Supplemental Digital Content is available in the text.
Key Words: immunomodulatory drugs, dual, cereblon, myeloma, immunohistochemistry
Abstract
Clinical interest in the measurement of Cereblon (CRBN), the primary target of the IMiDs immunomodulatory drugs lenalidomide and pomalidomide, has been fueled by its essential requirement for antitumor or immunomodulatory activity of both drugs in multiple myeloma (MM). However, limited analyses of clinical samples for CRBN gene expression or protein levels have utilized unvalidated reagents and assays, raising uncertainty about the interpretation of these results. We previously described a highly specific rabbit monoclonal antibody CRBN65 against 65-76 AA of human Cereblon. Here we describe a validated dual color bright-field Cereblon/CD138 immunohistochemical (IHC) assay utilizing CRBN65 and a commercial mouse monoclonal CD138 antibody. Sensitivity and specificity of the assay was determined and assay precision was shown for both cytoplasmic and nuclear Cereblon in MM bone marrow samples with coefficient of variation values of 5% and 2%, respectively. The dual IHC assay was effective for detecting a continuous range of Cereblon levels in 22 MM patient bone marrow core biopsies and aspirate clots, as shown by average cytoplasmic H-scores ranging from 63 to 267 and nuclear H-scores ranging from 17 to 250. Interpathologist comparison of MM sample H-scores by 3 pathologists demonstrated good concordance (R2=0.73). This dual assay demonstrated superior Cereblon IHC measurement in MM samples compared with the single IHC assay using a published commercial rabbit polyclonal Cereblon antibody and could be used to explore the potential utility of Cereblon as a biomarker in the clinic.
Treatment with thalidomide and IMiDs immunomodulatory drugs lenalidomide (LEN) and pomalidomide (POM) is associated with high rates of clinical remission and improved overall survival in multiple myeloma (MM) patients.1–3 Ito et al4 described the molecular target of thalidomide to be Cereblon, a substrate receptor for the Cullin Ring ubiquitin E3 ligase complex. Subsequent studies have shown that LEN and POM also bind Cereblon and their antiproliferative activity is mediated by Cereblon.5,6 Recently 3 independent groups demonstrated that LEN binds Cereblon and enhances selective ubiquitination and degradation of the transcription factors Ikaros and Aiolos resulting in toxicity to MM cells and activation of T-cell function.7–9 The central role of Cereblon as a direct target for thalidomide and the IMiD compounds has led some groups to hypothesize about its potential utility as a predictive biomarker of response and/or resistance to these agents.
A few studies have suggested that high CRBN gene expression before treatment is associated with more favorable response to IMiD compound treatment.10–13 However, we did not observe any correlation between CRBN mRNA or protein levels and intrinsic sensitivity or resistance to LEN in a diverse panel of MM cell lines.1 We have also shown a disconnect between CRBN mRNA and protein levels. Cereblon has multiple splice variants, if translated may not bind the IMiD agents. These variants are not discriminated by commercial gene chip or the polymerase chain reaction methods.1 Some studies have suggested that Cereblon protein levels as measured by immunohistochemical (IHC) staining with a commercial antibody (Proteintech) correlates with response to LEN and thalidomide in MM.14 However, we have shown that most commercially available Cereblon antibodies are nonspecific.1 Furthermore, methods to accurately identify MM cells amidst other bone marrow cells and to reproducibly quantify Cereblon protein in these MM cells have not been reported.
In an effort to establish a standardized and validated method to quantify Cereblon protein in MM bone marrow core biopsies and bone marrow aspirate clots, we developed a dual color, bright-field IHC assay using a specific rabbit monoclonal antibody CRBN65 against 65-76 AA of human Cereblon coupled with a commercially available anti-CD138 antibody to label MM cells. MM patient samples stained with this dual IHC assay were scored using the H-score method15 for a semiquantitative measurement of Cereblon protein levels. We show that the dual color CRBN65/CD138 IHC assay coupled with H-score assessment was specific, precise, and reproducible. This dual IHC assay described is thus far the most accurate and reliable IHC method to evaluate Cereblon protein levels in clinical MM patient bone marrow core biopsies and aspirate clots.
