Abstract
Regulated Apoptosis (Programmed Cell Death, PCD) maintains tissue homeostasis in adults, and ensures proper growth and morphogenesis of tissues during development of metazoans. Accordingly, defects in cellular processes triggering or executing apoptotic programs have been implicated in a variety of degenerative and neoplastic diseases. Here, we report the identification of DCAF12, an evolutionary conserved member of the WD40-motif repeat family of proteins, as a new regulator of apoptosis in Drosophila. We find that DCAF12 is required for Diap1 cleavage in response to pro-apoptotic signals, and is thus necessary and sufficient for RHG (Reaper, Hid, and Grim)-mediated apoptosis. Loss of DCAF12 perturbs the elimination of supernumerary or proliferation-impaired cells during development, and enhances tumor growth induced by loss of neoplastic tumor suppressors, highlighting the wide requirement for DCAF12 in PCD.
Keywords: DCAF12, Apoptosis, dIAP1 Cleavage
Introduction
The importance of PCD in sculpting tissues and ensuring proper growth during development, as well as in the maintenance of tissue homeostasis in adult metazoans, is well established (Elmore, 2007; Fuchs and Steller, 2011; Gyrd-Hansen and Meier, 2010). Drosophila metamorphosis has served as an informative and accessible model system for the characterization of genetic requirements for developmental apoptosis. During metamorphosis, larval tissues are eliminated through PCD and replaced by adult tissue derived from imaginal disks. Widespread Apoptosis is initiated after the third larval instar by a pulse of the steroid hormone Ecdysone (Yin and Thummel, 2005; Zirin et al., 2013). Other developmental and homeostatic processes that rely on apoptosis in Drosophila include development of the embryonic nervous system, fine-tuning of imaginal disk patterning, regulation of organ growth by cell competition in imaginal disks, and homeostatic control of epithelial integrity in the adult midgut epithelium (Cashio et al., 2005; Kuranaga, 2011).
The signaling mechanisms initiating apoptosis, as well as the executing proteolytic cascades are evolutionarily conserved from C. elegans to humans (Fuchs and Steller, 2011; Kuranaga, 2011). Extrinsic and intrinsic apoptotic signaling pathways are commonly distinguished. Extrinsic pathways respond to extracellular signals such as tumor necrosis factor alpha (TNFa). These ligands engage a proteolytic cascade involving oligomeric complexes of death-domain containing proteins at the receptor that lead to pro-Caspase-8 processing into active Caspase-8. Intrinsic pathways are induced by cellular damage, and rely on the release of Cytochrome C and other death-inducing molecules from mitochondria. Cytochrome C interacts with APAF-1 to form an oligomeric complex, the apoptosome, which engages and activates Caspase-9 (Riedl and Salvesen, 2007). Both pathways are thus activated by signal-induced aggregation and autoproteolytic activation of pro-Caspases, and converge on Caspase-3, the main protease involved in cellular degradation. The activity of these proteases is regulated by Inhibitors of Apoptosis (IAPs), which in turn are targets of pro- and anti-apoptotic signaling (Fuchs and Steller, 2011; Gyrd-Hansen and Meier, 2010; Kuranaga, 2011).
In Drosophila, the apical caspase Dronc (the Caspase-9 homologue), dIAP1, and the effector caspases DrICE and DCP-1 (caspase-3, -6, and -7 in mammals) are involved in developmental and stress-induced apoptosis. As in mammals, activation of Dronc is achieved by dARK/Apaf-1-mediated formation of an apoptosome, which recruits Dronc, stimulating its autoproteolytic cleavage. This cleavage promotes Dronc homodimerization, resulting in its activation (Yan et al., 2006). In contrast to its vertebrate homologues, Drosophila dARK/Apaf-1 does not seem to require Cytochrome C for apoptosome formation, indicating a divergent mechanism of apoptosome activation in flies (Dorstyn et al., 2004).
Apoptosome levels and stability are controlled in Drosophila cells by an antagonistic relationship between Dronc and dARK, in which dARK promotes the degradation of Dronc via the ubiquitin ligase activity of dIAP1 (Shapiro et al., 2008), while active Dronc induces cleavage and degradation of dARK (Akdemir et al., 2006). Dronc-induced apoptosis can therefore only be achieved when one of the dIAP1 inhibitors Reaper, Hid or Grim (encoded by the ‘RHG’ genes) is expressed (Wang et al., 1999). These molecules bind to dIAP1, destabilizing it. While this pathway is well understood, in vitro reconstitution studies have suggested that Dronc and dARK alone are not sufficient to form a fully functional apoptosome (Dorstyn and Kumar, 2008; Riedl and Salvesen, 2007; Yu et al., 2006), and it is therefore likely that additional molecules regulating Dronc/dARK – mediated activation of Caspases remain to be identified.
Here, we report the identification of DCAF12 as a regulator of apoptosis in Drosophila. DCAF12 (CG3313) is an evolutionary conserved member of the WD40-motif repeat family of proteins, which play diverse roles in signal transduction (Xu and Min, 2011). Genomic and proteomic studies have suggested that the human homologue of DCAF12 interacts with the CUL4/DDB1 ubiquitin ligase complex and may be involved in DNA repair and protein degradation, but no functional studies have been performed so far (Angers et al., 2006; Higa et al., 2006; Jin et al., 2006; Olma et al., 2009). We find that DCAF12 is necessary and sufficient for RHG (Reaper, Hid, and Grim)-mediated apoptosis in various Drosophila tissues. Highlighting its importance for developmental apoptosis, DCAF12 is transcriptionally induced at the onset of metamorphosis, and dcaf12 mutants die as pharate adults with phenotypes that resemble Drosophila Caspase-3 (drice) mutants. Biochemical and genetic analysis suggests that DCAF12 is required for dronc-mediated dIAP1 cleavage in response to pro-apoptotic signals. We further show that loss of DCAF12 significantly impacts the elimination of ddb1 deficient cells during development and enhances tumor growth induced by loss of neoplastic tumor suppressors. Our study thus introduces DCAF12 as a widely used, evolutionarily conserved regulator of apoptosis.
Material and Methods
Fly stocks and culture
Flies were maintained at 25C and 60% humidity on a 12h/12h light dark cycle unless otherwise specified. The composition of food (regular food) is as follows. For 1L water: 13.8 g agar, 22 g molasses, 80 g malt extract, 18 g (~2 % of total volume) Brewer’s yeast, 80 g corn flour, 10 g soy flour, 6.25 mL propionic acid, 2 g methyl-p-benzoate, 7.2 mL of Nipagin (20% in EtOH).
The following stocks were obtained from the Bloomington Drosophila Stock Center: - Patched-Gal4, Daughterless-Gal4, -, UAS-P35, GMR-Reaper, GMR-Hid, GMR-Grim, UAS-Diap1, -.
