Abstract
Objective
Combination antiretroviral therapy (ART) decreases the risk of sexual HIV transmission by suppressing blood and genital HIV RNA concentrations. We sought to determine HIV transmission risk prior to achieving complete viral suppression.
Design
Prospective cohort study.
Methods
Using data from the Partners PrEP Study, a prospective study of 4747 heterosexual HIV-serodiscordant couples in Kenya and Uganda, we examined multiple markers of HIV transmission risk during the first months after ART initiation: time to viral suppression in blood, persistence of HIV RNA in genital specimens, sexual risk behavior, pregnancy incidence, and HIV transmission using survival analysis and GEE logistic regression.
Results
The cumulative probabilities of achieving blood viral suppression (<80 copies/ml) 3, 6 and 9-months after ART initiation were 65.3%, 84.8% and 89.1%, respectively. Endocervical and seminal HIV RNA were detectable in 12% and 21% of samples obtained within 6-months of ART. Pregnancy incidence was 8.8 per 100 person-years during the first 6-months of ART, and sex unprotected by condoms was reported at 10.5% of visits. Among initially uninfected partners, HIV incidence before ART was 2.08 per 100 person-years (55 infections; 2644 person-years), 1.79 for 0–6 months after ART initiation (3 infections; 168 person-years), and 0.00 with >6 months of ART (0 infections; 167 person-years).
Conclusions
Residual HIV transmission risk persists during the first 6-months of ART, with incomplete viral suppression in blood and genital compartments. For HIV-serodiscordant couples in which the infected partner starts ART, other prevention options are needed, such as pre-exposure prophylaxis, until viral suppression is achieved.
Keywords: HIV transmission, antiretroviral therapy, viral suppression, serodiscordant couples
Introduction
Blood and genital HIV RNA concentrations are the prime determinant of HIV transmission risk1,2. Combination antiretroviral therapy (ART) prevents sexual transmission of HIV by suppressing HIV RNA in blood and genital secretions3. After ART initiation, the blood concentration of HIV RNA decreases in multiple overlapping phases4. During the first two phases of decay, blood viremia declines rapidly due to clearance of free virus, macrophages and productively infected and partially activated CD4+ T-cells, but HIV RNA is still detectable, suggesting residual risk of HIV transmission. In the third and fourth phases of decay, blood HIV RNA concentrations decline below the limit of quantification of standard assays5,6; typically, viral suppression occurs within six months after initiation of combination ART7.
Understanding the potential for HIV infectiousness during the period between ART initiation and complete viral suppression is a priority for patients, providers, and public health policymakers. As ART for HIV prevention is scaled up, data are needed to assess ART effectiveness during this risk period when sexual partners remain at risk of HIV acquisition. Within a prospective study among HIV serodiscordant couples, we evaluated measures of HIV transmission risk after ART initiation, focusing on residual risk during the first 6 months of ART, when the majority of patients typically achieve viral suppression.
Methods
Study population
This analysis utilized data from the Partners PrEP Study, a randomized clinical trial of daily oral antiretroviral pre-exposure prophylaxis (PrEP) to decrease HIV acquisition in heterosexual serodiscordant couples8. As previously reported, 4747 HIV serodiscordant couples from Kenya and Uganda were followed between 2008 and 20129. At study entry, couples were sexually active and planning to remain as a couple for the duration of the study. HIV-infected partners were not eligible for ART according to national guidelines at the time of enrollment. During follow-up, they received regular clinical and immunological monitoring and referrals for ART if they became eligible for treatment, initially at CD4 <200 cells/μL (Kenya) and <250 cells/μL (Uganda), which was revised to ≤350 cells/μL in both countries while the study was ongoing. ART use by HIV-infected partners and sexual behavior as reported by both members of the couple were assessed every three months.
All participants received a package of HIV prevention services including individual and couple risk-reduction counseling, free condoms, and screening and treatment of sexually transmitted infections. At all study sites, HIV-uninfected women were provided with contraception counseling and free contraceptives. Institutional review board approval was obtained from each collaborating institution and the University of Washington. All participants provided written informed consent in English or their local language.
