Key Points
Deletion of the erythroid enhancer of Bcl11a from the mouse genome does not affect viability or Bcl11a expression in nonerythroid lineages.
Elevated levels of γ-globin in Bcl11a enhancer–deleted mice are comparable to those in erythroid-specific Bcl11a gene knockout mice.
Abstract
BCL11A, a repressor of human fetal (γ-)globin expression, is required for immune and hematopoietic stem cell functions and brain development. Regulatory sequences within the gene, which are subject to genetic variation affecting fetal globin expression, display hallmarks of an erythroid enhancer in cell lines and transgenic mice. As such, this enhancer is a novel, attractive target for therapeutic gene editing. To explore the roles of such sequences in vivo, we generated mice in which the orthologous 10-kb intronic sequences were removed. Bcl11a enhancer–deleted mice, Bcl11a(Δenh), phenocopy the BCL11A-null state with respect to alterations of globin expression, yet are viable and exhibit no observable blood, brain, or other abnormalities. These preclinical findings provide strong in vivo support for genetic modification of the enhancer for therapy of hemoglobin disorders.
Introduction
The transcriptional repressor BCL11A is a principal regulator of the developmental switch from γ- to β-globin accompanying the fetal to adult erythropoiesis transition. Increased γ-globin reduces the clinical severity of the β-hemoglobinopathies, sickle-cell disease, and β-thalassemia caused by mutation or decreased expression of β-globin, respectively. Reactivation of γ-globin beyond the residual ∼1% fetal hemoglobin (HbF) (α2γ2) in normal adults is the most attractive therapeutic strategy. Genome-wide association studies identified BCL11A as a modifier of HbF level.1-3 Subsequent experiments in conditional mouse mutants and CD34+ primary human hematopoietic stem/progenitor cells validated BCL11A as a potent γ-globin repressor.4,5 In contrast to other regulators of γ-globin (GATA1, KLF1, and MYB6-8), BCL11A is dispensable for erythropoiesis and, in principle, is manipulatable for therapy. BCL11A in mice, however, is required for immune cells, hematopoietic stem cell (HSC) function and brain development.9-15 Rare patients who are haploinsufficient for BCL11A have appreciably increased HbF levels, but no other apparent hematopoietic disturbances, although they suffer from intellectual disability.16,17
The genome-wide association study–identified single-nucleotide polymorphisms associated with HbF levels reside within intron-2 of BCL11A. This region exhibits hallmarks of potential regulatory sequences, including DNase I hypersensitivity, characteristic epigenetic signature, and erythroid transcription factor binding.18 Human sequences (12 kb) from the intron function as an erythroid enhancer in human erythroid cell lines and transgenic mice.18,19 Deletion of the orthologous 10-kb mouse sequences in erythroleukemia cells leads to profound reduction in Bcl11a expression, whereas no effect is apparent in a Pro B-cell line. Whereas the human region has 3 DNase I hypersensitive sites (DHSs), the corresponding mouse region has 2. Additionally, the dominant functional sequences differ between the species. The human +58DHS is most potent, whereas in mouse it is the +62DHS ortholog.19
Primarily on the basis of ex vivo findings attesting to the specificity of enhancer sequences, the region has been proposed as a target for therapeutic gene editing in genetic management of the hemoglobin disorders. Potential requirements of the enhancer for nonerythroid cellular functions within or beyond the hematopoietic system cannot be formally excluded by prior cell studies. Although the human and mouse enhancers are evolutionarily and structurally distinct within the composite element, their general function as an erythroid regulatory element appears conserved. Here we present in vivo characterization of the mouse Bcl11a intronic enhancer element that demonstrates strict specificity for the erythroid lineage.
