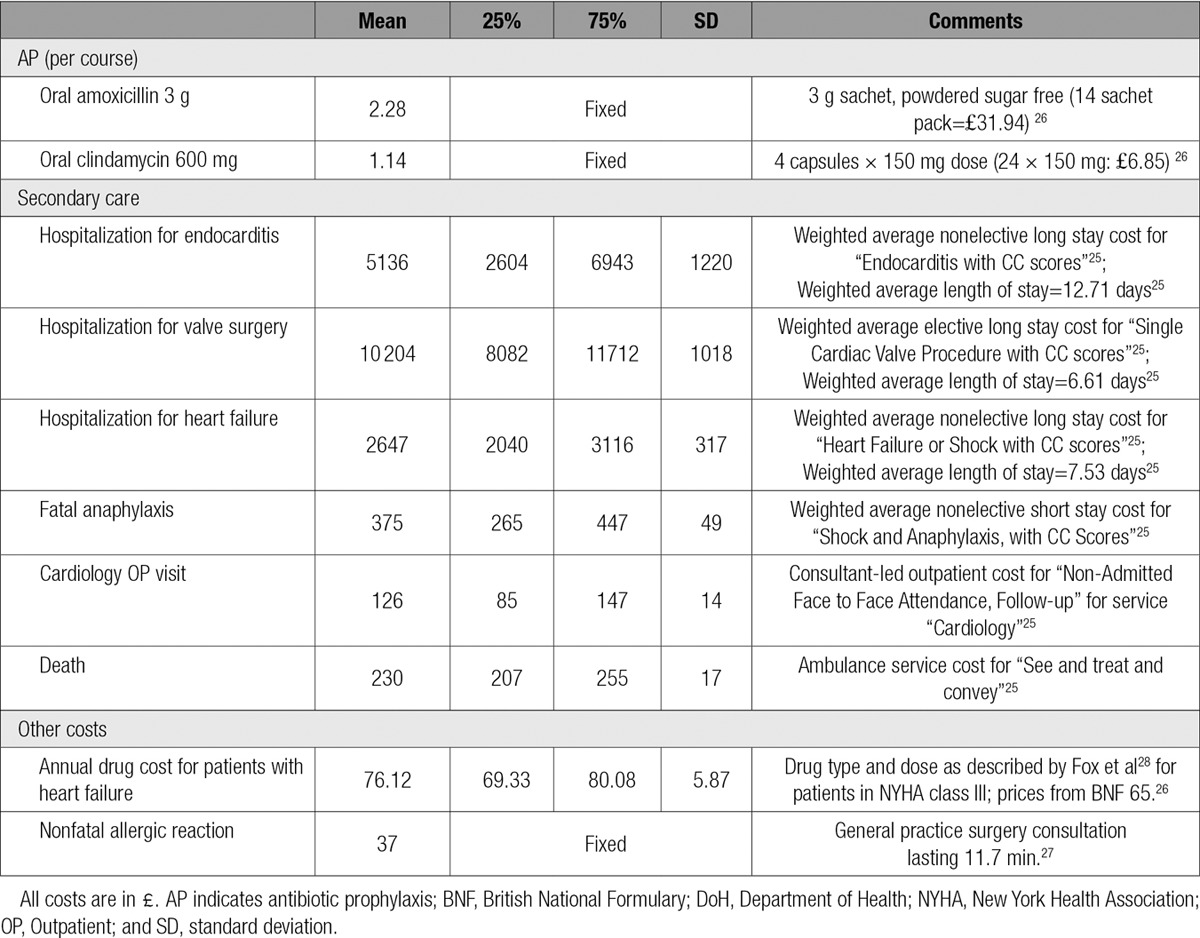
National sources for unit costs were used. The cost of amoxicillin (£2.28 [$3.03, €2.74]) and clindamycin (£1.14 [$1.52, €1.37]) were obtained from the British National Formulary (Number 65) for 2013.26 Secondary care costs were estimated using 2012 to 2013 National Reference costs.25,27 General practice consultation costs for AP adverse events were from Curtis.27 Exchange rates shown between UK£, US$, and the Euro € were calculated on the July 1, 2016 using the midmarket rate (£1=$1.33=€1.20).