Specimen collection and processing
At enrollment, every six months and at study exit, blood was collected from HIV-infected partners for HIV RNA quantification. Thus, blood was collected on a schedule relative to enrollment, not to ART initiation. Similarly, cervical specimens were collected at enrollment, annually and study exit. Swabs were collected by placing Dacron swabs into the endocervical canal and gently rotating twice, then were snipped distal from the base of the swab, inserted in aliquot vials, and frozen at −70°C within 5 hours of collection. Semen collection was scheduled at the six and twelve month visits, but samples could be obtained at any visit thereafter, depending on the preference of the male subject. At the prior visit, men were provided with a spermicide-free condom and sterile wide-mouth plastic container and instructed to abstain from ejaculation for 48–72 hours. Semen was collected during coitus on the day of the clinic visit, or through masturbation at the clinic or participant’s home, and brought into clinic within 5 hours of collection. Semen was centrifuged at 600–800g for 10 minutes within 4 hours of arrival in the laboratory. Seminal blood was separated from the cell pellet, aliquoted into cryovials and stored at −70°C10.
HIV serological testing for HIV-uninfected partners was performed monthly using dual rapid HIV antibody tests, and positive results confirmed by HIV EIA, Western blot and RNA polymerase chain reaction (PCR)11. Phylogenetic linkage between HIV seroconverters and their study partners was ascertained using HIV pol gene consensus sequencing. We used the Abbott m2000 Real-Time HIV assay (Abbott Diagnostics) to quantify HIV RNA in blood and genital samples. The lower limit of detection was 40 copies/mL in blood and semen plasma and 248 copies/swab in endocervical secretions. Urine pregnancy testing was conducted monthly for HIV-uninfected women and when clinically indicated for HIV-infected women.
Statistical analysis
To assess ongoing HIV transmission risk after ART initiation, we evaluated several measures: time to first viral suppression in blood, persistence of HIV RNA in genital secretions, self-reported sexual behavior, incidence of pregnancy, and phylogenetically linked HIV transmission within the couple. For analyses of viral suppression, sexual risk behavior and pregnancy incidence, we evaluated couples in which the HIV-infected partner initiated ART (N=1817). Analysis of HIV transmission was restricted to couples in the trial’s placebo arm, including those who did and did not initiate ART (N=1573). Blood viral suppression was defined as HIV RNA concentrations <40 copies/mL. Follow-up time was computed beginning on the date of the study visit at which ART use was first reported (thus, after ART had started). Kaplan-Meier methods were used to estimate the cumulative proportion achieving viral suppression. Persistence of HIV RNA in genital samples was described as the proportion with detectable HIV RNA.
Sexual risk behavior, pregnancy incidence and HIV transmission risk were quantified in three time periods: before ART initiation, during the first six months of ART, and after six months of ART. The frequency of self-reported condomless sex was calculated as the proportion of visits at which condomless sex was reported. Differences in the proportion of condomless sex between ART time periods were estimated using logistic regression with generalized estimating equations. Pregnancy incidence was computed by dividing the number of new pregnancies by follow up time at risk for pregnancy. Pregnancy and HIV incidence rates and 95% confidence intervals were estimated using exact methods assuming a Poisson distribution. All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC) and Stata 12.1 (StataCorp, College Station, TX).
Results
Participant characteristics
Of the 4747 HIV-infected persons followed in the Partners PrEP Study, 2184 were eligible for ART of which 1817 (83%) initiated ART (1062 women and 755 men). Most (98%) were married and the median ages of HIV-infected men and women were 40 years (interquartile range [IQR], 35–45) and 30 years (IQR, 25–36), respectively. At the study visit prior to reporting ART use, the median CD4 count and blood HIV RNA concentration were 288 cells/μL (IQR, 226–390) and 4.03 log10 copies/mL (IQR, 3.36–4.61) for HIV-infected women, and 262 cells/μL (IQR, 216–339) and 4.39 log10 copies/mL (IQR, 3.75–4.90) for HIV-infected men (p<0.001 for each variable comparing women and men).
Blood HIV RNA suppression
Of the 1817 ART initiators, 215 who reported ART use at the exit study visit and 10 who died or missed study visits after starting ART did not contribute to the analysis. The remaining 1592 were followed for 474 person-years to assess time to first viral suppression. The median time from date of the study visit at which ART use was first reported until first blood HIV RNA quantification was 3.1 months (IQR, 2.8–5.5). The cumulative probabilities of achieving blood viral suppression 3, 6, 9 and 12 months after the reported date of ART initiation were 65.3%, 84.8%, 89.1% and 90.9%, respectively (Figure 1).
Figure 1.