MATERIALS AND METHODS
Cell Lines and Tissues
MM cell line U266 was purchased from ATCC (American Type Culture Collection, Manassas, VA). DF15 cells were provided by John Shaughnessy (University of Arkansas, Little Rock, AR). POM-resistant DF15R cells and U266 short hairpin (shRNA) CRBN knockdown cell lines were generated and cultured as previously described.6 The FFPE tissue blocks from 22 MM patient cases (12 bone marrow core biopsies and 10 bone marrow aspirate clots) were purchased from Asterand (Asterand plc, Detroit, MI) and Avaden (Avaden BioSciences Inc., Scarsdale, NY). Multinormal human FFPE tissue microarray MNO341 containing 33 types of organs was purchased from Pantomics (Pantomics Inc., Richmond, CA).
Immunohistochemistry
All single IHC assays were performed on the Bond automated slide stainer (Leica Microsystems Inc., Buffalo Grove, IL) using the following primary antibodies: a rabbit monoclonal anti-Cereblon against amino acids 65-76 of human Cereblon (CRBN65) (Celgene Corporation) at a 1/2000 dilution, a rabbit polyclonal anti-Cereblon against recombinant C-terminal-300 amino acids of the human Cereblon (CRBN-PT) (Catalog No. 11435-1-AP; Proteintech Group Inc., Chicago, IL) at a 1/100 dilution, a mouse monoclonal anti-CD138 (clone MI15; Catalog No. M7228; Dako, Carpinteria, CA) at a 1/600 dilution for single color IHC and at a 1/1200 dilution for dual color IHC, and a rabbit polyclonal IgG isotype control (Jackson Immuno Research Labs, West Grove, PA) at 1.53 μg/mL.
Single IHC assays were performed as previously described.1 The dual CRBN65/CD138 IHC assay was performed on the Bond automated slide stainer by sequentially adding the primary antibodies and their detection systems (Table 1). Briefly, CRBN65 was added first followed by the Bond Polymer Refine Detection (DS9800; Leica Microsystems Inc.). A heating step was then introduced at 90°C for 5 minutes in ×1 Bond wash buffer to eliminate any possible cross-reactivity. The anti-CD138 antibody was added next followed by the Bond Polymer Refine Red Detection system (DS9390; Leica Microsystems Inc.). The Cereblon-DAB and CD138-Fast Red steps are integrated into 1 sequential double staining. After the dual IHC procedure, slides were counterstained with hematoxylin, rinsed in tap water, and baked in a 60°C oven for 20 minutes or until completely dry before being coverslipped by the Tissue-Tek Film Automated Coverslipper.
TABLE 1.
Dual Color CRBN65/CD138 Immunohistochemical (IHC) Assay Protocol
H-score Method
Cereblon immunoreactivity of CD138-positive MM cells was scored independently by 3 pathologists (S.C., D.E.H., and M.B.) using the H-score method.15 Briefly, the entire tumor is scored for Cereblon immunoreactivity based on range and intensity. Staining intensity is recorded on a scale of 0 to 3 for negative, mild, moderate, and strong immunoreactivity, respectively. Final scores range from 0 to 300 and are the sum of the products of percent of cells and intensity of staining to account for 100% of the tumor cells [H-score=(% at 1+)×1+(% at 2+)×2+(% at 3+)×3]. Because Cereblon can be found in the cytoplasm and in the nucleus, both compartments (nuclear and cytoplasmic) were scored separately. CRBN-PT single IHC stains were evaluated with the guidance of a serial section stained for CD138 to allow for approximate tumor cell identification. A total H-score (0 to 600) was generated for each case by addition of the H-scores for the nuclear and cytoplasmic compartments.