Patients with CHF or previous valve surgery were assumed to require 2 cardiology outpatient visits per year. Those with CHF were assumed to require treatment with angiotensin-converting enzyme/angiotensin II inhibitors, β-blockers, digoxin, and high-dose loop diuretics at typical daily doses.5,28 A summary of these unit costs is provided in Table 4.
Table 4.
Summary of Unit Costs
All costs were discounted by 3.5% as suggested by NICE 2013 technology appraisal guidelines.29 A range of sensitivity analyses was performed, including probabilistic sensitivity analysis, to reflect different aspects of uncertainty in the evidence.
Statistical Analysis
An initial decision tree model leading to a state transition model was used to estimate the cost per QALY gained of AP versus no AP over a time horizon of 50 years. One-way and probability sensitivity analysis and expected value of perfect information (EVPI) analysis were also conducted. We used Treeage Pro software (https://www.treeage.com) to construct the decision tree and state transition model and the Sheffield Accelerated Value of Information Tool (http://savi.shef.ac.uk/SAVI/) for the EVPI analysis.
This analysis was performed in compliance with the Consolidated Health Economic Evaluation Reporting Standard (CHEERS) guidelines for the reporting of health economic analyses.30 Ethics approval was not required for this study because it was confined to analysis of publicly available data containing no identifiable patient information.
Results
In comparison with no AP, both amoxicillin and clindamycin AP were associated with lower costs and better health outcomes (Table 5) for both high-risk and all-at-risk populations. In the all-at-risk group, there were mean cost savings of £2.47 (95% credible interval [CrI], £0.48–£6.96) ($3.29; CrI $0.64–$9.26; €2.96, CrI €0.58–€8.35) per person with amoxicillin AP and £3.65 (95% CrI, £0.69–£8.14) ($4.86; 95% CrI, $0.92–$10.83; €4.38, 95% CrI, €0.83–€9.77) with clindamycin AP, in comparison with no AP (the difference between the drugs being driven by the lower cost of clindamycin: £1.14 versus £2.28 [$1.52 versus $3.03; €1.37 versus €2.74]).26 With an estimated 2.24 million dental procedures per year in this population (Table 1), AP would lead to savings of £5.5 million to £8.2 million per annum ($7.3 million to $10.9 million; €6.6 million to €9.8 million). We calculated a mean health improvement of 0.0012 (95% CrI, 0.000–0.003) and 0.0010 (95% CrI, 0.000–0.002) QALYs per person for amoxicillin and clindamycin, respectively (equivalent to 2687 QALYs gained per annum at the population level if amoxicillin AP were used for all-at-risk patients).
Table 5.
Costs and Effects of Antibiotic Prophylaxis Versus No Antibiotic Prophylaxis: Base Case Analysis

These cost savings were substantially greater in the high-risk group at a mean of ≈£40 ($53.2; €48.00) per patient (or £4.0 million [$5.3 million; €4.8 million] per annum in England on the basis of an estimated 100 000 dental procedures in this population). The most effective strategy would be amoxicillin, leading to gains of 0.0107 QALYs per person (or 1071 QALYs for the population) per annum.
Sensitivity Analysis
A sensitivity analysis on the effectiveness of AP was performed by varying the number of additional IE cases associated with AP withdrawal from 35 per month (base case) to zero (implying AP has no protective effect). For the all-at-risk group, amoxicillin remained cost saving until the rate of IE cases avoided fell below 16.8 per month and cost-effective (at £20 000 [$26 600; €24 000] per QALY) until the rate fell below 2.76 per month (33.12 cases per year). In the same population, use of clindamycin was cost saving until the rate fell below 8·1 cases per month and cost-effective (at £20 000 per QALY) until the rate fell below 6.2 cases per month.