The following stocks were generous gifts:
ddb1PL12C : gift from Dr. Robert Duronio (Univ. of North Carolina)
scribble1: gift from Dr. Dirk Bohmann (Univ. of Rochester)
dcaf12RNAi(CG3313RNAi): from VDRC (Transformant ID:43758)
Tubulin-GeneSwitch-Gal4: a gift from Dr. Scott Pletcher (Univ. of Michigan)
UAS-Dronc and UAS-DroncDN (UAS-DroncCARD): gifts from Dr. Pascal Meier
eye-MARCM (eyelessGal4,UAS-FLP;act>y+>Gal4, UAS-GFP; FRT82B, tubGal80): a gift from Dr. Mirka Uhlirova (Univ. of Cologne)
Generation of transgenic flies
P-element mediated germ line transformation (Rubin and Spradling, 1982; Spradling and Rubin, 1982) was used to generate pUASt-dcaf12. The dcaf12 full-length cDNA containing 5′UTR and 3′UTR (for UAS-dcaf12) were amplified with Platinum® Taq DNA Polymerase High Fidelity (Sigma) using the primers below. The PCR products were cloned into TA-vector first (TOPO® TA Cloning® Kit, Invitrogen), verified by sequencing and then recloned into Not1 and Xba1 sites in the pUASt vector..
Forward (Not1): 5-GCGGCCGCTCGGTTCAGCAAACTTCAGTTCCG-3
Reverse (Xba1): 5-TCTAGAGGATCGGTATTTATGCTTACATTTACACT-3
Generation of deletion mutants
P-element mediated imprecise excision from the EP-CG3313 line (Bloomington #17350; P[EPgy]2CG3313EY05707) was used to generate dcaf12Δ1. After remobilizing the inserted P-element by crossing the original line to Δ2-3 transposase (P[ry[+t7.2]=Delta2-3]99B), the individual potential deletion alleles were balanced with TM3 or TM6. They were screened by PCR with the following primers. The amplified region by these primers was about 2 kb, and any PCR products shorter than this was assumed to be deletion alleles.
5′ Primer: TGT CTT GGC GGA ATA CAT ATG C
3′ Primer: TTG TGA AAG CGA TGG CCT A
After screening over 200 individual excised lines, one line with ~ 0.5 kb (Figure 1B) was identified– and sequenced to verify the breaking points. The deleted sequences were from 3R:7884610 to 3R:7841368 (1236 bps). This line was named dcaf12Δ1.
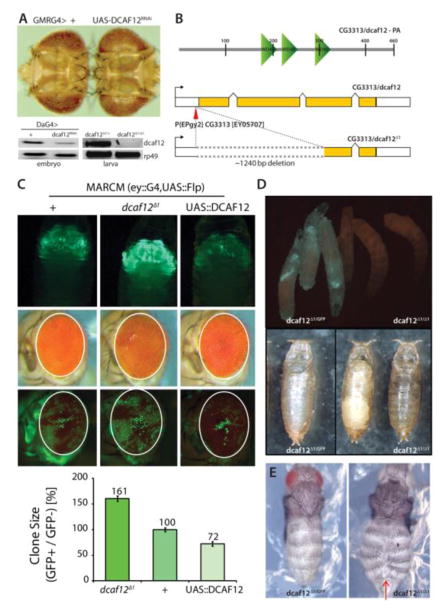
Figure 1. Effects of DCAF12 on retinal growth.
A) Knockdown of CG3313/dcaf12 in the developing retina causes tissue overgrowth. GMRGal4 expresses UAS-linked transgenes in all cells of the developing retina posterior to the morphogenetic furrow. The CG3313RNAi transgene effectively decreases CG3313 expression (lower panel, DA-Gal4 expresses UAS-linked transgenes ubiquitously). CG3313 transcripts are also undetectable in larvae of homozygous dcaf12Δ1/Δ1 mutant animals. The endogenous transcript of dcaf12 was amplified using RT-PCR (40 cycles).
B) Schematic representations of DCAF12 protein structure (upper) and of dcaf12Δ1 generated from EPgy2 element EY05707 by imprecise excision (lower). About 1240 nucleotides were deleted from the first to third exon in dcaf12Δ1. DCAF12 contains four WD40-repeats. dcaf12 sequence is 51% similar to human WDR40a/dcaf12 (ClustalW).
C) GFP-marked clones generated throughout eye development using the MARCM system (eyelessGal4, UAS-FLP; act>y+>Gal4, UAS-GFP; FRT82B, tubGal80). Upper: Overexpression of dcaf12 limits clonal growth, while clones homozygous for dcaf12Δ1 over-grow. Representative images of each genotype are shown. Despite the difference in clones, the entire eye structure is not significantly affected. Lower: Quantification of clone sizes using Image J and Phenocapture ®.
D) Comparison of sibling 3rd instar larvae heterozygous (left, carrying a GFP-expressing balancer chromosome) or homozygous for dcaf12Δ1 (right). Eggs were collected for ~ 4 hours and the crawling third instar larvae were examined at ~ 110 hours AEL.
dcaf12Δ1/Δ1 is lethal at pupal stage. Pupae of each genotype were examined ~124 hours AEL. The left panel shows an empty pupal case of a heterozygote (dcaf12Δ1/+ ), and the right panel shows arrested homozygous pupae. Heterozygous pupae emerged as adults ~120 AEL but homozygotes never emerged even after ~15days.
E) Abdominal closure defect in dcaf12Δ1/Δ1. Pharate adults of each genotype were dissected and examined. dcaf12Δ1 homozygotes exhibit frequent clefts in the dorsal abdominal cuticle, but no obvious phenotype was observed in dcaf12Δ1 heterozygotes.
Retinal UV sensitivity
The protocol from (Kelsey et al., 2012; Luo et al., 2007a) was directly adapted. Pupae of 24 hours APF were collected, and the pupal case in the head region was dissected to expose head and retinas. Only right retinas were treated with 12.5mJ/cm2 of UV radiation by UV crosslinker (Stratalinker 1800). To avoid light-dependent DNA repair, pupa were kept in the dark after irradiation until they emerged. The size of adult eyes was measured in Adobe Photoshop.
TUNEL staining
ApopTag kit (Chemicon International) S7101 and S7111 were used. Instead of glass slides, 500uL tubes were used to handle the samples for each step.