Western Blot Analysis
CRBN rabbit monoclonal antibody (CRBN65) production and validation was previously described by Lopez-Girona etal.6 Western blot analysis using CRBN65 and Proteintech CRBN-PT antibody was performed as previously described by Gandhi et al1 and Lopez-Girona et al.6
RESULTS
CRBN65 is a Highly Immunoreactive and Specific Antibody for IHC Measurement of Cereblon
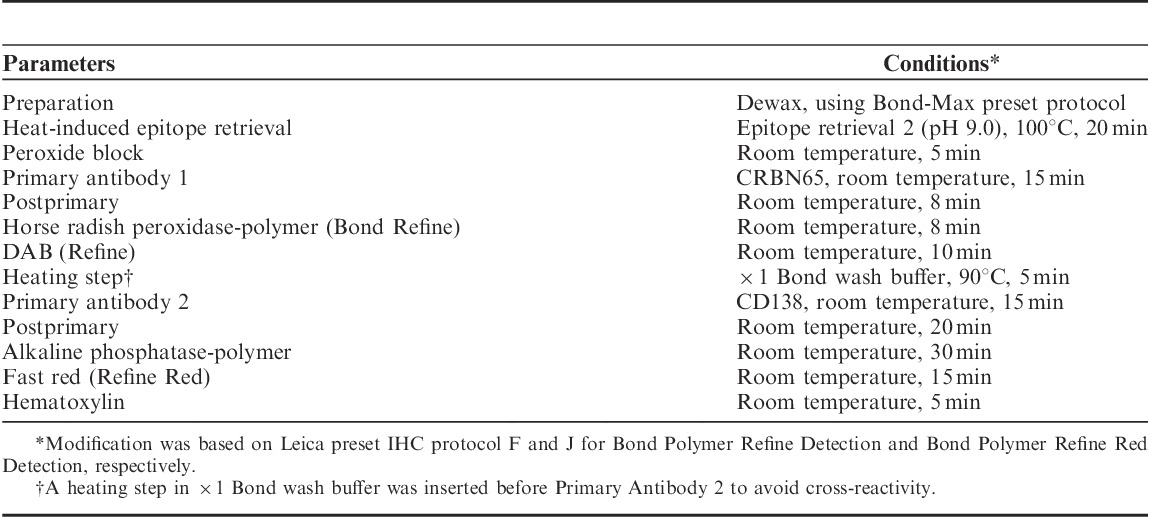
Previously we showed that CRBN65 IHC resulted in strong immunoreactivity to Cereblon in DF15 (high Cereblon) and very faint immunoreactivity in the POM-resistant DF15R cells (low Cereblon). The immunoreactivity patterns of CRBN65 correlated with Western blot analysis such that CRBN65 showed a single strong band corresponding to full-length 51 kDa Cereblon protein in DF15 but only a very faint band in DF15R.1 Huang et al14 have previously described Cereblon expression as measured by IHC using the rabbit polyclonal Cereblon antibody from Proteintech (CRBN-PT) to correlate with clinical response to thalidomide and LEN-based treatment in MM patients. Here we repeated the IHC and Western blot analysis using the rabbit monoclonal CRBN65 alongside CRBN-PT for a side-by-side comparison. CRBN65 had strong cytoplasmic and nuclear immunoreactivity in DF15 and negligible immunoreactivity in DF15R by IHC (Figs. 1A, B). Conversely, IHC staining of DF15 and DF15R with CRBN-PT did not differentiate the cell lines and did not show nuclear immunoreactivity (Figs. 1C, D). The embedded table in Figure 1 shows the detailed cytoplasmic and nuclear H-sores of the IHC staining using CRBN65 and CRBN-PT. Western blot analyses using CRBN65 and CRBN-PT were repeated and shown in Figure 1E for direct comparison that correlated with IHC staining using respective antibodies. Confirming our previous results, CRBN-PT seems to detect multiple nonspecific bands in addition to the 51 kDa Cereblon protein by Western blot analysis.1
FIGURE 1.
