Abstract
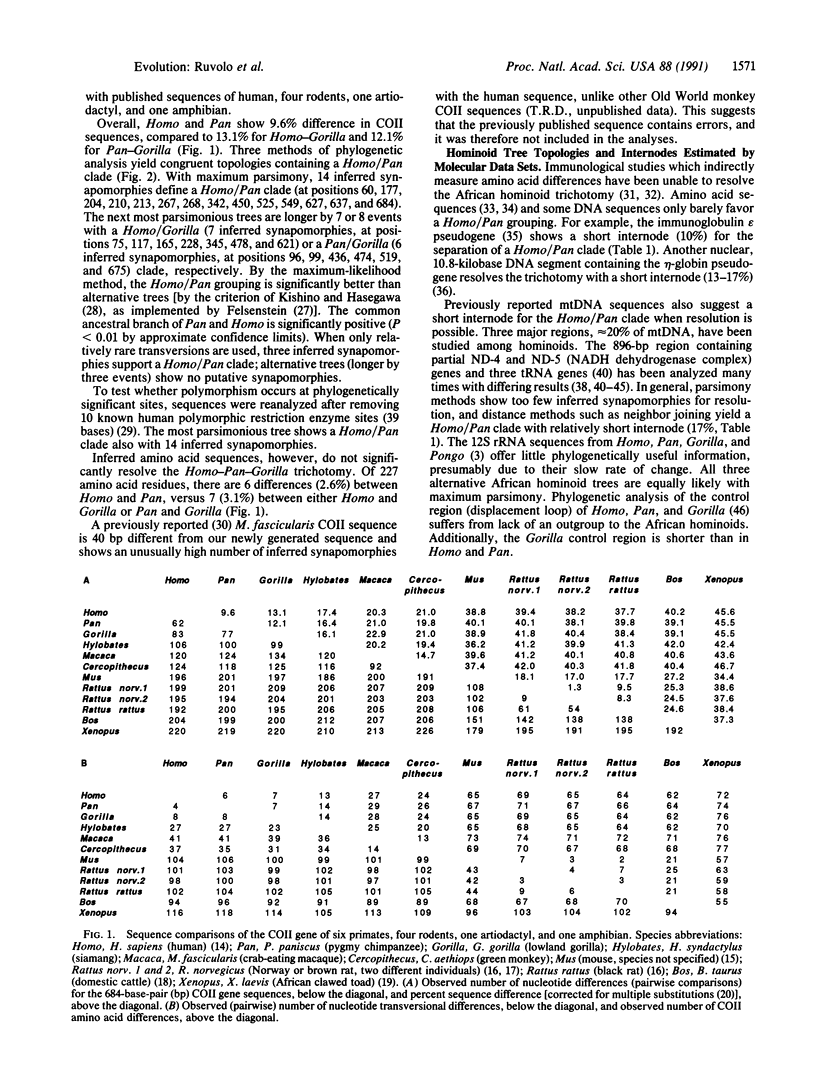
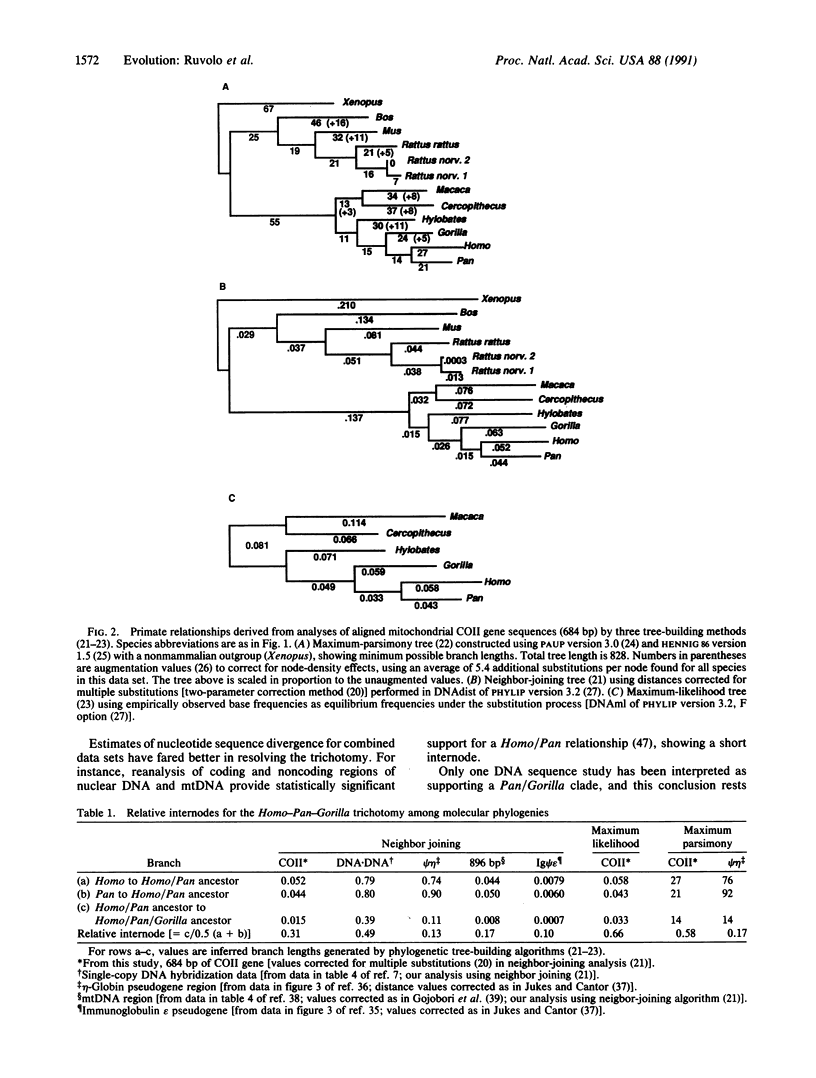
Mitochondrial DNA sequences encoding the cytochrome oxidase subunit II gene have been determined for five primate species, siamang (Hylobates syndactylus), lowland gorilla (Gorilla gorilla), pygmy chimpanzee (Pan paniscus), crab-eating macaque (Macaca fascicularis), and green monkey (Cercopithecus aethiops), and compared with published sequences of other primate and nonprimate species. Comparisons of cytochrome oxidase subunit II gene sequences provide clear-cut evidence from the mitochondrial genome for the separation of the African ape trichotomy into two evolutionary lineages, one leading to gorillas and the other to humans and chimpanzees. Several different tree-building methods support this same phylogenetic tree topology. The comparisons also yield trees in which a substantial length separates the divergence point of gorillas from that of humans and chimpanzees, suggesting that the lineage most immediately ancestral to humans and chimpanzees may have been in existence for a relatively long time.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Brown G. G., Simpson M. V. Novel features of animal mtDNA evolution as shown by sequences of two rat cytochrome oxidase subunit II genes. Proc Natl Acad Sci U S A. 1982 May;79(10):3246–3250. doi: 10.1073/pnas.79.10.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M. Polymorphism in mitochondrial DNA of humans as revealed by restriction endonuclease analysis. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3605–3609. doi: 10.1073/pnas.77.6.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M., Prager E. M., Wang A., Wilson A. C. Mitochondrial DNA sequences of primates: tempo and mode of evolution. J Mol Evol. 1982;18(4):225–239. doi: 10.1007/BF01734101. [DOI] [PubMed] [Google Scholar]
- Cann R. L., Brown W. M., Wilson A. C. Polymorphic sites and the mechanism of evolution in human mitochondrial DNA. Genetics. 1984 Mar;106(3):479–499. doi: 10.1093/genetics/106.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann R. L., Stoneking M., Wilson A. C. Mitochondrial DNA and human evolution. Nature. 1987 Jan 1;325(6099):31–36. doi: 10.1038/325031a0. [DOI] [PubMed] [Google Scholar]