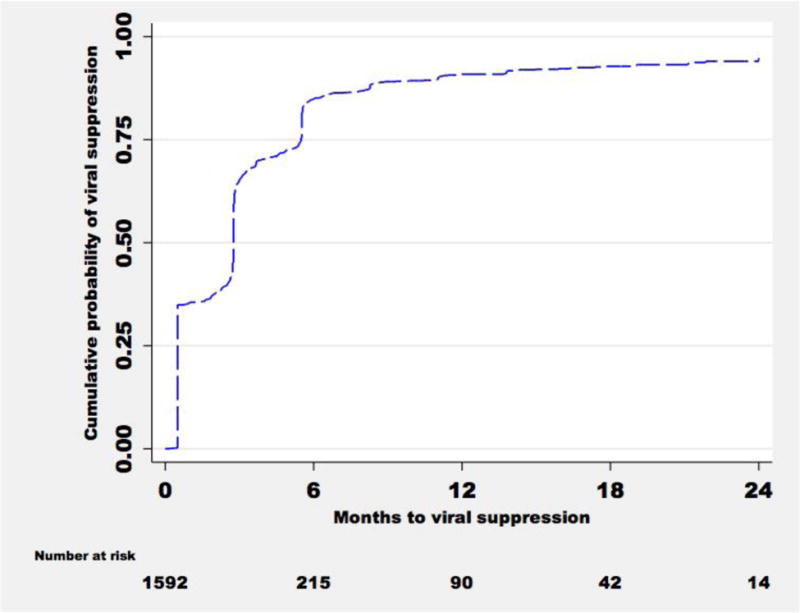
Cumulative probability of blood viral suppression from date of first visit at which ART use was reported
Genital HIV RNA levels
Of the 1062 women ART initiators, endocervical HIV RNA concentrations were available for 929 (87%). Of these, 492 women (53%) contributed one sample, 267 women (29%) contributed two samples, 149 women (16%) contributed three samples and 21 (2%) women contributed four samples. The median time from the reported date of ART initiation to the first endocervical HIV RNA quantification was 3.7 months (IQR, 0.4–8.3). During the first six months of ART, endocervical HIV RNA was detected in 12% (75/625) of swabs (Table 1) and the median quantity was 3.11 log10 copies/swab (IQR, 2.59–3.62). Among 454 swabs collected when blood HIV RNA concentrations were undetectable (<40 copies/mL), endocervical HIV RNA was detected in 8% (36 swabs from 36 women), and the median quantity was 3.14 log10 copies/swab (IQR, 2.88–3.53). When blood HIV RNA concentrations were detectable, endocervical HIV RNA was detected in 23% (39/171) of swabs.
Table 1.
Detection of HIV RNA in blood and genital secretions during the first six months of ART
| Blood HIV RNA | |||
|---|---|---|---|
|
| |||
| Genital HIV RNA | Detected | Not detected | Total |
|
| |||
| Endocervical swabs | |||
| Detected | 39 | 36 | 75 |
| Not detected | 132 | 418 | 549 |
|
| |||
| Total | 171 | 454 | 625 |
|
| |||
| Semen | |||
| Detected | 30 | 12 | 42 |
| Not detected | 42 | 120 | 162 |
|
| |||
| Total | 72 | 132 | 204 |
Of the 755 male ART initiators, 231 (31%) had semen samples available for HIV RNA quantification after initiating ART; 189 men (82%) contributed one sample and 41 (18%) contributed 2 samples. The median time from ART start to seminal HIV RNA quantification was 2.8 months (IQR, 0.0–4.6). During the first six months of ART, 21% (42/204) had detectable HIV RNA concentrations and the median quantity was 2.90 log10 copies/mL (IQR, 2.51–3.50). Among 132 semen samples collected after blood HIV RNA concentrations were suppressed, 9% (12 samples from 10 men) had detectable seminal HIV RNA and the median quantity detected was 2.60 log10 copies/mL. When blood HIV RNA concentrations were detectable, seminal HIV RNA was detected in 42% (30/72) of swabs.
Sexual risk behavior
The frequency of self-reported condomless sex with HIV-infected partners was 12.8% (15207/118499 visits) before ART initiation, 10.5% (882/8386 visits) during the first six months of ART and 9.1% (1038/11446 visits) after six months of ART (p=0.008 for 0–6 months of ART versus no ART). The pregnancy incidence rate among HIV-infected and uninfected women partners was 13.2 per 100 person-years (1215 pregnancies; 9191 person-years) prior to ART initiation, 8.8 per 100 person-years (79 pregnancies; 895 person-years) during the first six months of ART, and 8.0 per 100 person-years (95 pregnancies; 1183 person-years) after six months of ART (p=0.004 for 0–6 months of ART versus no ART).