Western blotting
Samples (whole pupae for figure 2–10 or adult males for figure 3–4(C)) were prepared in 2X SDS sample buffer (Laemmli Sample Buffer), resolved by 10% (for beta-actin, diap2, HA, and phospo-S6K) ~12% (for diap1 and caspase-3) SDS-PAGE, transferred to nitro nitrocellulose membrane using the semi-dry transfer method (Trans-Blot®, Bio-Rad), incubated with primary antibodies overnight in 1X TBS/T (1X TBS + 0.1% Tween-20 with 5% BSA), blocked in non-fat dry milk, washed in 1X TBS/T, incubated with secondary antibodies (1:5000 dilution, Goat Ani-Mouse, Rabbit (Bio-Rad), Guinea Pig (Jackson Laboratory) - HRP), detected by using horseradish-conjugated secondary antibodies, followed by the ECL detection system (GE Healthcare Life sciences or Pierce).
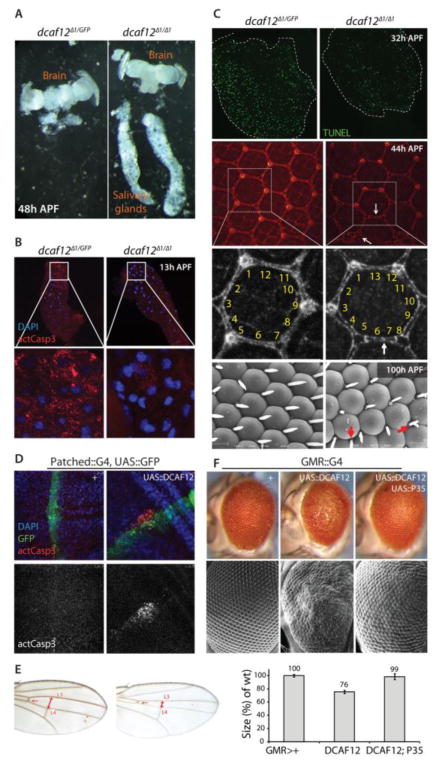
Figure 2. dcaf12 is required and sufficient to promote apoptosis in developing tissues.
A. Lack of salivary gland destruction in dcaf12Δ1 homozygous flies. Pupae of each genotype were dissected ~ 48 hours APF. While the developing retina and brain are indistinguishable between heterozygotes and homozygotes, larval salivary glands have been histolyzed and are absent from heterozygous flies, but still present in dcaf12Δ1 homozygotes.
B. Lack of active Caspase in larval salivary glands in dcaf12Δ1 homozygous flies. Salivary glands were collected at 13 hours APF and immunostained using anti-cleaved-Caspase-3 antibody (#9661 from Cell Signaling).
C. dcaf12 is required for apoptosis in the differentiating retina.
Top panels: TUNEL staining (green) was performed in pupal retina (32 hrs APF) of the indicated genotypes.
Middle panels: Pupal retina of indicated genotypes were collected at 44h APF and immunostained using anti-discs-large antibody to visualize cell boundaries. Extra cells and mis-specified cells in dcaf12Δ1/Δ1 are indicated by arrows.
Lower panels: Eyes of pharate adults of indicated genotypes were collected and visualized by scanning electron microscopy. Extra bristles are indicated by arrows in dcaf12Δ1/Δ1.
D. Increased apoptosis by overexpression of dcaf12 in wing disc using patched::Gal4. Expression domain indicated by co-expression of GFP (green). Cleaved-Caspase-3 antibody staining (red) was performed on 3rd instar larval wing imaginal discs.
E. Tissue size reduction by over-expression of dcaf12 between L3 and L4 veins using patched::Gal4. Note the reduced size of the intervein region between L3 and L4 veins.
F. P35-sensitive cell death phenotype in eyes over-expressing dcaf12 using GMR::Gal4. Eye sizes in each genotype were measured using Photoshop® and normalized to control. Student’s t test: p<0.001 between GMR-Gal4> w1118 and GMR-Gal4>UAS-dcaf12. Error bars represent the standard error of the mean.
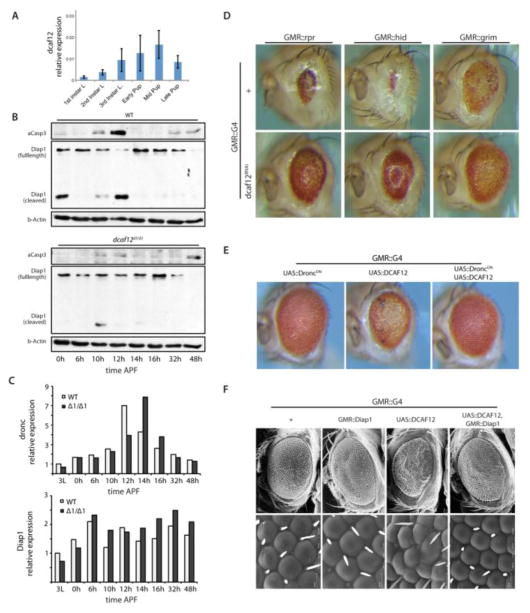
Figure 3. Interaction of dcaf12 with the canonical Caspase cascade.
A. Endogenous dcaf12 transcripts in wild type animals analyzed by qRT-PCR at designated time points during development (left panel). Expression normalized to actin5C.
B. Misregulation of the apoptotic signaling cascade in dcaf12Δ1/Δ1 mutants. Activation of apoptotic signaling during pupal development was assessed by detecting cleaved-caspase-3 (Cell Signaling #9661), total dIAP1, and cleaved dIAP1 (gift from Dr. Bruce Hay) by Western Blot. DIAP1 cleavage was observed in wild types (upper panel), but not in dcaf12Δ1/Δ1 mutants (lower panel). Beta-actin (Cell Signaling # 4967) was detected as loading control.
C. Expression pattern of dronc and diap1 in dcaf12Δ1/Δ1. Expression of dronc and diap1 were not significantly affected in dcaf12Δ1/Δ1 mutants. Total RNA was collected from 7~8 individual larvae or pupae of designated ages and used for qRT-PCR analysis. Expression was compared to actin5C and normalized to the value from wild type 3rd instar larvae.
D. Rescue of RHG-induced apoptotic phenotypes in the retina by knocking down dcaf12
E. Interaction of DCAF12 with dominant-negative Dronc (DroncDN, also known as Dronc-CARD). Expression of DroncDN rescues the apoptotic phenotype caused by over-expression of dcaf12.
F. Expression of diap1 rescues the apoptotic phenotype caused by overexpression of dcaf12. Eye structure was analyzed by scanning electron microscopy.
