Abstract
In this issue of Blood, using an elegant zebrafish-based model, Rossi et al demonstrated that vascular endothelial growth factor B (VEGFB), VEGFD, and placenta growth factor (PlGF) are able to sustain vascularization in the absence of VEGFA and generated dominant-negative VEGF mutants, thereby identifying new antiangiogenic strategies. There are multiple important points in this article which not only change our current understanding of the mechanisms of vascular growth in a complexity of vivo settings, but also advance the search for promising therapeutic approaches seeking to interfere with pathological vascularization.1
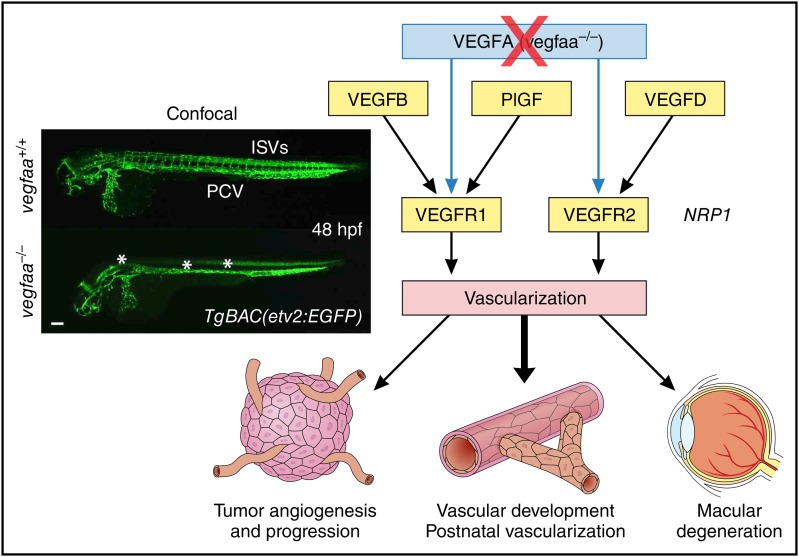
Results from zebrafish model demonstrate that VEGFB, PlGF, and VEGFD can compensate for the lack of VEGFA and solidify roles of individual VEGFRs in vivo, thereby opening new directions in treatment of human pathologies associated with vascularization. The inset has been adapted from Figure 1F in the article by Rossi et al that begins on page 2359, and shows confocal micrographs of 48-hpf TgBAC(etv2:EGFP) WT siblings and vegfaabns1 mutant zebrafish embryos in lateral view. Asterisks denote lack of ISVs, DA, and reduced CtA sprouting. CtA, central artery; DA, dorsal aorta; hpf, hours postfertilization; ISV, intersegmental vessel; PCV, posterior cardinal vein. Professional illustration by Patrick Lane, ScEYEnce Studios.
Discovery of VEGFA as a key permeability and angiogenic factor produced by tumors and driving excessive pathological vasculature created a new front in the battle against cancer and other diseases associated with hypervascularization.2,3 The prominent role of VEGFA in developmental vascular growth is underscored by severe defects leading to embryonic lethality caused by the loss of a single allele of VEGFA.4 Successful targeting of VEGFA with neutralizing antibodies is generally viewed as revolutionary and a turning point in antiangiogenic therapy.3 There are, however, situations when cancer develops resistance to VEGF inhibition, often by utilizing alternative proangiogenic pathways.5 VEGFA and other VEGF family members act through main receptors on the endothelial cells of blood vessels: VEGF receptor 1 (VEGFR1), VEGFR2, and Neuropilin 1 (NRP1) (see figure). Despite decades of research, the functional redundancy and exact in vivo roles of individual VEGF family members and their receptors is either unclear or controversial.6,7
Accordingly, Rossi et al aimed to assess whether and how various members of the VEGF family are able to support vascular development when VEGFA activity is disrupted. To this end, using a contemporary state-of-the-art genetic approach, Rossi et al created an efficient model by mutating vegfaa and vegfab in zebrafish where vasculature is visualized by enhanced green fluorescent protein (EGFP) expression. Thorough characterization of these mutants revealed that inactivation of vegfaa resulted in a number of severe vascular defects eventually causing lethality. This recapitulated the consequences of VEGFA loss in mammals,4 whereas the phenotype of the vegfab mutant was substantially milder. Injection of messenger RNA (mRNA) encoding Vegfaa-121 and -165 rescued the gross defects in arteriogenesis but not the formation of intersegmental vessels. This allowed the authors to conclude that Vegfaa might be dispensable in adult zebrafish, and then use the resulting mutants as a powerful tool for assessing the angiogenic redundancy of VEGF family members in physiologically appropriate settings. Thus, Rossi et al created and validated a new in vivo system for even wider screening of potentially proangiogenic molecules that are able to bypass the requirement for VEGFA. It is remarkable that in this system, vegfd, but not vegfc, was able to rescue vascular defects via VEGFR2.