Immunohistochemical (IHC) staining and Western blot analysis comparing Celgene CRBN65 antibody and Proteintech CRBN-PT in DF15 and DF15R cells. DF15 and DF15R IHC stained with CRBN65 (A, B) and CRBN-PT (C, D), respectively (objective ×40). Western blot analysis of DF15 and DF15R using CRBN65 and CRBN-PT antibodies (E). The embedded table shows the cytoplasmic and nuclear H-scores of DF15 and DF15R stained with CRBN65 and CRBN-PT.
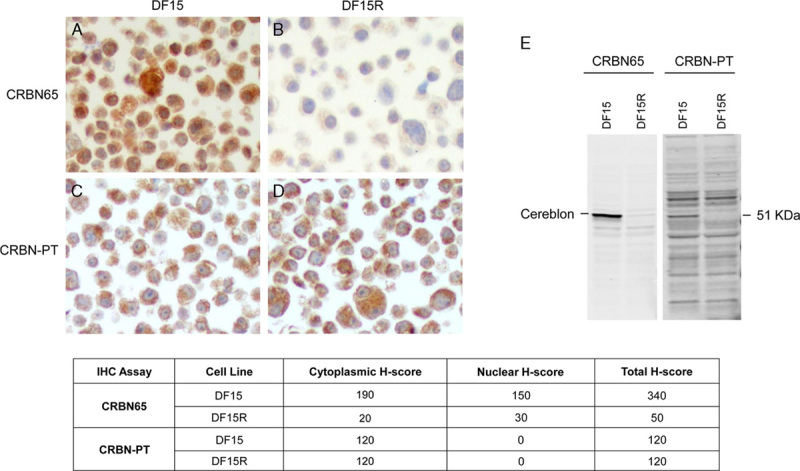
In addition, CRBN65 was more specific and accurate than CRBN-PT. We used a parental and shRNA-mediated knockdown of CRBN in stably transduced U266 MM cell lines that expressed medium to low amount of the protein. CRBN65 resulted in strong immunoreactivity in the parental U266 cell line, and medium and low levels in the CRBN shRNA medium (CRBNmedium) and low (CRBNlow) knockdown lines (Fig. 2A), which correlated with the corresponding Western blot analysis of U266 parental, CRBNmedium and CRBNlow cell lysates, respectively (Fig. 2B). In contrast, CRBN-PT immunoreactivity changed minimally in U266 CRBNlow cells compared with parental cell line (Fig. 2C), and Western blot analysis of parental and CRBNlow cell lysates using CRBN-PT yielded multiple strong bands in addition to the putative 51 kDa Cereblon protein band (Fig. 2D). The detailed H-scores of these cell pellets stained with CRBN65 and CRBN-PT are shown in the embedded table in Figure 2. These results demonstrated that CRBN65 is a highly specific antibody recognizing Cereblon and can accurately detect the different levels of Cereblon for the purpose of IHC analysis.
FIGURE 2.
Immunohistochemical (IHC) staining and Western blot analysis comparing Celgene CRBN65 and Proteintech CRBN-PT antibodies in U266 knockdown cell models. A, CRBN65 IHC in U266 parental, CRBN shRNA knockdown medium and low cell lines (objective ×40). B, Western blot of U266 parental, CRBN shRNA knockdown medium and low cell lines using CRBN65. C, CRBN-PT IHC in U266 parental and CRBN shRNA knockdown low cell lines (objective ×40). D, Western blot of U266 parental and CRBN shRNA knockdown low cell lines using CRBN-PT. The cytoplasmic and nuclear H-scores of the IHC staining of respective cell lines are shown in the embedded table.