In the high-risk group, amoxicillin remained cost saving provided the number of IE cases avoided with AP use was >0.74 per month and cost-effective (at £20 000 per QALY) provided the number of IE cases avoided was >0.12 per month (base case estimate 13.67 extra high-risk IE cases per month8) or 1.44 cases per year. For clindamycin, the corresponding values were 0.36 and 0.27, respectively.
Value of Information
Cost-effectiveness estimates are subject to uncertainty relating to values of the input parameters on clinical effectiveness, costs, and health outcomes. This uncertainty is a genuine concern because any decision could be incorrect: health benefits could be lost because of investment in a treatment that is not cost-effective. The value of eliminating all uncertainty, such that there is no risk of an incorrect decision, is called the Expected Value of Perfect Information (EVPI),31 which provides an estimate of the upper bound of the cost of any additional research that would reduce uncertainty. For the all-at-risk population, the EVPI is near zero (£9020 [$11 997; €10 824] for amoxicillin, £11 409 [$15 174; €13 691] for clindamycin over 10 years in England). This is because there is little uncertainty; AP is almost certainly cost-effective, and, therefore, reducing uncertainty in any of the input parameters would be unlikely to lead to a different conclusion. However, the clinical effectiveness of AP is subject to some uncertainty because of reliance on observational data and interrupted time-series analysis.8
Accordingly, we conducted an additional exploratory analysis in which assumptions concerning uncertainty around the efficacy of AP in the all-at-risk population were increased. The base case analysis assumes that withdrawal of AP led to 34.9 additional cases of IE per month. Therefore, a model averaging approach was used such that half the sample maintained this estimate and half used an estimate of zero (ie, AP has no effect). This resulted in AP (amoxicillin) being less cost saving (now £0.15 per person; 95% CrI, –£5.62 to £2.28 [$0.20; 95% CrI, –$7.48 to $2.74; €0.18, 95% CrI, –€6.74 to €2.74]) and more effective, although less so than in the base case (0.00061 QALYs; 95% CrI, 0.000–0.002). The probability of being cost saving is 0.48, and the probability of being cost-effective (at a £20 000 threshold) is 0.50. In this situation, the EVPI rises to £25.3 million ($33.7 million; €30.4 million), driven almost entirely by the introduced uncertainty concerning AP effectiveness.
Discussion
We recently estimated the impact of the withdrawal of AP on the incidence of IE in an interrupted time-series analysis of English data.8 This study provided unique evidence for evaluating the cost-effectiveness of AP, because the United Kingdom is the only country to have transitioned from the widespread use of AP to recommending its complete cessation. Using these figures as inputs to a cost-effectiveness analysis indicates that AP is likely to be not just cost-effective, but also cost saving. If AP were used in all those at risk of IE, then amoxicillin and clindamycin AP would result in estimated cost savings of £2.47 ($3.29; €2.96) and £3.65 ($4.86; €4.38) per patient and health gains of 0.0012 and 0.0010 QALYs, respectively. Overall, AP would result in an estimated saving of £5.5 to £8.2 million ($7.3 million to $10.9 million; €6.6 million to €9.8 million) and a health gain of 2687 QALYs in England per year. If AP were restricted to those at high risk of IE, the cost savings and health gain per person would be even greater at £40 ($53.20; €48.00) and 0.0107 QALYs. The overall benefit of using amoxicillin AP in high-risk patients would be a cost savings of £4.0 million ($5.3 million; €4.8 million) and a health gain of 1071 QALYs in England per year.
Because the recent time-series analysis was an observational study,8 we cannot be certain that the number of extra cases of IE identified was caused by the reduction in AP prescribing following the 2008 NICE guidelines.5 It is possible, therefore, that the number of IE cases prevented by AP is less than the identified 34.9 per month. To evaluate the cost-effectiveness of AP across a range of scenarios we performed a sensitivity analysis using a maximum effectiveness of preventing 35 IE cases per month and a minimum of preventing zero cases, ie, where AP is ineffective. Using this approach, we demonstrated that amoxicillin AP has to prevent only 2.76 cases per month in the all-at-risk group to be cost-effective and 16.8 cases per month to be cost saving. Moreover, amoxicillin AP is even more cost-effective in the high-risk group where only 0.12 cases per month need to be prevented for it to be cost-effective and 0.74 cases per month to be cost saving.