HIV incidence
We followed 1573 HIV serodiscordant couples enrolled in the placebo arm of the Partners PrEP Study for 2979 person-years. The HIV incidence rate among couples not on ART was 2.08 per 100 person-years (55 infections; 2644 person years), and 1.79 per 100 person-years (3 infections; 168 person years) during the first six months of ART use. There were no HIV transmissions during 167 person-years of follow up among couples exposed to ART for more than 6 months of ART (incidence rate 0.00; 95% CI: 0.00–2.20). All three ART-exposed HIV events were phylogenetically-linked female-to-male transmissions and occurred prior to complete viral suppression in blood and genital secretions. For one couple, the HIV-infected woman first reported ART use at the same study visit that her male partner tested seropositive for HIV. In the other two couples, HIV seroconversion occurred within six months after first report of ART use (Table 2).
Table 2.
Characteristics of women who transmitted HIV to male partners while on ART
| Variable | Couple 1 | Couple 2 | Couple 3 |
|---|---|---|---|
| Couple demographics (age, gender) | 40M, 50F | 53M, 31F | 32M, 29F |
| Enrollment plasma HIV RNA (copies/mL) | 332,514 | 20,188 | 694 |
| Pre-ART plasma HIV RNA (copies/mL) | 1,434,082 | 56,168 | 824 |
| Time from enrollment to ART initiation (months) | 14 | 22 | 25 |
| ART regimen | AZT/3TC/NVP | TDF/3TC/NVP | d4T/3TC/NVP |
| Time from reported date of ART initiation to HIV seroconversion (days) | 56 | 0 | 149 |
| Time from reported date of ART initiation to HIV RNA quantification (days) | 84 | 28 | 86 |
| Post ART plasma HIV RNA (copies/mL) ≤6 months >6 months |
738 <80 |
404 160 |
872 264 |
| Post ART endocervical HIV RNA (copies/swab) ≤6 months >6 months |
<248* <248 |
3166 <248 |
No samples available |
Discussion
HIV transmission risk is markedly reduced once effective ART has resulted in complete virologic suppression in blood and genital secretions. However, this prospective follow up of 1592 HIV serodiscordant couples after ART initiation by the HIV-infected partner demonstrates residual risk of HIV transmission during the first six months of ART, as measured by HIV in blood and genital secretions, behavioral risk, and direct measures of HIV transmission with three phylogenetically-linked transmissions occurring soon after the HIV-infected partner reported ART initiation. Thus, the first 6 months after ART initiation may be a period of transition and persistent risk, with declining markers of transmission but not yet minimized risk.
Rigorous clinical studies have demonstrated that ART significantly reduces HIV transmission risk in serodiscordant couples. A randomized trial and 5 observational studies followed 1672 serodiscordant couples for 5336 person-years12–17. Six phylogenetically-linked HIV transmission events were observed, of which at least 5 occurred within six months of ART initiation and the other occurred in the first year of ART18. In our study, all three men who acquired HIV did so within six months of the first report of ART use, when their female HIV-infected partners still had detectable blood and genital HIV RNA concentrations. One of these HIV transmission events was observed at the same visit ART use was first reported, and soon after ART was initiated. The HIV-infected partner’s blood and endocervical HIV RNA concentrations at this visit were 56168 copies/ml and 3166 copies/swab, respectively. Twenty eight days later, her blood HIV RNA concentration was 404 copies/ml, consistent with first and second phase decay kinetics when HIV is still detectable, and infectious.
HIV incidence during the first six months of ART use was similar to that among uninfected partners of HIV-infected persons not yet on ART. The effectiveness of ART for HIV prevention in our study is consistent with the PARTNER study, a European multi-center observational study of HIV transmission from infected partners on suppressive ART, in which no transmissions occurred during 894 couple-years of follow up19. The precision of the upper bound of HIV transmission risk on ART would be increased with additional HIV transmission events and person-years of follow up20.
The cumulative probabilities of achieving blood viral suppression three, six and nine months after starting ART were 65%, 85%, and 89%, respectively, suggesting residual transmission risk soon after ART initiation. Importantly, the majority of HIV-infected partners achieved complete viral suppression by six months after ART initiation. The fraction achieving viral suppression after 12 months in our study is similar to other cohorts from sub-Saharan Africa in which ~85% were fully suppressed after one year on ART14,16.