Dual Color CRBN65/CD138 IHC Assay Precision
We determined assay precision of the dual CRBN65/CD138 IHC assay on 9 sequential sections of bone marrow from the same MM patient (MM13). Three separate runs were undertaken on 3 consecutive days, with each run consisting of 3 slides. Cytoplasmic and nuclear Cereblon H-scores from the 9 slides were generated by 1 pathologist (S.C.) and the mean and SD were calculated. The precision of the dual assay was expressed as a coefficient of variation (CV) calculated from the mean and the SD of the H-scores from the 9 slides across 3 runs. The CV values for cytoplasmic and nuclear H-scores were 5% and 2%, respectively (see Figure, Supplemental Digital Content 1, http://links.lww.com/AIMM/A83, which demonstrates dual CRBN65/CD138 IHC assay precision). This is within the Food and Drug Administration and National Cancer Institute guidelines for analytical assays that recommend CV values below 15%.16,17
CRBN65 Antibody Detection of a Range of Cereblon Levels in Various Human Normal Tissues
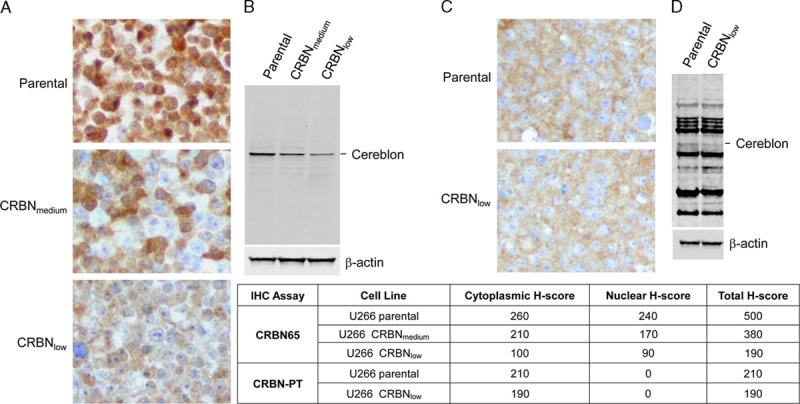
A tissue microarray MNO341 containing normal human tissues was stained with CRBN65 and CRBN-PT antibodies for comparison. The CRBN65 antibody was able to detect various Cereblon expression levels across different tissue types, whereas the CRBN-PT antibody showed overall weak immunoreactivity in all normal tissues tested. CRBN65 IHC showed strong Cereblon immunoreactivity in liver, and weak immunoreactivity in heart (Figs. 3A, B). In contrast CRBN-PT revealed only slightly higher Cereblon immunoreactivity in liver than in heart (Figs. 3C, D). Negative isotype controls are shown in Figures 3E and F. These results clearly show that CRBN65 had a broader detection range than CRBN-PT.
FIGURE 3.
Immunohistochemical (IHC) staining comparing Celgene CRBN65 and Proteintech CRBN-PT antibodies in normal human tissues. CRBN65 IHC in normal liver (A) and normal heart (B); CRBN-PT IHC in normal liver (C) and normal heart (D); rabbit IgG isotype control in normal liver (E) and normal heart (F) (objective ×40).