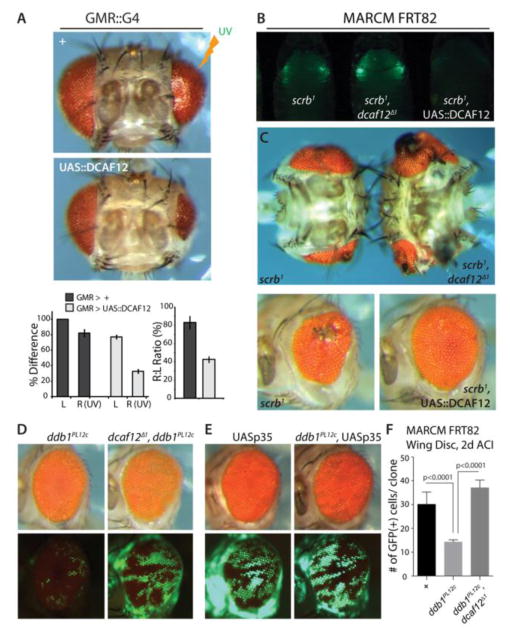
Figure 4. dcaf12 in the control of tissue homeostasis.
A. Overexpression of dcaf12 sensitizes retina to UV-induced cell death. Tissue loss after UV irradiation in pupae (at 24 hours APF) was quantified by measuring size of the right (irradiated; 17.5mJ of UV radiation using Stratalinker 1800) and left (non-irradiated) adult retina. Error bars represent the standard error of the mean. In all cases p<0.01 from Student’s t-test.
B, C. dcaf12 genedose influences tumor-like phonotype of scribble mutant cells. GFP-marked mutant clones were generated using an eye-specific MARCM system (eyeless::Gal4, UAS::FLP; act>y+>Gal4, UAS::GFP; FRT82B, tub::Gal80). Clonal growth can be observed in mid-pupal stages (B). Consistent with the differences in clonal growth, the tumor-like phenotype in the adult retina was strongly influenced by the dcaf12 genedose (C).
D, E. Rescue of ddb1 loss of function phenotype by overexpression of P35 or loss of dcaf12
The ddb1 hypomorphic allele ddb1PL12c strongly limits clonal growth in the eye. Loss of dcaf12 (D), or over-expression of P35 (E) in these clones rescues clonal growth.
F. Rescue of ddb1PL12c loss of function phenotype by loss of dcaf12. Quantification of clone sizes in third instar wing discs (2 days after clone induction, ACI). GFP-marked clones were generated using MARCM (hs::FLP, UAS::GFP;;tub::Gal4, FRT82B, tub::Gal80). Error bars represent the standard error of the mean. P values from Student’s t-test.
The following primary antibodies were used.
Diap1: a gift from Dr. Bruce Hay (CalTech); 1:2,000
Cleaved caspase-3: Cell Signaling (#9661); 1:1,000
beta-Actin: Cell Signaling (#4967); 1:1,000
Immunostaining and Microscopy
Tissues (salivary glands and retina-head complex) were dissected in PBS, fixed in PP (4% paraformaldehyde, 0.1% Tween-20, and 0.1% Triton X-100 in PBS) washed in PBTr (PBS + 0.1% Triton X-100), blocked in BBTr (PBTr + 0.1% BSA) for 1 hour, and incubated overnight with primary antibodies diluted in BBTr. Next day, the samples were washed in BBTr and incubated overnight with appropriate 2nd antibodies. Next day, the samples were washed in BBTr and mounted with Moviol for observation under confocal microscope (Leica SP5). Primary antibodies used in this study are anti-activated-caspase 3 (Cell Signaling #9661; 1:100) and anti-dics-large (DSHB, 4F3 anti-discs large; 1:50).
Real Time PCR
RNA was prepared from whole flies, whole pupae, or whole larvae using Trizol reagent (Invitrogen). About 2~5 ug of RNA was used to synthesize cDNA by oligodT and Superscript reverse transcriptase II (Invitrogen). The resulting cDNA was diluted by 20~50 times to be used for real-time PCR using SYBR green in a Biorad IQ5 machine. The following primers were used.
| actin5C: | Forward: 5′ - CTCGCCACTTGCGTTTACAGT - 3 Reverse: 5′ - TCCATATCGTCCCAGTTGGTC - 3 |
| dcaf12 | Forward 5-TATGCCGTTGGATGCCGTTCCTAT-3 Reverse 5-TATTGCCAAACGCCCGCATAGTTG-3 |
| dronc | Forward 5- ACCCTTTATCTCGCTAAACGAAC-3 Reverse 5- TCAACGACACCCACATAAGG-3 |
| diap1 | Forward 5- GCTACTCCCTCGACAAACAG -3 Reverse 5- AGGAATGCCGTATTGTACTCG -3 |
Results and Discussion
DCAF12 regulates tissue growth during development
In genetic studies aimed at identifying novel regulators of apoptosis in the Drosophila retina (Kelsey et al., 2012; Luo et al., 2007b; Nielsen et al., 2008), we found that knocking down a gene named CG3313 by RNAi in the developing retina resulted in an overgrown and ‘rough’ eye phenotype (Fig. 1A). CG3313 encodes a previously uncharacterized homologue of human Wdr40a/DCAF12, and we therefore renamed the gene dcaf12. Drosophila and human WDR40a are 43% similar at the amino acid level. To study the function of DCAF12 in detail, we generated a deletion allele for dcaf12 by imprecise excision of a P-element. We mobilized a P[EPgy2] insertion in the 5-UTR of dcaf12 (allele CG3313EY05707), and obtained a deletion of 1240 bp that encompasses the first three exons of dcaf12 without affecting any neighboring genes (allele named dcaf12Δ1; Fig. 1B). dcaf12 transcripts were not detectable in larvae homozygous for dcaf12Δ1, confirming that this is a null allele (Fig. 1A).
Homozygosity for dcaf12Δ1 leads to pupal lethality, and this phenotype could be rescued fully by a transgene expressing dcaf12 from a UAS promoter (Fig. S2A, B), further confirming that dcaf12Δ1 is a loss of function allele. Over-expressing this transgene throughout development in the whole animal in a wild-type background resulted in significant size reduction of the emerging adults, suggesting that DCAF12 is sufficient to limit growth (Fig. S2C). To assess the effects of loss of dcaf12 on retinal growth, we generated homozygous dcaf12Δ1 mutant cell clones using Mosaic Analysis with a Repressible Cell Marker (MARCM) (Lee and Luo, 1999). To ensure widespread recombination, we used FLP recombinase driven by the eyeless promoter (Newsome et al., 2000). In a wild-type background, this strategy results in about 50% of all adult ommatidia expressing GFP. dcaf12Δ1 homozygous cells, however, appeared to outcompete heterozygous cells, resulting in a strong increase in GFP fluorescence in the head during pupal development, and in adult retinas in which mutant GFP+ cells were 1.6 fold more abundant than GFP- cells (Figure 1C). We confirmed these results using the EGUF system, in which retinal cells are killed by expression of Hid, and recombination generates cells without Hid expression (Stowers and Schwarz, 1999). dcaf12 mutant cells were able to restore the retina to a larger size than wild-type cells (Fig. S3B). Similarly, when the dcaf12Δ1mutation was recombined against a chromosome carrying a recessive lethal (l(3)cl) marked by w+, the homozygous dcaf12 mutant cells outcompeted heterozygous cells to a greater extent than wild-type cells in control experiments (Fig. S3B). These experiments support the notion that loss of DCAF12 results in over-proliferation or enhanced maintenance of retinal cells. DCAF12 over-expressing cells, on the other hand, were outcompeted by wild-type cells (Fig. 1C). At the same time, we did not observe any changes in the pattern of cell proliferation in developing eye discs deficient for dcaf12 (Fig. S3A), indicating that this gene influences cell numbers in developing tissues without affecting proliferation.