Another important finding is that growth factors Pgfb (analog of mammalian PlGF) and Vegfbb (analog of mammalian VEGFB), which are dispensable for vascular development,8 were able to compensate for missing Vegfaa. Therefore, targeting the PlGF/VEGFR1 pathway during VEGFA blockade seems to be well supported by in vivo genetic evidence, thereby addressing yet another controversial topic.8,9 Furthermore, using a series of Vegfaa mutants generated based on the crystal structure of VEGF-VEGFR2 and VEGF-NRP1 complexes, Rossi et al established the key role for Vegfaa-VEGFR2 interaction rather than the Vegfaa-Nrp1 axis in vasculogenesis. These experiments used a combination of genetic approaches in vivo with a structure-function analysis of growth factor interactions with their respective receptors, and thereby provided a mechanism to answer important questions in the field of vascular biology, including but not limited to the relative contributions of VEGFR2 vs Nrp1 and the role of Vegfaa-165 vs Vegfaa-121 in vascular development. Notably, similar experiments in mammals are either extremely time-consuming or practically impossible. Because these conclusions are derived from a well-justified in vivo model with distinctive lack of bias, their interpretation allows the settling of a number of previous controversies. Therefore, the importance of the results described by Rossi et al is impossible to overestimate.
Separate attention needs to be given to the described role of VEGFR1 in the rescue of vegfaa mutants. Not only wild-type (WT) but also a mutant form of Pgfb that is unable to interact with VEGFR2 or Nrp1 rescued vascularization defects, emphasizing the key role for VEGFR1 in Pgfb signaling. After pinning down the role of individual VEGFRs in vascularization, it is tempting to speculate that the targeting of kinase activity in these receptors to block angiogenesis might present a certain advantage. However, besides rather diffuse specificity of many tyrosine kinase inhibitors, there is another potential problem with this approach. In mammals, endothelial VEGF operates via a VEGFR2-dependent autocrine loop, supporting survival and homeostasis of the endothelium itself.10 Considering this mechanism, extracellular VEGF neutralization or trapping seems to be safe, whereas the complete blockade of VEGFR2 tyrosine kinase activity by intracellular inhibitors is expected to cause endothelial damage and thrombotic complications. Again, Rossi et al scored big by constructing a dominant-negative “trap” for Vegfaa, which abolished vascularization in WT embryos and, most remarkably, interfered with the activity of other VEGF family members.
One might argue that zebrafish studies cannot be perfectly translated to mammalian biology and even less so to human biology. However, only comprehensive functional in vivo screening like the one described by Rossi et al will yield “true” hits and such comprehensive screening is almost impossible to conduct in mammal models. Obviously, validation of the chosen candidates in mammalian models will certainly solidify their therapeutic value. In any scenario, these zebrafish tools and newly identified VEGF forms have enormous potential for both vascular biology research and the design of new proangiogenic as well as antiangiogenic strategies in humans.
Footnotes
Conflict-of-interest disclosure: The author declares no competing financial interests.
REFERENCES
- 1.Rossi A, Gauvrit S, Marass M, Pan L, Moens CB, Stainier DYR. Regulation of Vegf signaling by natural and synthetic ligands. Blood. 2016;128(19):2359–2366. doi: 10.1182/blood-2016-04-711192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dewhirst MW, Ashcraft KA. Implications of increase in vascular permeability in tumors by VEGF: a commentary on the pioneering work of Harold Dvorak. Cancer Res. 2016;76(11):3118–3120. doi: 10.1158/0008-5472.CAN-16-1292. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS. 2005;(94) doi: 10.1007/3-7643-7311-3_15. 209-231. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N, Carver-Moore K, Chen H, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380(6573):439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 5.Bartolotti M, Franceschi E, Poggi R, Tosoni A, Di Battista M, Brandes AA. Resistance to antiangiogenic therapies. Future Oncol. 2014;10(8):1417–1425. doi: 10.2217/fon.14.57. [DOI] [PubMed] [Google Scholar]
- 6.Fischer C, Jonckx B, Mazzone M, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131(3):463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 7.Bais C, Wu X, Yao J, et al. PlGF blockade does not inhibit angiogenesis during primary tumor growth. Cell. 2010;141(1):166–177. doi: 10.1016/j.cell.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 8.Luttun A, Tjwa M, Carmeliet P. Placental growth factor (PlGF) and its receptor Flt-1 (VEGFR-1): novel therapeutic targets for angiogenic disorders. Ann N Y Acad Sci. 2002;979:80–93. doi: 10.1111/j.1749-6632.2002.tb04870.x. [DOI] [PubMed] [Google Scholar]
- 9.Malik AK, Baldwin ME, Peale F, et al. Redundant roles of VEGF-B and PlGF during selective VEGF-A blockade in mice. Blood. 2006;107(2):550–557. doi: 10.1182/blood-2005-05-2047. [DOI] [PubMed] [Google Scholar]
- 10.Lee S, Chen TT, Barber CL, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130(4):691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]