Study design
All animal experiments were approved by the Boston Children’s Hospital Institutional Animal Care and Use Committee. Enhancer deletion [Bcl11a (Δenh)] or single +62DHSΔ mice were made through modification of CJ9 mouse embryonic stem cells (ESCs) by transient cotransfection of pX330-Cas9 plasmids (Addgene #80445) containing guide RNA sequences 5′-CCAGGAGCCTCAAGCAAGCC-3′ and 5′-CGACTGGCTGATGACATCAC-3′ or 5′-TCGCTGCCTTCAGTTCTGCT-3′ and 5′-CTATTTCCATTGGTGGATAC-3′, respectively. Chimeric mice were bred with females transgenic for the entire human β-globin gene locus (β-YAC).20
Neonates were transcardially perfused with formaldehyde and brains prepared for immunocytochemistry as previously described.13
Embryonic fetal livers from timed matings, adult peripheral blood from tail vein, and adult bone marrow (BM) were collected, prepared for flow cytometry or RNA extraction, and analyzed as previously described.15,21 Whole BM was transplanted into lethally irradiated first-generation B6Sjl mice (CD45.1; Taconic) crossed with 129SVE (CD45.2; Taconic) hybrids.
Results and discussion
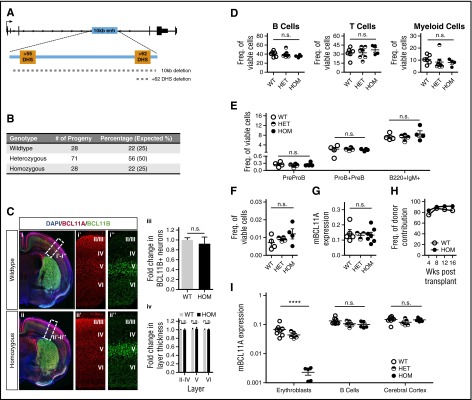
Mouse ESCs with deletion of the 10-kb enhancer were used to generate germ-line transmitting chimeric mice (Figure 1A). In contrast to Bcl11a gene knockout mice that die as neonates,9 mice homozygous for the enhancer deletion allele [Bcl11a(Δenh)] were viable (Figure 1B). Bcl11a(Δenh) mice were indistinguishable from wild-type littermates. Male and female Bcl11a(Δenh) mice bred unremarkably, and mothers were able to nurse pups to maturity, indicating functional mammary development.22 To further explore any extrahematopoietic effects, neonatal brains were analyzed by immunocytochemistry. Importantly, Bcl11a expression was unchanged in the neocortex of Bcl11a(Δenh) mice (Figure 1C). Moreover, although Bcl11a null mice exhibit defects in cortical projection neuron subtype identity, leading to altered thickness of cortical layers V and VI,12,13 these abnormalities are not present in Bcl11a(Δenh) mice.
Figure 1.
Loss of the Bcl11a erythroid enhancer has no effect outside of the erythroid compartment. (A) Diagram of the murine Bcl11a locus showing the location of the intronic 10-kb element and the 2 orthologous DHSs. Dashed lines indicate position of the deletions made in the Bcl11a(Δenh) and single +62DHS deletion mouse lines. (B) Bcl11a(Δenh) mice are viable. Heterozygous enhancer deleted female and male mice were bred together. Numbers of combined progeny and their genotypes from these breedings were tallied. Expected percentages based on normal Mendelian genetics are indicated in parentheses. (C) The integrity of the cortical and subcortical organization of Bcl11a(Δenh) mice is maintained. At postnatal day 4 (P4), mice were perfused with 4% paraformaldehyde for preparation of brain specimens. (i and ii) After sectioning, tissue was stained for nuclei (DAPI-blue), BCL11A-red (mouse anti-BCL11A clone 14B5 [Abcam]), and BCL11B-green (rat anti-BCL11B [Abcam]). (i’ and ii’) BCL11A expression in Bcl11a(Δenh) mice is comparable to wild-type levels. (i’’ and ii”) The intensity of BCL11B staining establishes the boundary between neocortical layers V and VI. (iii) The number of BCL11B positive neurons is unaffected by the loss of the Bcl11a enhancer. (iv) Quantification of the thickness of the BCL11B defined cortical layers indicates no significant difference in the depth of each layer. Cells positive for indicated marker(s) were counted in a box of predefined size and applied to both wild-type and Bcl11a(Δenh) single confocal slices. Each layer was defined as a