We observed ongoing high-risk sexual behavior during the first six months of ART as shown by self-reported sex unprotected by condoms and pregnancy incidence. The proportion reporting condomless sex in our study is comparable with that reported from a West African cohort in which 10–13% of HIV-infected persons on ART reported sex unprotected by condoms with serodiscordant partners21. The observed incidence of pregnancy in mutually-disclosed serodiscordant couples with access to comprehensive HIV prevention services is comparable to annual pregnancy rates reported in other African cohorts22,23. HIV serodiscordant couples may practice condomless sex because of desire for children, condom fatigue or perception of low risk of HIV transmission in dyadic heterosexual partnerships24,25.
Our results suggest that strategies to reduce HIV risk prior to complete viral suppression are needed. Combining ART and PrEP for HIV prevention results in near elimination of HIV transmission in African serodiscordant couples26. As ART for HIV prevention is scaled up worldwide, and couples are made aware of the treatment and prevention benefits of ART, providers should counsel serodiscordant couples about residual risk of HIV transmission during the first six months of ART, and encourage use of additional HIV prevention services, including PrEP.
The strengths of our study include the large prospective multi-national cohort, the serodiscordant couple design permitting assessment of sexual behavior, HIV infectiousness and phylogenetic linkage of HIV transmission events to avoid misclassification of ART effectiveness. Our study has limitations. We relied on self-report of ART use at quarterly scheduled visits, and cannot precisely estimate the interval between ART start and HIV transmission to susceptible partners. Most of the person-time at risk in our study was accrued before ART initiation, limiting the precision of the upper bound of our point estimate for HIV transmission risk on ART. Viral load testing was done after the study ended, and participants were not provided with results in real-time, as routine viral load testing was not standard of care in the study settings.
In conclusion, among African HIV serodiscordant couples, we observed residual risk of HIV transmission, measured through virologic and behavioral outcomes, during the first six months of ART. During the transition to ART, other prevention options such as PrEP are needed for HIV serodiscordant couples in which the infected partner delays, declines or is starting treatment. Ongoing studies are designed to provide further evidence of ART effectiveness for HIV prevention.
Acknowledgments
Source of Funding: This study was supported through research grants from the Bill & Melinda Gates Foundation (OPP47674), the National Institute of Mental Health of the US National Institutes of Health (R01 MH095507) and University of Washington Centers for AIDS Research (P30-AI-27757). The contents are solely the views of the authors and do not necessarily represent those of the funding organizations.
Footnotes
These data were reported, in part, at the 2015 Conference on Retroviruses and Opportunistic Infections, Seattle, WA, USA (abstract #989)
Conflicts of Interest:
The authors report no conflicts of interest.
Author contributions
AM and JMB designed the study and wrote the first draft. AM performed the statistical analyses. All authors contributed to data collection, interpretation of the results and the writing of the manuscript, and all approved the final draft.
References
- 1.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 2.Baeten JM, Kahle E, Lingappa JR, et al. Genital HIV RNA predicts risk of heterosexual HIV transmission. Sci Transl Med. 2011;3(77):77ra29. doi: 10.1126/scitranslmed.3001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perelson AS, Essunger P, Cao Y, et al. Decay characteristics of HIV-infected compartments during combination therapy. Nature. 1997;387(6629):188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 5.Hilldorfer BB, Cillo AR, Besson GJ, Bedison MA, Mellors JW. New tools for quantifying HIV reservoirs: blood RNA single copy assays and beyond. Curr HIV/AIDS Rep. 2012;9(1):91–100. doi: 10.1007/s11904-011-0104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrade A, Rosenkranz SL, Cillo AR, et al. Three distinct phases of HIV RNA decay in treatment-naive patients receiving raltegravir-based antiretroviral therapy: ACTG A5248. J Infect Dis. 2013;208(6):884–891. doi: 10.1093/infdis/jit272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308(4):387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 8.Mujugira A, Baeten JM, Donnell D, et al. Characteristics of HIV Serodiscordant Couples Enrolled in a Clinical Trial of