These data suggest that a strategy of directing AP at those at high risk of IE is likely to be cost-effective or cost saving, even at very low rates of AP clinical effectiveness. This conflicts with the NICE health economic analysis of AP,5 which used older data on the incidence of adverse drug reactions after the use of parenteral penicillins.5 More recent data suggest that fatal anaphylaxis is exceedingly rare, and there have been no reports of fatal anaphylaxis after oral amoxicillin AP in the world literature.32 The incidence of adverse reactions after amoxicillin AP is extremely low (0 fatal, 22.62 nonfatal reactions per million prescriptions).9,32 Although low, reactions to clindamycin AP were higher than anticipated, suggesting that an alternative AP regimen for those allergic to penicillin would be desirable.9 Our data suggest that AP needs only minimal clinical effectiveness to be cost-effective, because it is so cheap in comparison with the substantial cost and health implications of IE.
International guideline committees have highlighted the lack of evidence for the benefit of AP and called for randomized clinical trials (RCTs) to provide that evidence.4,6,7 However, ethical issues and the high cost of performing an RCT have prevented such a study to date. Skepticism concerning noncontrolled data represents a genuine source of uncertainty about the cost and clinical effectiveness of AP that underpin any cost-effectiveness analysis. This uncertainty conveys a risk that the advice given by guideline committees is wrong. However, there is also a cost associated with performing the RCTs needed to eliminate that uncertainty. EVPI analysis provides an estimate of the maximum amount it is worth spending to reduce that uncertainty.31 If there is genuine uncertainty about whether AP is effective or not, then the value of an RCT becomes substantial. Exploratory analysis using the at-risk population of England over a 10-year period estimates the EVPI of amoxicillin at £25.3 million ($33.7 million; €30.4 million). Therefore, although such a study may be costly, its value may well outweigh its cost.
The main limitations of this study are the lack of RCT data and the resulting need to use observational studies to identify the input parameters for health economic analysis. In particular, our data on the effectiveness of AP are based on the increase in IE cases and fall in AP prescribing that occurred after the introduction of the 2008 NICE guideline.8 Although this study demonstrated a temporal association between the fall in AP prescribing and increasing IE incidence, it did not prove a causal link. Hence, we undertook a sensitivity analysis to examine the cost-effectiveness of AP if the level of AP clinical effectiveness was less than anticipated. Furthermore, we used pre- and post-NICE prescribing figures as proxies for the use of AP even though compliance is never 100%. A final limitation is that HES data were used to populate most transitional probability estimates in our model; events occurring outside the hospital setting were not captured. IE is also complicated by a number of high-cost serious outcomes, eg, stroke and renal failure,14,15 that we were unable to take into account. Our analysis is likely, therefore, to have underestimated the impact of IE and cost-effectiveness of AP. Although our analysis is specific to England, its findings are likely to be broadly applicable to other advanced healthcare systems. However, cost-effectiveness may be even greater in nations with higher healthcare costs (eg, United States) but lower in those where healthcare costs are cheaper.
Conclusion
Because of the serious consequences and high costs associated with IE and the comparatively low costs associated with AP, this analysis demonstrates that AP is likely to be very cost-effective (and even cost saving) in preventing IE, particularly for those at high risk, even when the number of prevented IE cases is very low. Our data suggest that European and American guidelines recommending AP use in high-risk individuals are likely to be cost-effective.