Dual Color CRBN65/CD138 IHC Assay Detects a Range of Cereblon Levels in MM Patient Samples
Twenty-two commercial MM patient samples (12 FFPE bone marrow core biopsies and 10 FFPE bone marrow aspirate clots) were stained with the dual color CRBN65/CD138 IHC assay and scored independently by 3 pathologists (S.C., D.E.H., and M.B.) using the H-score method. Myeloma core biopsies and aspirate clots yielded similar results using the same assay conditions. Both cytoplasmic and nuclear Cereblon H-scores were recorded for the CD138+ cells in each sample. A continuous range of Cereblon levels was detectable using this dual IHC assay in both cytoplasmic and nuclear compartments. The average cytoplasmic H-scores ranged from 63 to 267, average nuclear H-scores ranged from 17 to 250, and total H-score (sum of cytoplasmic and nuclear H-scores) ranged from 84 to 500 in the 22 MM cases evaluated (Table 2). Interobserver agreement among 3 pathologists was good, with average R2=0.73 (see Figure, Supplemental Digital Content 2, http://links.lww.com/AIMM/A84, which demonstrates H-score agreement between different pathologist pairs). MM bone marrow core biopsies with high, medium, and low Cereblon detected by the dual CRBN65/CD138, single CRBN65, and single CD138 assays are shown in Figure 4. The single CRBN-PT IHC did not detect nuclear Cereblon and was unable to demonstrate significant difference in cytoplasmic Cereblon immunoreactivity in the same MM cases with high, medium and low Cereblon levels (data not shown). Similarly, dual CRBN65 IHC assay detected high, medium, and low Cereblon in MM bone marrow aspirate clots (see Figure, Supplemental Digital Content 3, http://links.lww.com/AIMM/A85, which shows dual color CRBN65/CD138 IHC staining in MM aspirate clots). Single CRBN-PT IHC failed to yield the dynamic detection range in these clots (data not shown).
TABLE 2.
Cereblon H-scores of the Dual CRBN65/CD138 Immunohistochemical Assay in 22 Multiple Myeloma (MM) Patients
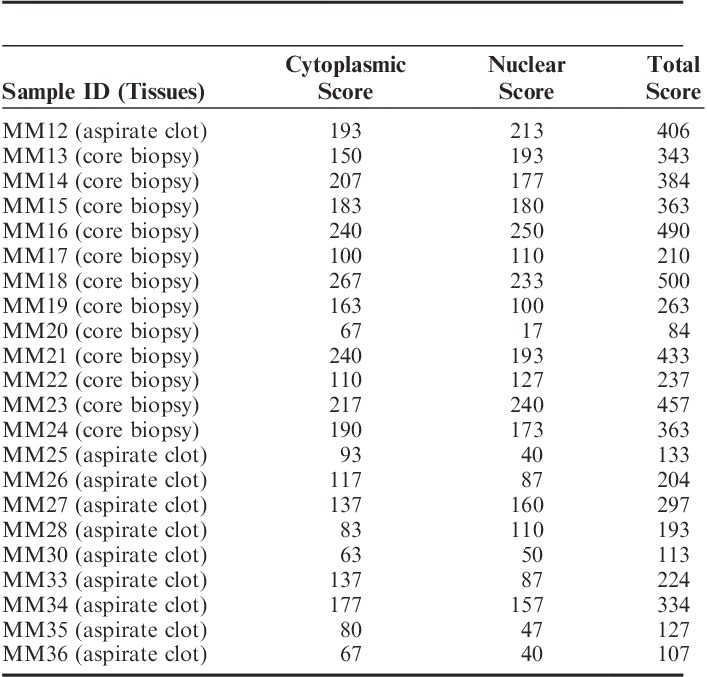
FIGURE 4.