percentage of the total cortical thickness. Sections were mounted using DAPI-Fluoromount G (Southern Biotech) and images obtained at room temperature by a Zeiss AxioCam MRm camera attached to a Zeiss LSM 700 microscope using ZEN Black 2011 acquisition software (Zeiss). Images were processed for brightness and cropped using Photoshop. All transformations were applied evenly across all images. (D) Mature blood cell production and HSCs were unaffected by the loss of the 10-kb Bcl11a enhancer. Peripheral blood was harvested from the tail veins of 4-month-old animals and analyzed by flow cytometry for B cells (B220+/CD19+), T cells (CD3e+/NK1-1−), and myeloid cells (Gr-1+/Mac1+). (E) Bcl11a(Δenh) mice had normal numbers of B-cell progenitors (PreProB: B220+, CD43+, IgM−, AA4-1+, CD19−; ProB+PreB: B220+, CD43+, IgM−, AA4-1+, CD19+) and differentiated B cells (B220+, IgM+). (F) Sixteen-week Bcl11a(Δenh) mice have normal BM HSC frequencies as defined by Lineage−, Sca-1+, c-kit+ CD48−, Flt3−, and CD150+. (G) Bcl11a expression in HSCs does not change in Bcl11a(Δenh) mice. Greater than 5000 HSCs (Lineage−, Sca-1+, c-kit+ CD48−, and CD150high) were sorted from 32- to 35-week-old mouse BM and RNA isolated. Quantitative polymerase chain reaction (qPCR) shows no difference in Bcl11a expression between the genotypes when normalized to GAPDH. (H) Bcl11a(Δenh) HSCs are functional. Whole BM was isolated from either wild-type (CD45.2) or Bcl11a(Δenh) (CD45.2) mice and transplanted into lethally irradiated recipients (CD45.1/.2). Engraftment was measured as the CD45.2+ percentage of total viable cells. (I) The 10-kb enhancer only affects erythroid Bcl11a transcription. Date of vaginal plug detection was designated E0.5. RNA analysis of Bcl11a expression in embryonic day 16.5 (E16.5) tissues shows decreased Bcl11a expression in the erythroid compartment of homozygous Bcl11a(Δenh) mice, but not in B cells or brain tissue. (Data are represented as mean ± standard error of the mean; ****P < .0001; n.s., not significant; all conditions represent n ≥ 4). DAPI, 4′,6-diamidino-2-phenylindole.
Bcl11a is expressed in several hematopoietic lineages. We first assessed the distribution of the mature hematopoietic lineages in the peripheral blood of adult mice using flow cytometry. There were no significant differences between wild-type and Bcl11a(Δenh) mice (Figure 1D). Most relevant, B-cell numbers were unaffected by enhancer loss. In addition, analysis of the (BM) lymphoid compartment revealed normal distribution of differentiating B-cell progenitors and HSC frequency (Figure 1E-F). Bcl11a RNA expression was unchanged in Bcl11a(Δenh) HSCs (Figure 1G). To interrogate the fitness of Bcl11a(Δenh) HSCs, whole BM cells were transplanted into lethally irradiated recipient mice. Engraftment was >90% 16 weeks after transplantation (Figure 1H). Under similar conditions, Bcl11a−/− HSCs exhibited poor contribution to hematopoiesis with barely detectable BM donor chimerism at 13 weeks posttransplant.10,11,15
To determine the extent to which Bcl11a expression in different lineages is dependent on the enhancer, we obtained embryonic day 16.5 (E16.5) wild-type, heterozygous, and homozygous Bcl11a(Δenh) fetuses. B cells and erythroid cells were sorted from the fetal liver based on double positive staining for CD19/B220 or CD71/Ter119, respectively. In addition, cortex tissue was dissected from the fetal brain. Bcl11a expression in erythroid cells of homozygous Bcl11a(Δenh) mice was reduced >30-fold (Figure 1I). In stark contrast, Bcl11a RNA expression was unchanged in cortex and sorted B cells upon enhancer deletion.
