Abstract
In Northeastern Brazil visceral leishmaniasis is endemic with lethal cases among humans and dogs. Treatment is toxic and 5–10% of humans die despite treatment. The aim of this work was to survey natural active compounds to find new molecules with high activity and low toxicity against Leishmania infantum chagasi. The compounds thymol and eugenol were chosen to be starting compounds to synthesize acetyl and benzoyl derivatives and to test their antileishmanial activity in vitro and in vivo against L. i. chagasi. A screening assay using luciferase-expressing promastigotes was used to measure the growth inhibition of promastigotes, and an ELISA in situ was performed to evaluate the growth inhibition of amastigote. For the in vivo assay, thymol and eugenol derivatives were given IP to BALB/c mice at 100 mg/kg/day for 30 days. The thymol derivatives demonstrated the greater activity than the eugenol derivatives, and benzoyl- thymol was the best inhibitor (8.67 ± 0.28 μg/mL). All compounds demonstrated similar activity against amastigotes, and acetyl-thymol was more active than thymol and the positive control drug amphotericin B. Immunohistochemistry demonstrated the presence of Leishmania amastigote only in the spleen but not the liver of mice treated with acetyl-thymol. Thus, these synthesized derivatives demonstrated anti-leishmanial activity both in vitro and in vivo. These may constitute useful compounds to generate new agents for treatment of leishmaniasis.
Keywords: Leishmania infatum chagasi, Thymol, Eugenol
1. Introduction
The treatment of leishmaniasis has been carried out with pentavalent antimonials such as antimoniate N-methyl glucamine (Glucantime®) and sodium stibogluconate (Pentostan®) for more than sixty years. These are the drugs of first choice for the treatment of visceral leishmaniasis in Latin America and the Mediterranean region. The antimonials drugs are toxic, not always effective, and used in prolonged.1 In geographical regions where there is documented Leishmania to antimony compounds, alternate drugs such as pentamidine, amphotericin B, liposomal formulation are used in the treatment of these patients.2 Antileishmanial drug development follows three lines: first, exploration of the parasite metabolic pathways to find targets and develop synthetic compounds; second, study of other drugs that are already on the market with hitherto unknown antileishmanial activity (e.g., cancer drugs), and third, focus on the use of medicinal plants as a source of anti-protozoan molecules.3 As part of the search for new and better drugs with improved activity and low toxicity compared to existing regimens, the Programme for Tropical Diseases of the World Health Organization (WHO) has targeted research using plants to treat leishmaniasis essential and high priority.4
Plants are important sources of compounds for drug discovery, especially for antiparasitic drugs due to the occurrence of human parasitic infections in regions where medicinal plants are in use.5 Research using herbal resources can lead to the identification of secondary metabolites that can either serve as valuable drugs in themselves, or lead to the development of new therapeutic substances with similar structure.6 Many studies have validated the effect of natural products as potential sources of new and selective agents for the treatment of tropical diseases caused by protozoa and other parasites.7
Thymol and eugenol are largely used as flavoring agents for food because they are the main constituents of oregano and cloves, respectively, and they display many pharmacological actions including antimicrobial,8,9 anti-inflammatory,10,11 fungicidal,12,13 and antioxidant activity.14,15 Common medicinal plants used by local populations include Ocimum gratissimum, whose essential oil and main constituent eugenol displays anthelmintic and antimicrobial activities,16,17 and Lippia sidoides, which is rich in the general antiseptic thymol.18
The cytotoxicities and antileishmanial activities of thymol and its hemisynthetic derivatives, as well as quinoline–triclosan and quinoline–eugenol hybrids have been demonstrated both in vitro and in vivo.19,20 Lippia sidoides Cham. Essential oil was shown to display in vitro cytotoxicity and antileishmanial activity,21 and the antileishmanial activity of Eugenol-rich essential oil from Ocimum gratissimum was also reported.22
In Northeastern Brazil leishmaniasis is an endemic disease, causing lethal cases among humans and dogs. The Brazilian Health Care Ministry cites the incidence of VL as 2 cases per 100,000 habitants. 23 The incidence of canine leishmaniasis is underestimated due to the incomplete communication between the private clinics vets and the authorities. Due to concern about the lack of efficacious antileishmanial drugs, we surveyed natural active compounds to find new molecules with enhanced activity against Leishmania and low toxicity for host cells. Based on this, thymol and eugenol were chosen as lead compounds from which acetyl and benzoyl derivatives could be synthesized and tested for their antileishmanial activity in vitro and in vivo against Leishmania infantum chagasi life stages.