- Djian P., Green H. Vectorial expansion of the involucrin gene and the relatedness of the hominoids. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8447–8451. doi: 10.1073/pnas.86.21.8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981;17(6):368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Bruschi M. The evolution of prokaryotic ferredoxins--with a general method correcting for unobserved substitutions in less branched lineages. Mol Biol Evol. 1987 Jul;4(4):381–394. doi: 10.1093/oxfordjournals.molbev.a040452. [DOI] [PubMed] [Google Scholar]
- Foran D. R., Hixson J. E., Brown W. M. Comparisons of ape and human sequences that regulate mitochondrial DNA transcription and D-loop DNA synthesis. Nucleic Acids Res. 1988 Jul 11;16(13):5841–5861. doi: 10.1093/nar/16.13.5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODMAN M. Immunochemistry of the primates and primate evolution. Ann N Y Acad Sci. 1962 Dec 28;102:219–234. doi: 10.1111/j.1749-6632.1962.tb13641.x. [DOI] [PubMed] [Google Scholar]
- Gojobori T., Ishii K., Nei M. Estimation of average number of nucleotide substitutions when the rate of substitution varies with nucleotide. J Mol Evol. 1982;18(6):414–423. doi: 10.1007/BF01840889. [DOI] [PubMed] [Google Scholar]
- Gonzalez I. L., Sylvester J. E., Smith T. F., Stambolian D., Schmickel R. D. Ribosomal RNA gene sequences and hominoid phylogeny. Mol Biol Evol. 1990 May;7(3):203–219. doi: 10.1093/oxfordjournals.molbev.a040600. [DOI] [PubMed] [Google Scholar]
- Goodman M., Braunitzer G., Stangl A., Schrank B. Evidence on human origins from haemoglobins of African apes. Nature. 1983 Jun 9;303(5917):546–548. doi: 10.1038/303546a0. [DOI] [PubMed] [Google Scholar]
- Goodman M., Koop B. F., Czelusniak J., Fitch D. H., Tagle D. A., Slightom J. L. Molecular phylogeny of the family of apes and humans. Genome. 1989;31(1):316–335. doi: 10.1139/g89-050. [DOI] [PubMed] [Google Scholar]