DCAF12 is required and sufficient for developmental apoptosis during metamorphosis
dcaf12Δ1 homozygotes develop to third instar without any apparent phenotypes, and reach the white prepupal stage at the same rate compared to their heterozygous siblings. However, homozygous mutant animals do not complete metamorphosis, but arrest during pupal development and eventually die (Fig. 1D). Although a small portion of mutant pupae survive to pharate adults, we did not observe a single adult escaper among more than a thousand mutant flies. DCAF12 is thus essential for completing pupal development. Interestingly, homozygous mutant pharate adults display cuticle closure phenotypes that are consistent with defects in the replacement of larval epidermal cells by histoblasts (Fig. 1E) (Madhavan and Madhavan, 1980). Loss of drICE causes similar phenotypes, suggesting potential defects in apoptosis during metamorphosis in dcaf12 deficient animals (Muro et al., 2006).
To explore a possible role for DCAF12 in pupal apoptosis, we analyzed histolysis of larval salivary glands. During early metamorphosis, around 12h APF (After Puparium Formation), larval salivary glands start to be degraded by apoptosis, and by 16h APF this larval tissue is completely histolyzed (Yin et al., 2007). This removal of larval salivary glands failed in dcaf12Δ1/Δ1 homozygotes, and salivary glands remained morphologically intact at least until 48h APF (Fig. 2A). Brain and differentiating retina at 40h~48h APF showed no gross differences between wild types and dcaf12Δ1/Δ1 mutants (Fig. 2A), indicating that differentiation of imaginal tissues is broadly normal in these mutants. These results suggested that dcaf12 is specifically required for timely degradation of larval tissue during metamorphosis, a phenotype that is also observed in drIce, dronc and dark mutants. (Daish et al., 2004; Mills et al., 2006; Muro et al., 2006). Supporting this view, activation of the caspase cascade in salivary glands, as determined by antibody staining against active caspase-3, was significantly delayed in dcaf12 homozygotes (Fig. 2B).
To assess if the function of DCAF12 is limited to histolyzing tissues, or whether apoptotic processes in developing adult tissues are also affected, we focused on the developing retina. In this tissue, about one third of all cells generated during larval development will not be incorporated into ommatidia, and these cells need to be removed by apoptosis. Apoptosis in the retina reaches its peak at 32 hours after puparium formation (Brachmann and Cagan, 2003; Cagan and Ready, 1989; Wolff and Ready, 1991). In a fully developed pupal eye, 12 cells surround each ommatidium: 6 secondary pigment cells, 3 tertiary pigment cells, and 3 bristle cells (Brachmann and Cagan, 2003). Among these 12 cells, the secondary and the tertiary cells are targeted for apoptosis (Brachmann and Cagan, 2003; Cadigan and Nusse, 1996). When apoptosis is impaired, excessive cells are observed in the adult retina, resulting in mild ‘rough eye’ phenotypes. We observed the same phenotypes in dcaf12 mutants: strongly decreased retinal apoptosis at 32 hours APF (as observed by TUNEL staining) was accompanied by excessive inter-ommatididal cells at 44 h APF, and resulted in eyes with supernumerary bristles in the adult (Fig. 2C).
These results indicated that dcaf12 is required for apoptosis in various contexts. dcaf12 over-expression is also sufficient to induce apoptosis, as when dcaf12 was expressed between the L3 and L4 veins in the developing wing disc using patched-Gal4, the size of this region was significantly reduced in the adult, and increased active-Caspase 3 staining was observed in the patched expression domain in the 3rd instar larval wing disc (Fig. 2D, E). Similarly, over-expression of dcaf12 in the developing retina using GMR-Gal4 (Hay et al., 1997) resulted in strong size reduction and disruption of normal patterning in the adult eye (Fig. 2F). This phenotype was rescued by co-expression of the viral inhibitor of apoptosis p35, confirming that over-expressing dcaf12 is sufficient to ectopically induce Caspase-mediated cell death (Fig. 2F).
DCAF12 activates the canonical caspase cascade by regulating dIAP1 Cleavage
Apoptotic cell death during metamorphosis is induced by 20-hydroxyecdysone (ecdysone), which regulates the expression of core components of the apoptotic cascade. A high titer of Ecdysone induces reaper, hid, dronc and drice, and represses transcription of diap1 (Yin et al., 2007). Interestingly, the transcriptional profile of dcaf12 recapitulates the expression of Ecdysone-responsive genes: dcaf12 expression gradually increases in pupae and reaches its peak during the mid-pupal stage (Fig. 3A). Further highlighting the importance of dcaf12 in the induction of apoptosis during development, we observed that global activation of apoptosis during metamorphosis is lost in dcaf12Δ1/Δ1 homozygous pupae, as evidenced by the lack of Caspase-3 activation and by reduced Diap1 cleavage and maintenance of high levels of full-length Diap1 at 12 hours after puparium formation (Fig. 3B; Diap1 cleavage is a consequence of Drice and Dronc activation (Muro et al., 2005)). Importantly, transcript levels of dronc or diap1 were unaffected by dcaf12 deficiency (Fig. 3C).
To place DCAF12 into the regulatory cascade promoting cell death, we performed epistatic analysis: Knockdown of dcaf12 rescued RHG-induced apoptosis (Fig. 3D), while over-expression of dominant-negative Dronc (Meier et al., 2000) was sufficient to rescue size and structure of retinae in which dcaf12 was over-expressed (Fig. 3E). These observations strongly suggest that DCAF12 is an integral component of the canonical RHG-induced apoptotic pathway, acting between RHG proteins and Dark-DRONC activation. RHG proteins induce apoptosis by binding to and destabilizing Diap1, facilitating Dark-mediated apoptosome formation and Dronc activation. Based on its interaction with RHG genes and Dronc, and on the lack of Diap1 cleavage in dcaf12 deficient pupae, DCAF12 may thus be involved in the inhibition of Diap1 by RHG proteins, or in apoptosome formation by stabilization of Dark and/or Dronc. Accordingly, Diap1 over-expression is sufficient to strongly suppress DCAF12-mediated apoptosis (Fig. 3F).