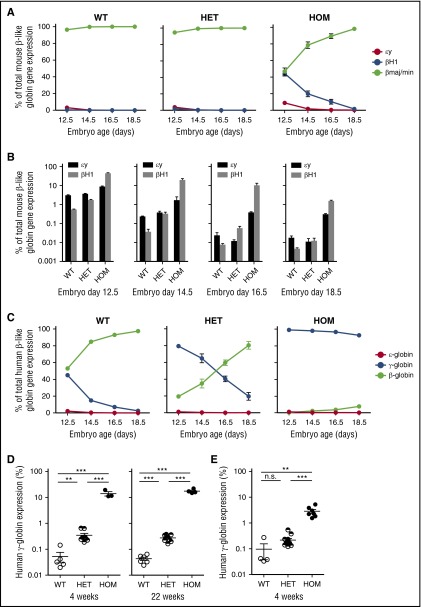
To evaluate the consequence of deleting the 10-kb enhancer on mouse globin gene expression, we harvested fetal liver at several developmental stages. RNA analysis revealed a delay in the silencing of the 2 embryonic globin genes, εy and βH1 (Figure 2A-B). To assess the effect of enhancer deletion on human globin gene levels in an in vivo mouse model, we bred Bcl11a(Δenh) mice to mice transgenic for the entire human β-globin gene locus (β-YAC).20 During normal β-YAC mouse development, γ-globin is expressed in yolk sac and silenced by E16.5. Analysis of Bcl11a(Δenh) embryos showed an absence of γ-globin gene silencing during development (Figure 2C). At 4 and 22 weeks, γ-globin was silenced to 20% of total human globin expression; however, wild-type β-YAC mice expressed 100-fold less γ-globin (Figure 2D). These derepressed levels were similar to those seen in conditional gene knockout mice.21 Mice harboring a deletion of the +62DHS, which accounts for a substantial portion of enhancer function in a murine erythroid cell line, exhibited less robust repression of γ-globin, suggesting that the remaining upstream +55DHS in the mouse enhancer contributes to overall enhancer activity (Figure 2E).
Figure 2.
Deletion of erythroid specific enhancer sequences delays embryonic and fetal globin gene silencing. (A) Fetal liver was dissected from indicated days of embryonic development. qPCR was done on RNA from the dissected tissue. Globin genes were normalized to GAPDH expression. Delayed hemoglobin switching of the mouse embryonic globins εy and βH1 was observed in Bcl11a(Δenh) mice. (B) Derepression of mouse embryonic globins (εy and βH1) in developmental erythropoiesis as assessed by qPCR. (C) Bcl11a(Δenh) mice were mated with β-YAC mice in order to evaluate human globin gene expression. Bcl11a(Δenh) mice failed to silence γ-globin gene expression during embryonic development. (D) qPCR analysis of RNA from 4-week and 22-week adult mice peripheral blood showed incomplete silencing of γ-globin. (E) Deletion of the in vitro defined core enhancer (+62DHS) region also fails to completely silence γ-globin; however, derepression is not to the level seen in Bcl11a(Δenh) mice. (Data are represented as mean ± standard error of the mean; ***P < .001; **P < .01; n.s., not significant; all conditions represent n ≥ 4.)
The data presented here establish exquisite erythroid in vivo lineage specificity of the Bcl11a intronic enhancer. Sequences within the 10-kb region are not required for proper brain or B-cell development. Most notable is the ostensibly normal development and function of HSCs in the absence of the intronic enhancer. These findings are encouraging with respect to the proposed strategy of genome editing of the Bcl11a enhancer in the context of HSCs for induction of HbF in the major hemoglobinopathies.