Sources of Funding
This study was supported by the National Institute for Dental and Craniofacial Research (NIDCR) (NIH R03 grant Ref: 1R03DE023092-01) http://www.nidcr.nih.gov. The views expressed in this publication are those of the authors and not necessarily those of the funders. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Disclosures
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_discosure.pdf and declare that (1) Dr Thornhill received support from the National Institute for Dental and Craniofacial Research [NIH R03 grant Ref: 1R03DE023092-01] for the submitted work; (2) none of the authors have a relationship with companies that might have an interest in the submitted work in the previous 3 years; (3) none of the authors spouses, partners or children have a financial relationship that may be relevant to the submitted work; and (4) Drs Franklin, Wailoo, Jones, and Thornhill have no nonfinancial interests that may be relevant to the submitted work. Drs Baddour and Lockhart are members of the American Heart Association’s Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease and were involved in producing the 2007 American Heart Association guideline on prevention of infective endocarditis. Dr Prendergast was a member of the Task Force that produced the 2009 European Society of Cardiology guidelines on the prevention, diagnosis, and treatment of infective endocarditis, and also acted as a consultant to the committee that produced the 2008 NICE clinical guideline 64 on prophylaxis against infective endocarditis, and Dr Dayer was a consultant to the review committee that produced the 2015 update to NICE clinical guideline 64 on prophylaxis against infective endocarditis.
Supplementary Material
Footnotes
Sources of Funding, see page 1577
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.116.022047/-/DC1.
Circulation is available at http://circ.ahajournals.org.
Clinical Perspective
What Is New?
This study uses recent data to evaluate the cost-effectiveness of antibiotic prophylaxis (AP) in preventing infective endocarditis (IE).
It demonstrates that AP before invasive dental procedures for those at moderate or high risk of IE is very cost-effective, in fact, cost saving.
Cost-effectiveness is even greater when AP is confined to those at high risk of IE.
For high-risk individuals, AP is cost-effective even if it only prevents 1.44 cases of IE per year.
What Are the Clinical Implications?
If AP was reinstated in England for those at moderate or high risk of IE, it could save £5.5 to £8.2 million ($7.3–$10.9 million; €6.6–€9.8 million) and result in health gains >2600 quality-adjusted life-years per year.
AP is even more cost-effective for those at high risk of IE. Restricting AP to those at high risk would result in cost savings of £4.0 million ($5.32 million; €4.8 million) and health gains of >1070 quality-adjusted life-years per year in England because of the smaller number of individuals at high risk.
These findings support the cost-effectiveness of guidelines recommending AP use, in particular, in high-risk individuals.
References
- 1.Lewis T, Grant R. Observations relating to subacute infective endocarditis. Heart. 1923;10:21–77. [Google Scholar]
- 2.Jones TD, Baumgartner L, Bellows MT, Breese BB, Kuttner AG, McCarty M, Rammelkamp CH. Prevention of rheumatic fever and bacterial endocarditis through control of streptococcal infections. Circulation. 1955;11:317–320. [Google Scholar]
- 3.Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I, Moreillon P, de Jesus Antunes M, Thilen U, Lekakis J, Lengyel M, Müller L, Naber CK, Nihoyannopoulos P, Moritz A, Zamorano JL ESC Committee for Practice Guidelines. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J. 2009;30:2369–2413. doi: 10.1093/eurheartj/ehp285. doi: 10.1093/eurheartj/ehp285. [DOI] [PubMed] [Google Scholar]