Several post-translational regulatory processes have been described that can affect dIAP1 function (Ditzel and Meier, 2005; Vucic et al., 2011). E3 ubiquitin ligase-mediated degradation of dIAP1 (Xu et al., 2009) is unlikely to be affected by dcaf12, since total protein levels of dIAP1 were comparable throughout early pupal development in wild-type and dcaf12 mutants (Fig. 3B). Furthermore, over-expression of dcaf12 did not affect total protein levels of Diap1 (data not shown). Our data support a role for dcaf12 in regulating of Dronc activity, potentially by promoting autoproteolytic cleavage and/or stabilization of active Dronc dimers (Xu et al., 2009; Yan et al., 2006). The mechanism by which cleaved Dronc is stabilized is not understood, and it will be interesting to test if DCAF12 facilitates this stabilization.
DCAF12 may further be involved in the formation of the active apoptosome: while the Drosophila apoptosome comprises only Dronc and Dark, overexpression of neither is sufficient to strongly induce apoptosis. Only when dark and dronc are co-expressed, they function synergistically to induce apoptosis (Meier et al., 2000; Shapiro et al., 2008; Xu et al., 2009). Dorstyn et al. have suggested that Dark alone is not sufficient for Dronc activation and that additional molecules have to exist that enhance Dark-mediated Dronc activation (Dorstyn and Kumar, 2008).
It is possible that DCAF12 is such an enhancing molecule. Supporting this view, DCAF12 over-expression strongly sensitizes cells to genotoxic stress: Exposure of the developing pupal retina to UV-light at 24 hours APF causes widespread apoptosis in the affected eye, resulting in adults that exhibit major tissue loss in the affected eye (Kelsey et al., 2012; Luo et al., 2007b; Nielsen et al., 2008). In these experiments, the relative size of the irradiated and the non-irradiated control eye can serve as a measure of DNA damage-induced apoptosis (Kelsey et al., 2012; Luo et al., 2007b; Nielsen et al., 2008). Over-expression of dcaf12 using GMR::G4 dramatically increased the sensitivity of the retina to UV irradiation (Fig. 4A), consistent with a role for DCAF12 in facilitating rather than inducing apoptosis. Interestingly, the peak of dcaf12 expression during pupal development coincides with the period of maximal UV sensitivity (Kelsey et al., 2012; Luo et al., 2007b; Nielsen et al., 2008), indicating that natural fluctuations in dcaf12 expression can influence the responsiveness of cells to apoptotic stimuli.
DCAF12 thus seems to act as a pro-apoptotic molecule only in the presence of other pro-apoptotic stimuli. In mammalian cells, Cytochrome c binds to the WD40 domain of Apaf-1 to fully activate the apoptosome. It will be interesting to test if DCAF12 can bind to the WD40 domain of Dark, thus taking the place of Cytochrome C in the activation of the Drosophila apoptosome.
DCAF12: a critical regulator of tissue homeostasis
Our data presented above indicate that DCAF12 is required for developmental apoptosis during metamorphosis. At the same time, dcaf12 mutant cells outcompete wild-type cells in the developing eye-disc. Since tissue homeostasis during development relies on processes in which cells with a growth advantage kill neighboring, ‘unfit’ cells (Johnston, 2009), these results suggested that DCAF12 is also required for cell death in conditions in which tissue homeostasis is maintained by regulated apoptosis. To test this idea directly, we assessed two conditions in which cell death plays an important homeostatic role: cell death induced by mutations in neoplastic tumor suppressors that perturb epithelial integrity, and death induced by genotoxic stress (Figure 4B–F). In Drosophila imaginal discs, the loss of neoplastic tumor suppressors can cause tumors that disrupt epithelial structure. Apoptotic processes usually limit the extent of this overgrowth, as in cells mutant for the scribble tumor suppressor, which are usually eliminated by JNK-induced apoptosis in developing imaginal discs (Brumby and Richardson, 2003; Uhlirova et al., 2005). When apoptosis is impaired in such cells, significant tissue overgrowth is observed in the adult. Most of the overgrown tissue in these conditions is contributed by non-mutant cells, which are induced to over-proliferate by the scrib mutant cells (Brumby and Richardson, 2003; Uhlirova et al., 2005). On the other hand, when apoptosis of scrib mutant cells is enhanced, tissue integrity is improved (Brumby and Richardson, 2003; Uhlirova et al., 2005). We observed similar responses in scrib, dcaf12 double-mutant clones (increased tissue overgrowth) and in scrib mutant clones over-expressing dcaf12 (enhanced tissue integrity; Fig. 4 B,C). DCAF12 is thus sufficient and required to promote tissue integrity in conditions of compromised epithelial integrity.
We further tested whether dcaf12 is required for apoptosis in response to genotoxic stress by assessing whether cell death caused by ddb1 deficiency would be affected by the dcaf12 genedose (Fig. 4 D–F). DDB1 binds to DNA damaged by UV irradiation and is involved in nucleotide excision repair (Iovine et al., 2011b). It forms a complex with the CUL4 E3 ubiquitin ligase, acting as a linker between CUL4 and adapter molecules that specify target proteins to be degraded (Iovine et al., 2011a; Iovine et al., 2011b; Scrima et al., 2011). In Drosophila and mammals, loss of ddb1 results in loss of genome integrity and subsequent apoptosis (Cang et al., 2006; Takata et al., 2004a; Takata et al., 2004b; Wakasugi et al., 2007). Using the MARCM system, we found that loss of dcaf12 prevented the apoptotic phenotype of the hypomorphic ddb1 allele ddb1PL12c, allowing ddb1 mutant cells to survive and contribute to the adult eye (Fig. 4 D). The extent of this rescue was similar to a rescue caused by over-expression of P35 (Fig. 4E), suggesting that the pro-apoptotic function of DCAF12 was critical for this interaction. We observed a similar interaction between DCAF12 and ddb1 in the developing wing disk (Fig. 4F).
Interestingly, biochemical and genomic studies have identified mammalian WDR40a/DCAF12 as a physical binding partner of DDB1 (Angers et al., 2006; Higa et al., 2006; Jin et al., 2006; Olma et al., 2009). In a separate study, we have observed genetic interactions between dcaf12, ddb1, cul4, and the ddb1/cul4 binding partner cdt2 in the control of cell cycle exit in enteroblasts of the Drosophila intestinal stem cell lineage (Kim et al., submitted). The results obtained in that study, and the interaction of dcaf12 with the ddb1 hypomorphic allele shown here, suggest that DCAF12 negatively influences the DDB1/CUL4 complex.
Based on the data presented here, we propose that DCAF12 plays a critical role in the execution of apoptosis in both developmental and pathological contexts. Our data suggest that expression of dcaf12 may be induced by ecdysone during metamorphosis along with other core components of the apoptotic machinery. DCAF12 is required for execution of the core pro-apoptotic signaling cascade, promoting the proteolytic processing of Diap1 and ensuring full activation of Drice and execution of Drice-mediated apoptosis.