One caveat to our findings is that, by necessity, in vivo studies have assessed the specificity of the in situ mouse Bcl11a enhancer, rather than that of human. Although the detailed architectures of the mouse and human enhancers differ,19 evidence to this point indicates that the orthologous regions subserve similar functions for the species. The human 12-kb enhancer directs lineage- and stage-restricted expression in transgenic mice, providing support for functional conservation.18 The current studies constitute comprehensive examination of regulatory sequences as part of preclinical assessment of potential risks associated with gene editing of the BCL11A enhancer for therapy of the β-hemoglobinopathies.
Acknowledgments
The authors thank the Dana-Farber Cancer Institute Flow Cytometry Core for cell sorting and F. Godinho for technical help.
E.C.S. was supported by a Jane Coffin Childs Memorial Fund for Medical Research Fellowship and a Burroughs Wellcome Fund Postdoctoral Enrichment Program Award. S.L. is supported by a Leukemia & Lymphoma Society Fellow Award. M.B.W. and L.C.G. were supported by National Institute of Neurological Disorders and Stroke, National Institutes of Health individual National Research Service Awards (grants NS064730 and NS080343); the National Institute of Neurological Disorders and Stroke, National Institutes of Health (grant NS075672) (J.D.M.); and the DEARS Foundation (J.D.M.), with additional infrastructure support by the National Institute of Neurological Disorders and Stroke, National Institutes of Health (grants NS045523, NS041590, and NS093376) (J.D.M.). J.D.M. is an Allen Distinguished Investigator of the Paul G. Allen Frontiers Group. L.C.G. is also supported by the Harvard Medical Scientist Training Program. D.E.B. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (grants K08DK093705 and R03DK109232); the Charles H. Hood Foundation; the American Society of Hematology; the Burroughs Wellcome Fund; and Cooley’s Anemia Foundation. S.H.O. is supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (grant P01HL032262) and National Institute of Diabetes and Digestive and Kidney Diseases (grant P30DK049216) (Center of Excellence in Molecular Hematology).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.C.S., D.E.B., and S.H.O. conceived the study; E.C.S., M.N., Y.F., D.E.B., and S.H.O. designed and produced the mice; E.C.S., S.L., and D.M.C. performed hematopoietic analyses; E.C.S., M.B.W., L.C.G., F.S., and J.D.M. contributed to the brain analyses; and E.C.S. and S.H.O. wrote the manuscript with input from all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stuart H. Orkin, 44 Binney St, Boston, MA 02115; e-mail: stuart_orkin@dfci.harvard.edu.
References
- 1.Lettre G, Sankaran VG, Bezerra MA, et al. DNA polymorphisms at the BCL11A, HBS1L-MYB, and beta-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc Natl Acad Sci USA. 2008;105(33):11869–11874. doi: 10.1073/pnas.0804799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menzel S, Garner C, Gut I, et al. A QTL influencing F cell production maps to a gene encoding a zinc-finger protein on chromosome 2p15. Nat Genet. 2007;39(10):1197–1199. doi: 10.1038/ng2108. [DOI] [PubMed] [Google Scholar]
- 3.Uda M, Galanello R, Sanna S, et al. Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta-thalassemia. Proc Natl Acad Sci USA. 2008;105(5):1620–1625. doi: 10.1073/pnas.0711566105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sankaran VG, Menne TF, Xu J, et al. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science. 2008;322(5909):1839–1842. doi: 10.1126/science.1165409. [DOI] [PubMed] [Google Scholar]
- 5.Sankaran VG, Xu J, Ragoczy T, et al. Developmental and species-divergent globin switching are driven by BCL11A. Nature. 2009;460(7259):1093–1097. doi: 10.1038/nature08243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perkins AC, Gaensler KM, Orkin SH. Silencing of human fetal globin expression is impaired in the absence of the adult beta-globin gene activator protein EKLF. Proc Natl Acad Sci USA. 1996;93(22):12267–12271. doi: 10.1073/pnas.93.22.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wijgerde M, Gribnau J, Trimborn T, et al. The role of EKLF in human beta-globin gene competition. Genes Dev. 1996;10(22):2894–2902. doi: 10.1101/gad.10.22.2894. [DOI] [PubMed] [Google Scholar]