- Goodman M., Tagle D. A., Fitch D. H., Bailey W., Czelusniak J., Koop B. F., Benson P., Slightom J. L. Primate evolution at the DNA level and a classification of hominoids. J Mol Evol. 1990 Mar;30(3):260–266. doi: 10.1007/BF02099995. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M., Kishino H., Yano T. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol. 1985;22(2):160–174. doi: 10.1007/BF02101694. [DOI] [PubMed] [Google Scholar]
- Hayasaka K., Gojobori T., Horai S. Molecular phylogeny and evolution of primate mitochondrial DNA. Mol Biol Evol. 1988 Nov;5(6):626–644. doi: 10.1093/oxfordjournals.molbev.a040524. [DOI] [PubMed] [Google Scholar]
- Heidecker G., Messing J., Gronenborn B. A versatile primer for DNA sequencing in the M13mp2 cloning system. Gene. 1980 Jun;10(1):69–73. doi: 10.1016/0378-1119(80)90145-6. [DOI] [PubMed] [Google Scholar]
- Hixson J. E., Brown W. M. A comparison of the small ribosomal RNA genes from the mitochondrial DNA of the great apes and humans: sequence, structure, evolution, and phylogenetic implications. Mol Biol Evol. 1986 Jan;3(1):1–18. doi: 10.1093/oxfordjournals.molbev.a040379. [DOI] [PubMed] [Google Scholar]
- Holmquist R., Miyamoto M. M., Goodman M. Analysis of higher-primate phylogeny from transversion differences in nuclear and mitochondrial DNA by Lake's methods of evolutionary parsimony and operator metrics. Mol Biol Evol. 1988 May;5(3):217–236. doi: 10.1093/oxfordjournals.molbev.a040494. [DOI] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980 Dec;16(2):111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Kishino H., Hasegawa M. Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in hominoidea. J Mol Evol. 1989 Aug;29(2):170–179. doi: 10.1007/BF02100115. [DOI] [PubMed] [Google Scholar]
- Koop B. F., Tagle D. A., Goodman M., Slightom J. L. A molecular view of primate phylogeny and important systematic and evolutionary questions. Mol Biol Evol. 1989 Nov;6(6):580–612. doi: 10.1093/oxfordjournals.molbev.a040574. [DOI] [PubMed] [Google Scholar]
- Miyamoto M. M., Slightom J. L., Goodman M. Phylogenetic relations of humans and African apes from DNA sequences in the psi eta-globin region. Science. 1987 Oct 16;238(4825):369–373. doi: 10.1126/science.3116671. [DOI] [PubMed] [Google Scholar]