Conclusions
This study introduces the conserved WD40-motif repeat family protein DCAF12 as an evolutionarily conserved regulator of programmed cell death (PCD). Using Drosophila melanogaster as a model system, it is shown that DCAF12 is required for PCD in a range of developmental contexts, acting to regulate the core apoptotic machinery. DCAF12 is thus indispensable for normal development, influences tissue growth, and mitigates the loss of tissue homeostasis in neoplastic contexts.
Supplementary Material
Highlights.
DCAF12 regulates tissue growth during development
DCAF12 regulates developmental apoptosis
DCAF12 activates the canonical caspase cascade by regulating dIAP1 Cleavage
DCAF12 is required for tissue homeostasis under stress conditions
Acknowledgments
This work was supported by the National Eye Institute (R01 EY018177 to H.J.). We would like to thank Olga Dunaevsky for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akdemir F, Farkas R, Chen P, Juhasz G, Medved’ova L, Sass M, Wang L, Wang X, Chittaranjan S, Gorski SM, Rodriguez A, Abrams JM. Autophagy occurs upstream or parallel to the apoptosome during histolytic cell death. Development. 2006;133:1457–1465. doi: 10.1242/dev.02332. [DOI] [PubMed] [Google Scholar]
- Angers S, Li T, Yi X, MacCoss MJ, Moon RT, Zheng N. Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Nature. 2006;443:590–593. doi: 10.1038/nature05175. [DOI] [PubMed] [Google Scholar]
- Brachmann CB, Cagan RL. Patterning the fly eye: the role of apoptosis. Trends Genet. 2003;19:91–96. doi: 10.1016/S0168-9525(02)00041-0. [DOI] [PubMed] [Google Scholar]
- Brumby AM, Richardson HE. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. Embo J. 2003;22:5769–5779. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. wingless signaling in the Drosophila eye and embryonic epidermis. Development. 1996;122:2801–2812. doi: 10.1242/dev.122.9.2801. [DOI] [PubMed] [Google Scholar]
- Cagan RL, Ready DF. The emergence of order in the Drosophila pupal retina. Developmental biology. 1989;136:346–362. doi: 10.1016/0012-1606(89)90261-3. [DOI] [PubMed] [Google Scholar]
- Cang Y, Zhang J, Nicholas SA, Bastien J, Li B, Zhou P, Goff SP. Deletion of DDB1 in mouse brain and lens leads to p53-dependent elimination of proliferating cells. Cell. 2006;127:929–940. doi: 10.1016/j.cell.2006.09.045. [DOI] [PubMed] [Google Scholar]
- Cashio P, Lee TV, Bergmann A. Genetic control of programmed cell death in Drosophila melanogaster. Seminars in cell & developmental biology. 2005;16:225–235. doi: 10.1016/j.semcdb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Daish TJ, Mills K, Kumar S. Drosophila caspase DRONC is required for specific developmental cell death pathways and stress-induced apoptosis. Dev Cell. 2004;7:909–915. doi: 10.1016/j.devcel.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Ditzel M, Meier P. Ubiquitylation in apoptosis: DIAP1’s (N-)en(d)igma. Cell death and differentiation. 2005;12:1208–1212. doi: 10.1038/sj.cdd.4401711. [DOI] [PubMed] [Google Scholar]
- Dorstyn L, Kumar S. A biochemical analysis of the activation of the Drosophila caspase DRONC. Cell death and differentiation. 2008;15:461–470. doi: 10.1038/sj.cdd.4402288. [DOI] [PubMed] [Google Scholar]
- Dorstyn L, Mills K, Lazebnik Y, Kumar S. The two cytochrome c species, DC3 and DC4, are not required for caspase activation and apoptosis in Drosophila cells. J Cell Biol. 2004;167:405–410. doi: 10.1083/jcb.200408054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicologic pathology. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyrd-Hansen M, Meier P. IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nature reviews Cancer. 2010;10:561–574. doi: 10.1038/nrc2889. [DOI] [PubMed] [Google Scholar]
- Hay BA, Maile R, Rubin GM. P element insertion-dependent gene activation in the Drosophila eye. Proc Natl Acad Sci U S A. 1997;94:5195–5200. doi: 10.1073/pnas.94.10.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa LA, Wu M, Ye T, Kobayashi R, Sun H, Zhang H. CUL4-DDB1 ubiquitin ligase interacts with multiple WD40-repeat proteins and regulates histone methylation. Nature cell biology. 2006;8:1277–1283. doi: 10.1038/ncb1490. [DOI] [PubMed] [Google Scholar]
- Iovine B, Iannella ML, Bevilacqua MA. Damage-specific DNA binding protein 1 (DDB1) is involved in ubiquitin-mediated proteolysis of p27Kip1 in response to UV irradiation. Biochimie. 2011a;93:867–875. doi: 10.1016/j.biochi.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Iovine B, Iannella ML, Bevilacqua MA. Damage-specific DNA binding protein 1 (DDB1): a protein with a wide range of functions. Int J Biochem Cell Biol. 2011b;43:1664–1667. doi: 10.1016/j.biocel.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Jin J, Arias EE, Chen J, Harper JW, Walter JC. A family of diverse Cul4-Ddb1-interacting proteins includes Cdt2, which is required for S phase destruction of the replication factor Cdt1. Mol Cell. 2006;23:709–721. doi: 10.1016/j.molcel.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Johnston LA. Competitive interactions between cells: death, growth, and geography. Science. 2009;324:1679–1682. doi: 10.1126/science.1163862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey EM, Luo X, Bruckner K, Jasper H. Schnurri regulates hemocyte function to promote tissue recovery after DNA damage. Journal of cell science. 2012;125:1393–1400. doi: 10.1242/jcs.095323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuranaga E. Caspase signaling in animal development. Dev Growth Differ. 2011;53:137–148. doi: 10.1111/j.1440-169X.2010.01237.x. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Luo X, Puig O, Hyun J, Bohmann D, Jasper H. Foxo and Fos regulate the decision between cell death and survival in response to UV irradiation. The EMBO journal. 2007a;26:380–390. doi: 10.1038/sj.emboj.7601484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Puig O, Hyun J, Bohmann D, Jasper H. Foxo and Fos regulate the decision between cell death and survival in response to UV irradiation. The EMBO journal. 2007b;26:380–390. doi: 10.1038/sj.emboj.7601484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan MM, Madhavan K. Morphogenesis of the epidermis of adult abdomen of Drosophila. J Embryol Exp Morphol. 1980;60:1–31. [PubMed] [Google Scholar]