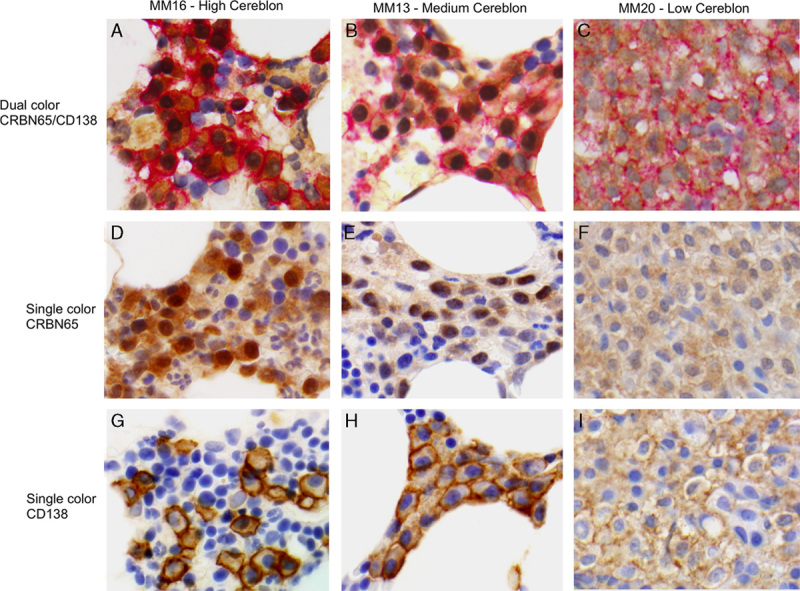
Representative images of multiple myeloma (MM) core biopsies stained with dual CRBN65/CD138, single CRBN65, and single CD138 immunohistochemical (IHC) assays. Dual CRBN65/CD138 IHC in MM16 with high Cereblon (A), MM13 with medium Cereblon (B), and MM20 with low Cereblon immunoreactivity (C). Single CRBN65 IHC in MM16 (D), MM13 (E), and MM20 (F). Single CD138 IHC in MM16 (G), MM13 (H), and MM20 (I) (objective ×60).
DISCUSSION
Following the identification of Cereblon as the primary target of the IMiD immunomodulatory compounds, researchers and clinicians have been searching for ways to detect Cereblon levels in patient tissue samples to understand its role in IMiD compounds treatment response and relapse. Recent publications have attempted to correlate high Cereblon protein or gene expression levels with more favorable clinical outcome of MM patients treated with thalidomide, LEN or POM-based regimens.10,12,14 However, results generated by comparing gene expression data with clinical response are questionable due to (1) lack of correlation between CRBN mRNA and protein levels and (2) complexity of CRBN gene expression due to the presence of splice variants some of which lack the drug binding region. Determining protein levels in MM cells has also posed many challenges. First, most commercially available antibodies for Cereblon IHC are not specific.1 Second, it is difficult to identify MM tumor cells in patient bone marrow biopsies or aspirate clots with low tumor density. IHC for the CD138 plasma cell surface marker has been used for identifying MM cells but locating the same cells in a serial section stained for Cereblon is challenging, especially in samples with a low density of tumor cells. Importantly, accurate MM tumor cell identification is essential in the case of MM patients because Cereblon is expressed by many normal bone marrow cell types.
To address these challenges, we have developed and validated a new, dual color, bright-field IHC assay using the highly specific CRBN65 antibody coupled with an anti-CD138 antibody, and scored using the H-score method for use on formalin-fixed, paraffin-embedded MM bone marrow clots and biopsies. The CRBN65 antibody showed specificity in DF15 (high Cereblon) and DF15R (low Cereblon), accurately reflected Cereblon protein levels in U266 CRBN knockdown cell lines, and demonstrated a good detection range in normal tissues (liver vs. heart). In addition, the CRBN65/CD138 dual IHC assay was able to detect a range of Cereblon staining intensity in 22 patient-derived MM core biopsies and aspirate clots. Conversely, the commercial CRBN-PT antibody was unable to detect differences in Cereblon immunoreactivity in DF15 versus DF15R, U266 CRBN shRNA cell model, normal tissues (liver vs. heart), or MM patient samples. These data indicate that Cereblon immunoreactivity detected using the CRBN-PT antibody do not represent accurate Cereblon protein levels. Furthermore, the ability to detect cytoplasmic as well as nuclear Cereblon with CRBN65 may help define the biological relevance of Cereblon cellular localization in future studies. We believe that the dual color CRBN65/CD138 IHC assay will allow for accurate assessment of Cereblon levels in MM patients. In this assay development we opted for the semiquantitative H-score method and found good interpathologist agreement for both cytoplasmic and nuclear immunoreactivity. Interpathologist correlation also improved after training using specific guidelines to illustrate staining intensity (data not shown). Finally, the dual IHC assay was developed for bone marrow core biopsies and aspirate clots fixed in 10% neutral buffered formalin, therefore antibody dilutions as well as other assay parameters may need to be adjusted for tissues fixed with other formalin containing fixatives.