- Pamilo P., Nei M. Relationships between gene trees and species trees. Mol Biol Evol. 1988 Sep;5(5):568–583. doi: 10.1093/oxfordjournals.molbev.a040517. [DOI] [PubMed] [Google Scholar]
- Ramharack R., Deeley R. G. Structure and evolution of primate cytochrome c oxidase subunit II gene. J Biol Chem. 1987 Oct 15;262(29):14014–14021. [PubMed] [Google Scholar]
- Roe B. A., Ma D. P., Wilson R. K., Wong J. F. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 1985 Aug 15;260(17):9759–9774. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarich V. M., Wilson A. C. Immunological time scale for hominid evolution. Science. 1967 Dec 1;158(3805):1200–1203. doi: 10.1126/science.158.3805.1200. [DOI] [PubMed] [Google Scholar]
- Sibley C. G., Ahlquist J. E. DNA hybridization evidence of hominoid phylogeny: results from an expanded data set. J Mol Evol. 1987;26(1-2):99–121. doi: 10.1007/BF02111285. [DOI] [PubMed] [Google Scholar]
- Sibley C. G., Ahlquist J. E. The phylogeny of the hominoid primates, as indicated by DNA-DNA hybridization. J Mol Evol. 1984;20(1):2–15. doi: 10.1007/BF02101980. [DOI] [PubMed] [Google Scholar]
- Sibley C. G., Comstock J. A., Ahlquist J. E. DNA hybridization evidence of hominoid phylogeny: a reanalysis of the data. J Mol Evol. 1990 Mar;30(3):202–236. doi: 10.1007/BF02099992. [DOI] [PubMed] [Google Scholar]
- Tseng H., Green H. The involucrin gene of the owl monkey: origin of the early region. Mol Biol Evol. 1989 Sep;6(5):460–468. doi: 10.1093/oxfordjournals.molbev.a040563. [DOI] [PubMed] [Google Scholar]
- Ueda S., Watanabe Y., Saitou N., Omoto K., Hayashida H., Miyata T., Hisajima H., Honjo T. Nucleotide sequences of immunoglobulin-epsilon pseudogenes in man and apes and their phylogenetic relationships. J Mol Biol. 1989 Jan 5;205(1):85–90. doi: 10.1016/0022-2836(89)90366-5. [DOI] [PubMed] [Google Scholar]
- Van Valen L. M. Our ancestors. Nature. 1989 Mar 23;338(6213):304–304. doi: 10.1038/338304b0. [DOI] [PubMed] [Google Scholar]
- Williams S. A., Goodman M. A statistical test that supports a human/chimpanzee clade based on noncoding DNA sequence data. Mol Biol Evol. 1989 Jul;6(4):325–330. doi: 10.1093/oxfordjournals.molbev.a040556. [DOI] [PubMed] [Google Scholar]