- Meier P, Silke J, Leevers SJ, Evan GI. The Drosophila caspase DRONC is regulated by DIAP1. Embo J. 2000;19:598–611. doi: 10.1093/emboj/19.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K, Daish T, Harvey KF, Pfleger CM, Hariharan IK, Kumar S. The Drosophila melanogaster Apaf-1 homologue ARK is required for most, but not all, programmed cell death. J Cell Biol. 2006;172:809–815. doi: 10.1083/jcb.200512126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro I, Berry DL, Huh JR, Chen CH, Huang H, Yoo SJ, Guo M, Baehrecke EH, Hay BA. The Drosophila caspase Ice is important for many apoptotic cell deaths and for spermatid individualization, a nonapoptotic process. Development. 2006;133:3305–3315. doi: 10.1242/dev.02495. [DOI] [PubMed] [Google Scholar]
- Muro I, Means JC, Clem RJ. Cleavage of the apoptosis inhibitor DIAP1 by the apical caspase DRONC in both normal and apoptotic Drosophila cells. J Biol Chem. 2005;280:18683–18688. doi: 10.1074/jbc.M501206200. [DOI] [PubMed] [Google Scholar]
- Newsome TP, Asling B, Dickson BJ. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000;127:851–860. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- Nielsen MD, Luo X, Biteau B, Syverson K, Jasper H. 14-3-3 Epsilon antagonizes FoxO to control growth, apoptosis and longevity in Drosophila. Aging Cell. 2008;7:688–699. doi: 10.1111/j.1474-9726.2008.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olma MH, Roy M, Le Bihan T, Sumara I, Maerki S, Larsen B, Quadroni M, Peter M, Tyers M, Pintard L. An interaction network of the mammalian COP9 signalosome identifies Dda1 as a core subunit of multiple Cul4-based E3 ligases. Journal of cell science. 2009;122:1035–1044. doi: 10.1242/jcs.043539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl SJ, Salvesen GS. The apoptosome: signalling platform of cell death. Nature reviews Molecular cell biology. 2007;8:405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- Rubin GM, Spradling AC. Genetic transformation of Drosophila with transposable element vectors. Science (New York, N Y ) 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Scrima A, Fischer ES, Lingaraju GM, Bohm K, Cavadini S, Thoma NH. Detecting UV-lesions in the genome: The modular CRL4 ubiquitin ligase does it best! FEBS Lett. 2011;585:2818–2825. doi: 10.1016/j.febslet.2011.04.064. [DOI] [PubMed] [Google Scholar]
- Shapiro PJ, Hsu HH, Jung H, Robbins ES, Ryoo HD. Regulation of the Drosophila apoptosome through feedback inhibition. Nature cell biology. 2008;10:1440–1446. doi: 10.1038/ncb1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling AC, Rubin GM. Transposition of cloned P elements into Drosophila germ line chromosomes. Science (New York, N Y ) 1982;218:341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Stowers RS, Schwarz TL. A genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype. Genetics. 1999;152:1631–1639. doi: 10.1093/genetics/152.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata K, Shimanouchi K, Yamaguchi M, Murakami S, Ishikawa G, Takeuchi R, Kanai Y, Ruike T, Nakamura R, Abe Y, Sakaguchi K. Damaged DNA binding protein 1 in Drosophila defense reactions. Biochem Biophys Res Commun. 2004a;323:1024–1031. doi: 10.1016/j.bbrc.2004.08.182. [DOI] [PubMed] [Google Scholar]
- Takata K, Yoshida H, Yamaguchi M, Sakaguchi K. Drosophila damaged DNA-binding protein 1 is an essential factor for development. Genetics. 2004b;168:855–865. doi: 10.1534/genetics.103.025965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlirova M, Jasper H, Bohmann D. Non-cell-autonomous induction of tissue overgrowth by JNK/Ras cooperation in a Drosophila tumor model. Proc Natl Acad Sci U S A. 2005;102:13123–13128. doi: 10.1073/pnas.0504170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucic D, Dixit VM, Wertz IE. Ubiquitylation in apoptosis: a post-translational modification at the edge of life and death. Nature reviews Molecular cell biology. 2011;12:439–452. doi: 10.1038/nrm3143. [DOI] [PubMed] [Google Scholar]
- Wakasugi M, Matsuura K, Nagasawa A, Fu D, Shimizu H, Yamamoto K, Takeda S, Matsunaga T. DDB1 gene disruption causes a severe growth defect and apoptosis in chicken DT40 cells. Biochem Biophys Res Commun. 2007;364:771–777. doi: 10.1016/j.bbrc.2007.10.063. [DOI] [PubMed] [Google Scholar]
- Wang SL, Hawkins CJ, Yoo SJ, Muller HA, Hay BA. The Drosophila caspase inhibitor DIAP1 is essential for cell survival and is negatively regulated by HID. Cell. 1999;98:453–463. doi: 10.1016/s0092-8674(00)81974-1. [DOI] [PubMed] [Google Scholar]
- Wolff T, Ready DF. Cell death in normal and rough eye mutants of Drosophila. Development (Cambridge, England) 1991;113:825–839. doi: 10.1242/dev.113.3.825. [DOI] [PubMed] [Google Scholar]
- Xu C, Min J. Structure and function of WD40 domain proteins. Protein Cell. 2011;2:202–214. doi: 10.1007/s13238-011-1018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Woodfield SE, Lee TV, Fan Y, Antonio C, Bergmann A. Genetic control of programmed cell death (apoptosis) in Drosophila. Fly. 2009;3:78–90. doi: 10.4161/fly.3.1.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan N, Huh JR, Schirf V, Demeler B, Hay BA, Shi Y. Structure and activation mechanism of the Drosophila initiator caspase Dronc. J Biol Chem. 2006;281:8667–8674. doi: 10.1074/jbc.M513232200. [DOI] [PubMed] [Google Scholar]
- Yin VP, Thummel CS. Mechanisms of steroid-triggered programmed cell death in Drosophila. Seminars in cell & developmental biology. 2005;16:237–243. doi: 10.1016/j.semcdb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Yin VP, Thummel CS, Bashirullah A. Down-regulation of inhibitor of apoptosis levels provides competence for steroid-triggered cell death. J Cell Biol. 2007;178:85–92. doi: 10.1083/jcb.200703206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Wang L, Acehan D, Wang X, Akey CW. Three-dimensional structure of a double apoptosome formed by the Drosophila Apaf-1 related killer. J Mol Biol. 2006;355:577–589. doi: 10.1016/j.jmb.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Zirin J, Cheng D, Dhanyasi N, Cho J, Dura JM, Vijayraghavan K, Perrimon N. Ecdysone signaling at metamorphosis triggers apoptosis of Drosophila abdominal muscles. Developmental biology. 2013;383:275–284. doi: 10.1016/j.ydbio.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.