- 4.Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M, Bolger A, Cabell CH, Takahashi M, Baltimore RS, Newburger JW, Strom BL, Tani LY, Gerber M, Bonow RO, Pallasch T, Shulman ST, Rowley AH, Burns JC, Ferrieri P, Gardner T, Goff D, Durack DT American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; American Heart Association Council on Cardiovascular Disease in the Young; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Surgery and Anesthesia; Quality of Care and Outcomes Research Interdisciplinary Working Group. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116:1736–1754. doi: 10.1161/CIRCULATIONAHA.106.183095. doi: 10.1161/CIRCULATIONAHA.106.183095. [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence (NICE) Prophylaxis against infective endocarditis. 2008:NICE Clinical Guideline No 64. https://www.nice.org.uk/guidance/cg64. Accessed October 19, 2016. [PubMed]
- 6.National Institute for Health and Care Excellence (NICE) Prophylaxis against infective endocarditis. 2015:NICE Clinical Guideline No 64. https://www.nice.org.uk/guidance/cg64. Accessed October 19, 2016. [PubMed]
- 7.Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Tornos Mas P, Vilacosta I, Zamorano JL, Erol Ç, Nihoyannopoulos P, Aboyans V, Agewall S, Athanassopoulos G, Aytekin S, Benzer W, Bueno H, Broekhuizen L, Carerj S, Cosyns B, De Backer J, De Bonis M, Dimopoulos K, Donal E, Drexel H, Flachskampf FA, Hall R, Halvorsen S, Hoen B, Kirchhof P, Lainscak M, Leite-Moreira AF, Lip GY, Mestres CA, Piepoli MF, Punjabi PP, Rapezzi C, Rosenhek R, Siebens K, Tamargo J, Walker DM Document Reviewers. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36:3075–3128. doi: 10.1093/eurheartj/ehv319. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 8.Dayer MJ, Jones S, Prendergast B, Baddour LM, Lockhart PB, Thornhill MH. Incidence of infective endocarditis in England, 2000-13: a secular trend, interrupted time-series analysis. Lancet. 2015;385:1219–1228. doi: 10.1016/S0140-6736(14)62007-9. doi: 10.1016/S0140-6736(14)62007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thornhill MH, Dayer MJ, Prendergast B, Baddour LM, Jones S, Lockhart PB. Incidence and nature of adverse reactions to antibiotics used as endocarditis prophylaxis. J Antimicrob Chemother. 2015;70:2382–2388. doi: 10.1093/jac/dkv115. doi: 10.1093/jac/dkv115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duval X, Alla F, Hoen B, Danielou F, Larrieu S, Delahaye F, Leport C, Briançon S. Estimated risk of endocarditis in adults with predisposing cardiac conditions undergoing dental procedures with or without antibiotic prophylaxis. Clin Infect Dis. 2006;42:e102–e107. doi: 10.1086/504385. doi: 10.1086/504385. [DOI] [PubMed] [Google Scholar]
- 11.Health and Social Care Information Centre. Hospital Episode Statistics. http://www.hscic.gov.uk/hes. Accessed January 11, 2016.
- 12.Office of National Statistics (ONS) Population prediction statistics for 2012/2013. 2013. http://www.ons.gov.uk/ons/rel/pop-estimate/population-estimates-for-uk–england-and-wales–scotland-and-northern-ireland/2013/stb—mid-2013-uk-population-estimates.html Accessed January 11, 2016.
- 13.Edwards MB, Ratnatunga CP, Dore CJ, Taylor KM. Thirty-day mortality and long-term survival following surgery for prosthetic endocarditis: a study from the UK heart valve registry. Eur J Cardiothorac Surg. 1998;14:156–164. doi: 10.1016/s1010-7940(98)00148-1. [DOI] [PubMed] [Google Scholar]
- 14.Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387:882–893. doi: 10.1016/S0140-6736(15)00067-7. doi: 10.1016/S0140-6736(15)00067-7. [DOI] [PubMed] [Google Scholar]
- 15.Hoen B, Duval X. Infective endocarditis. N Engl J Med. 2013;369:785. doi: 10.1056/NEJMc1307282. doi: 10.1056/NEJMc1307282. [DOI] [PubMed] [Google Scholar]
- 16.Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG, Jr, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST, Chu VH, Falcó V, Holland DJ, Jones P, Klein JL, Raymond NJ, Read KM, Tripodi MF, Utili R, Wang A, Woods CW, Cabell CH International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169:463–473. doi: 10.1001/archinternmed.2008.603. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kind P, Hardman G, Macran S. UK population norms for EQ-5D. 1999;2015. http://www.york.ac.uk/media/che/documents/papers/discussionpapers/CHE%20Discussion%20Paper%20172.pdf Accessed January 11, 2016.