2. Materials and methods
2.1. Acetylation reaction
Eugenol and thymol were purchased from VETEC, in Fortaleza, Brazil. A mixture of acetic anhydride (6 g) and pyridine (2 g) was added to eugenol or thymol (3 g). The mixture was left stirring for 24 h at room temperature, and then cold water was added (20 mL). The solution was neutralized to pH 7.0. The reaction mixture was transferred to a separating funnel and washed three times with chloroform (20 mL). The chloroform layer containing acetylated material was washed with water and then dried with anhydrous sodium sulfate. The solvent was evaporated under reduced pressure.
2.2. Benzoylation reaction
Eugenol (8.2 g or 0.05 mol) or thymol (7.5 g or 0.05 mol) was dissolved in 40 mL of 5% NaOH in the cold, and benzoyl chloride (7 g/5.8 ml, i.e., 0.05 mol) was added. The mixture was vigorously mixed until the odor of benzoyl chloride disappeared (20–25 min). The solid product was filtered on a Buchner funnel, washed with cold water, recrystallized with 60 mL of rectified spirit, and collected on a filter.24
2.3. Analysis of synthesized derivatives
The crude synthesized derivatives were subjected to silica gel column chromatography and eluted with mixtures of hexane, dichloromethane, ethyl acetate and methanol with increasing polarity. The fractions were collected and compared by TLC. The chemical structures of the purified compounds were confirmed by spectroscopic analysis of the nuclear magnetic resonance spectra recorded on a Bruker Avance DRX-500 spectrometer, in CDCl3.
O-acetyl-thymol (AT): 1H NMR (δ, ppm, J Hz): 7.25 (H-3, d, J = 7.7 Hz), 7.02 (H-4, d, J = 7.7), 6.85 (H-6, s), 3.02 (H-7, heptete, J = 6.9 Hz), 1.29 (H-8, d, J = 6.9 Hz), 1.29 (H-9, d, J = 6.9 Hz), 2.34 (H-10, s). 13C NMR (δ, ppm, CHCl3): 148.8 (C-1), 116.5 (C-2), 126.0 (C-3), 126.0 (C-4), 134.9 (C-5), 122.1 (C-6), 34.4 (C-7), 24.1 (C-8), 24.1 (C-9), 21.3 (C-10), 170.3 (C-11), 20.0 (C-12).
O-benzoyl-thymol (BT); 1H NMR (δ, ppm, CHCl3): 7.28 (H-3, d, J = 7.7 Hz), 7.12 (H-4, d, J = 7.7 Hz), 7.0 (H-6, s), 8.29 (H-13, m), 7.59 (H-14, m), 7.68 (H-15, m), 7.59 (H-16, m), 8.29 (H-17, m). 13C NMR (δ, ppm, CHCl3): 135.5 (C-1), 138.6 (C-2), 126.3 (C-3), 126.0 (C-4), 134.9 (C-5), 122.1 (C-6), 34.4 (C-7), 24.1 (C-8), 24.1 (C-9), 21.3 (C-10), 166.8 (C-11), 148.5 (C-12), 129.7 (C-13), 128.4 (C-14), 133.3 (C-15), 128.4 (C-16), 129.7 (C-17).
O-acetyl-eugenol (AE): 1H NMR (δ, ppm, CHCl3): 6.9 (H-3, s), 6.7 (H-5, d, J = 7.9 Hz), 6.9 (H-6, d, J = 7.9 Hz), 3.4 (H-7, d, J = 6.7), 5.9 (H-8, m), 5.1 (H-9, m), 3.8 (H-10, s, OCH3), 2.3 (H-12, s, CH3). 13C NMR (δ, ppm, CCCl3): 138 (C-1), 150.8 (C-2), 112.7 (C-3), 138.9 (C-4), 120.6 (C-5), 122.5 (C-6), 40 (C-7), 137 (C-8), 116.1 (C-9), 55.7 (C-10), 169.1 (C-11), 20.60 (C-12).
O-benzoyl-eugenol (BE): 1H NMR (δ, ppm, CHCl3): 6.8 (H-3, m), 6.8 (H-5, m), 7.1 (H-6, d, J = 7.6 Hz), 3.4 (H-7, d, J = 6.2 Hz), 6.0 (H-8, m), 5.2 (H-9, m), 3.8 (H-10, OCH3), 8.2 (H-13, m), 7.5 (H-14, m), 7.6 (H-15, m), 7.5 (H-16, m), 8.2 (H-17, m). 13C NMR (δ, ppm, CCCl3): 129.44 (C-1), 151.03 (C-2), 112.78 (C-3), 138.93 (C-4), 120.62 (C-5), 122.55 (C-6), 40.00 (C-7), 137.00 (C-8), 116.02 (C-9), 55.74 (C-10), 164.75 (C-11), 138.14 (C-12), 130.16 (C-13), 128.3 (C-14), 133.28 (C-15), 128.3 (C-16), 130.16 (C-17).