Antiretroviral Pre-Exposure Prophylaxis for HIV Prevention. PLoS One. 2011;6(10):e25828. doi: 10.1371/journal.pone.0025828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baeten JM, Donnell D, Mugo NR, et al. Single-agent tenofovir versus combination emtricitabine plus tenofovir for pre-exposure prophylaxis for HIV acquisition: an update of data from a randomised, double-blind, phase 3 trial. Lancet Infect Dis. 2014;14(11):1055–1064. doi: 10.1016/S1473-3099(14)70937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourlet T, Levy R, Maertens A, et al. Detection and characterization of hepatitis C virus RNA in seminal blood and spermatozoon fractions of semen from patients attempting medically assisted conception. J Clin Microbiol. 2002;40(9):3252–3255. doi: 10.1128/JCM.40.9.3252-3255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen M, Chen Y, McCauley T, et al. Final results of the HPTN 052 randomized controlled trial: antiretroviral therapy prevents HIV transmission. 8th IAS Conference on HIV Pathogenesis, Treatment and Prevention; 19–22 July 2015; Vancouver, Canada. 2015. [Google Scholar]
- 13.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375(9731):2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apondi R, Bunnell R, Ekwaru JP, et al. Sexual behavior and HIV transmission risk of Ugandan adults taking antiretroviral therapy: 3 year follow-up. AIDS. 2011;25(10):1317–1327. doi: 10.1097/QAD.0b013e328347f775. [DOI] [PubMed] [Google Scholar]
- 15.Melo MG, Santos BR, De Cassia Lira R, et al. Sexual transmission of HIV among serodiscordant couples in Porto Alegre, southern Brazil. Sex Transm Dis. 2008;35(11):912–915. doi: 10.1097/OLQ.0b013e31817e2491. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds SJ, Makumbi F, Nakigozi G, et al. HIV transmission among HIV discordant couples before and after the introduction of antiretroviral therapy. AIDS. 2011;25(4):473–477. doi: 10.1097/QAD.0b013e3283437c2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Romero J, Castilla J, Hernando V, Rodriguez C, Garcia S. Combined antiretroviral treatment and heterosexual transmission of HIV: cross sectional and prospective cohort study. BMJ. 2010;340:c2205. doi: 10.1136/bmj.c2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Supervie V, Viard JP, Costagliola D, Breban R. Heterosexual risk of HIV transmission per sexual act under combined antiretroviral therapy: systematic review and bayesian modeling. Clin Infect Dis. 2014;59(1):115–122. doi: 10.1093/cid/ciu223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodger A, Cambiano V, Bruun T, Vernazza P, Lundgren J. HIV transmission risk through condomless sex if the HIV positive partner is on suppressive ART: PARTNER study. Conference On Retroviruses And Opportunistic Infections (CROI 2014); March 3–6, 2014; Boston, MA, USA. [Google Scholar]
- 20.Rodger AJ, Bruun T, Vernazza P, et al. Further research needed to support a policy of antiretroviral therapy as an HIV prevention initiative. Antivir Ther. 2013;18(3):285–287. doi: 10.3851/IMP2609. [DOI] [PubMed] [Google Scholar]
- 21.Jean K, Gabillard D, Moh R, et al. Effect of early antiretroviral therapy on sexual behaviors and HIV transmission risk among adults with diverse heterosexual partnership statuses in Cote d’Ivoire. J Infect Dis. 2014;209(3):431–440. doi: 10.1093/infdis/jit470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heffron R, Were E, Celum C, et al. A prospective study of contraceptive use among African women in HIV serodiscordant partnerships. Sex Transm Dis. 2010;37(10):621–628. doi: 10.1097/OLQ.0b013e3181e1a162. [DOI] [PubMed] [Google Scholar]
- 23.Kaida A, Matthews LT, Kanters S, et al. Incidence and predictors of pregnancy among a cohort of HIV-positive women initiating antiretroviral therapy in Mbarara, Uganda. PLoS One. 2013;8(5):e63411. doi: 10.1371/journal.pone.0063411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mujugira A, Heffron R, Celum C, Mugo N, Nakku-Joloba E, Baeten JM. Fertility Intentions and Interest in Early Antiretroviral Therapy Among East African HIV-Infected Individuals in Serodiscordant Partnerships. J Acquir Immune Defic Syndr. 2013;63(1):e33–35. doi: 10.1097/QAI.0b013e318288bb32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ngure K, Mugo N, Celum C, et al. A qualitative study of barriers to consistent condom use among HIV serodiscordant couples in Kenya. AIDS Care. 2012;24(4):509–516. doi: 10.1080/09540121.2011.613911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baeten JM, Heffron R, Kidoguchi L, et al. Near Elimination of HIV Transmission in a Demonstration Project of PrEP and ART. Conference on Retroviruses and Opportunistic Infections (CROI 2015); February 23–26, 2015; Seattle, Washington. 2015. [Google Scholar]