- 18.Kirsch J, McGuire A. Establishing health state valuations for disease specific states: an example from heart disease. Health Econ. 2000;9:149–158. doi: 10.1002/(sici)1099-1050(200003)9:2<149::aid-hec501>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 19.Alexiou C, Langley SM, Stafford H, Haw MP, Livesey SA, Monro JL. Surgical treatment of infective mitral valve endocarditis: predictors of early and late outcome. J Heart Valve Dis. 2000;9:327–334. [PubMed] [Google Scholar]
- 20.Calvert MJ, Freemantle N, Cleland JG. The impact of chronic heart failure on health-related quality of life data acquired in the baseline phase of the CARE-HF study. Eur J Heart Fail. 2005;7:243–251. doi: 10.1016/j.ejheart.2005.01.012. doi: 10.1016/j.ejheart.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Jamieson WR, Allen P, Miyagishima RT, Gerein AN, Munro AI, Burr LH, Tyers GF. The Carpentier-Edwards standard porcine bioprosthesis. A first-generation tissue valve with excellent long-term clinical performance. J Thorac Cardiovasc Surg. 1990;99:543–561. [PubMed] [Google Scholar]
- 22.Pomerantzeff PM, de Almeida Brandão CM, Albuquerque JM, Pomerantzeff PY, Takeda F, Oliveira SA. Mitral valve annuloplasty with a bovine pericardial strip–18-year results. Clinics (Sao Paulo) 2005;60:305–310. doi: 10.1590/s1807-59322005000400008. doi: /S1807-59322005000400008. [DOI] [PubMed] [Google Scholar]
- 23.McAlister F, Ezekowitz J, Wiebe N, Rowe B, Spooner C, Crumley E, Hartling L, Kaul P, Nichol G, Klassen T. Cardiac resynchronization therapy for congestive heart failure. Evid Rep Technol Assess (Summ) 2004;106:1–8. [PMC free article] [PubMed] [Google Scholar]
- 24.National Institute for Health and Care Excellence (NICE) Chronic Heart Failure: management of chronic heart failure in adults in primary and secondary care. 2003;2015. www.nice.org.uk/guidance/cg5. Accessed January 11, 2016.
- 25.Department of Health (DoH) National Schedule of Reference Costs 2012–13. 2013;2015. https://www.gov.uk/government/publications/nhs-reference-costs-2012-to-2013. Accessed January 11, 2016.
- 26.Joint Formulary Committee. British National Formulary. 65 ed. London: BMJ Group and Pharmaceutical Press; 2013. [Google Scholar]
- 27.Curtis L. Unit costs of health and social care 2013. 2013;2015. http://www.pssru.ac.uk/project-pages/unit-costs/2013/. Accessed January 8, 2016.
- 28.Fox M, Mealing S, Anderson R, Dean J, Stein K, Price A, Taylor RS. The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model. Health Technol Assess. 2007;11:iii–iv, ix. doi: 10.3310/hta11470. [DOI] [PubMed] [Google Scholar]
- 29.National Institute for Health and Care Excellence (NICE) Guide to methods of technology appraisal. 2013;2015. http://publications.nice.org.uk/guide-to-the-methods-of-technology-appraisal-2013-pmg9. Accessed January 8, 2016. [PubMed]
- 30.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E CHEERS Task Force. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health. 2013;16:e1–e5. doi: 10.1016/j.jval.2013.02.010. doi: 10.1016/j.jval.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Felli JC, Hazen GB. Sensitivity analysis and the expected value of perfect information. Med Decis Making. 1998;18:95–109. doi: 10.1177/0272989X9801800117. [DOI] [PubMed] [Google Scholar]
- 32.Lee P, Shanson D. Results of a UK survey of fatal anaphylaxis after oral amoxicillin. J Antimicrob Chemother. 2007;60:1172–1173. doi: 10.1093/jac/dkm315. doi: 10.1093/jac/dkm315. [DOI] [PubMed] [Google Scholar]