- 8.Xu J, Sankaran VG, Ni M, et al. Transcriptional silencing of gamma-globin by BCL11A involves long-range interactions and cooperation with SOX6. Genes Dev. 2010;24(8):783–798. doi: 10.1101/gad.1897310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu P, Keller JR, Ortiz M, et al. Bcl11a is essential for normal lymphoid development. Nat Immunol. 2003;4(6):525–532. doi: 10.1038/ni925. [DOI] [PubMed] [Google Scholar]
- 10.Tsang JC, Yu Y, Burke S, et al. Single-cell transcriptomic reconstruction reveals cell cycle and multi-lineage differentiation defects in Bcl11a-deficient hematopoietic stem cells. Genome Biol. 2015;16:178. doi: 10.1186/s13059-015-0739-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brendel C, Guda S, Renella R, et al. Lineage-specific BCL11A knockdown circumvents toxicities and reverses sickle phenotype. J Clin Invest. 2016;126(10):3868–3878. doi: 10.1172/JCI87885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greig LC, Woodworth MB, Greppi C, Macklis JD. Ctip1 controls acquisition of sensory area identity and establishment of sensory input fields in the developing neocortex. Neuron. 2016;90(2):261–277. doi: 10.1016/j.neuron.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodworth MB, Greig LC, Liu KX, Ippolito GC, Tucker HO, Macklis JD. Ctip1 regulates the balance between specification of distinct projection neuron subtypes in deep cortical layers. Cell Reports. 2016;15(5):999–1012. doi: 10.1016/j.celrep.2016.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ippolito GC, Dekker JD, Wang YH, et al. Dendritic cell fate is determined by BCL11A. Proc Natl Acad Sci USA. 2014;111(11):E998–E1006. doi: 10.1073/pnas.1319228111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luc S, Huang J, McEldoon JL, et al. Bcl11a deficiency leads to hematopoietic stem cell defects with an aging-like phenotype. Cell Reports. 2016;16(12):3181–3194. doi: 10.1016/j.celrep.2016.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basak A, Hancarova M, Ulirsch JC, et al. BCL11A deletions result in fetal hemoglobin persistence and neurodevelopmental alterations. J Clin Invest. 2015;125(6):2363–2368. doi: 10.1172/JCI81163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funnell AP, Prontera P, Ottaviani V, et al. 2p15-p16.1 microdeletions encompassing and proximal to BCL11A are associated with elevated HbF in addition to neurologic impairment. Blood. 2015;126(1):89–93. doi: 10.1182/blood-2015-04-638528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauer DE, Kamran SC, Lessard S, et al. An erythroid enhancer of BCL11A subject to genetic variation determines fetal hemoglobin level. Science. 2013;342(6155):253–257. doi: 10.1126/science.1242088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canver MC, Smith EC, Sher F, et al. BCL11A enhancer dissection by Cas9-mediated in situ saturating mutagenesis. Nature. 2015;527(7577):192–197. doi: 10.1038/nature15521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porcu S, Kitamura M, Witkowska E, et al. The human beta globin locus introduced by YAC transfer exhibits a specific and reproducible pattern of developmental regulation in transgenic mice. Blood. 1997;90(11):4602–4609. [PubMed] [Google Scholar]
- 21.Xu J, Peng C, Sankaran VG, et al. Correction of sickle cell disease in adult mice by interference with fetal hemoglobin silencing. Science. 2011;334(6058):993–996. doi: 10.1126/science.1211053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khaled WT, Choon Lee S, Stingl J, et al. BCL11A is a triple-negative breast cancer gene with critical functions in stem and progenitor cells. Nat Commun. 2015;6:5987. doi: 10.1038/ncomms6987. [DOI] [PMC free article] [PubMed] [Google Scholar]