In conclusion, data summarized here highlight the requirement for standardization in the evaluation of Cereblon expression in clinical samples. We validated a dual color, bright-field, CRBN65/CD138 IHC assay incorporating a semiquantitative H-score that is specific, accurate, and reliable in measuring Cereblon protein levels in bone marrow core biopsies and aspirate clots from MM patients. This dual IHC assay could be utilized to assess the potential value of Cereblon as a biomarker in MM patients treated with IMiD-based therapies.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.appliedimmunohist.com.
ACKNOWLEDGMENTS
The authors thank John Shaughnessy (University of Arkansas, Little Rock, AR) for the DF15 cell line. The authors thank Kenye Sebastian and Evelyn Diaz for their excellent technical assistance, and thank Ti Cai and James Hartke for helpful discussions and critical reading of the manuscript.
Footnotes
All authors except D.E.H. are employees of Celgene and all Celgene employees have equity ownership in Celgene Corporation. D.E.H. is a Celgene consultant.
REFERENCES
- 1.Gandhi AK, Mendy D, Waldman M, et al. Measuring cereblon as a biomarker of response or resistance to lenalidomide and pomalidomide requires use of standardized reagents and understanding of gene complexity. Br J Haematol. 2014;164:233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. [DOI] [PubMed] [Google Scholar]
- 4.Ito T, Ando H, Suzuki T, et al. Identification of a primary target of thalidomide teratogenicity. Science (New York, N Y). 2010;327:1345–1350. [DOI] [PubMed] [Google Scholar]
- 5.Zhu YX, Braggio E, Shi CX, et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood. 2011;118:4771–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Girona A, Mendy D, Ito T, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26:2326–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu G, Middleton RE, Sun H, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science (New York, N Y). 2014;343:305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kronke J, Udeshi ND, Narla A, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science (New York, N Y). 2014;343:301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandhi AK, Kang J, Havens CG, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN). Br J Haematol. 2014;164:811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klimowicz A, Neri P, Belch A, et al. High cereblon protein expression correlates with improved response and survival in myeloma patients treated with lenalidomide. Blood. 2012;120:931. [Abstract]. [Google Scholar]
- 11.Broyl A, Kuiper R, van DM, et al. High cereblon expression is associated with better survival in patients with newly diagnosed multiple myeloma treated with thalidomide maintenance. Blood. 2013;121:624–627. [DOI] [PubMed] [Google Scholar]
- 12.Heintel D, Rocci A, Ludwig H, et al. High expression of cereblon (CRBN) is associated with improved clinical response in patients with multiple myeloma treated with lenalidomide and dexamethasone. Br J Haematol. 2013;161:695–700. [DOI] [PubMed] [Google Scholar]
- 13.Schuster SR, Kortuem KM, Zhu YX, et al. The clinical significance of cereblon expression in multiple myeloma. Leuk Res. 2014;38:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang SY, Lin CW, Lin HH, et al. Expression of cereblon protein assessed by immunohistochemical staining in myeloma cells is associated with superior response of thalidomide- and lenalidomide-based treatment, but not bortezomib-based treatment, in patients with multiple myeloma. Ann Hematol. 2014;93:1371–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarty KS, Jr, Miller LS, Cox EB, et al. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985;109:716–721. [PubMed] [Google Scholar]
- 16.Guidelines for industry, text on validation of analytical procedures. ICH-Q2A, 1995. Available at: http://www.labcompliance.de/documents/international/ich/h-307-ich-fda-methods-terminology-ichq2a.pdf. Accessed March 20, 2014.
- 17.National Cancer Institute. Requirements for pharmacokinetic and biomarker methods development. 1999. Available at: http://prevention.cancer.gov/files/clinical-trials/biomarkers.pdf. Accessed March 20, 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.appliedimmunohist.com.