2.4. Antileishmanial activity
A Brazilian strain of L. infantum chagasi (Lic; MHOM/BR/00/1669) was originally isolated from a patient with visceral leishmaniasis. A strain expressing luciferase (Lic-luc), was generated from a hamster-passaged clinical isolate in the lab of MEW as described by Thalhofer et al.25, using the integrating vector pIR1-SAT kindly provided by Dr. Steve Beverley (Washington University, St. Louis). The promastigote form of Lic-Luc was cultured in a hemoflagellate-modified MEM (HOMEM), supplemented with 10% fetal bovine serum at 26 °C. Thymol, acetyl-thymol, benzoyl-thymol, eugenol, acetyl-eugenol and benzoyl-eugenol were dissolved in DMSO at a concentration of 0.2% and diluted in HOMEM in 96-well microplates. The assay was performed at concentrations of 100, 50, 25, 12.5 and 6.25 μg/mL. Control wells contained DMSO or no additives. Promastigotes were counted in a Neubauer hemocytometer and seeded at 1 × 106/well. After 24 h at 26 °C incubation in drug or control conditions, the number of remaining viable promastigotes was assessed using a luciferase assay. Triplicate samples from each condition were tested for luciferase activity in a 96-well plate (adapted methodology from Lang et al.26). Pentamidine (Sigma-Aldrich, St. Louis, MO, USA) was used as the positive control drug. The optical density (OD) was determined in a Fluostar Omega (BMG Labtech) at 620 nm.
The drug activity against amastigotes was measured using a modified ELISA to detect intracellular parasites as a measure of the growth of L. i. chagasi in the murine macrophage RAW 264.7 cell line (ATCC TIB-71) in the absence or presence of drug (method adapted from Piazza et al.27). 4 × 105 RAW 264.7 cells in RPMI-1640, 10% foetal bovine serum, were added to each well of a 96-well plate. After 24 h they adhered to plates, and L. i. chagasi promastigotes were added at a 1:10 (macrophage/promastigote) ratio at 37 °C, 5% CO2. No adherent promastigotes were removed by rinsing after 2 h, and macrophages were returned to the CO2 incubator for an additional 72 h. Stock solutions of all of the compounds were prepared at a concentration of 0.2% in ethanol. An additional five concentrations were obtained by two-fold serial-dilution. The compound solutions were added to microplates containing the confluent layer of cells with amastigotes for 48 h at 37 °C in a humidified 5% CO2 incubator. Macrophages incubated without drugs were used as the control, and amphotericin B (Sigma-Aldrich, St. Louis, MO, USA) was used as the positive control drug at the same concentration as the other compounds. Murine macrophage RAW 264.7 cells were incubated with 0.01% saponin in PBSA, containing 1% bovine serum albumin, for 30 min. The wells were then blocked for 30 min with 5% nonfat milk (Nestlé) in PBS. In the next step, the cells were incubated in serum from a rabbit immunized with a saline extract of L. i. chagasi promastigotes, collected 30 days after the infection, at 37 °C for 1 h. The serum employed was diluted 1:500 and pre-absorbed with 10% foetal calf serum (FCS) at 22 °C for 1 h. The wells were washed three times with 0.05% Tween-20 in PBSA, and peroxidase-conjugated goat anti-rabbit IgG (Sigma Chemical Co.) diluted 1:5000 in 5% nonfat milk was added and incubated at 37 °C for l h. After the wells were washed, o-phenylenediamine (0.4 mg/mL) and 0.05% H202 were added. The reaction was stopped by the addition of 1M HC1. The plates were read at 492 nm in a Multiskan MS (UniScience) ELISA reader.
2.5. Macrophage cytotoxicity assay
A MTT assay adapted from Tempone et al.28 was used. Murine macrophages-RAW 264.7 cells were seeded at 4 × 104/well in 96-well microplates and incubated at 37 °C for 48 h in the presence of the compounds, dissolved previously in ethanol at a concentration of 0.2% and diluted with M199 medium to the highest concentration of 100 μg/mL. The microplates were incubated for 48 h at 37 °C in a 5% CO2 humidified incubator. Control cells were incubated in the presence of DMSO without the drugs, or DMSO plus amphotericin B or pentamidine (standard drugs). The viability of the macrophages was determined with the MTT assay, as described above, macrophage morphology remained intact by light microscopy.
2.6. Animals and infection
This study involved 35 21-day-old male BALB/c mice, kept according to institutional guidelines, which were inoculated intra-peritoneally (given IP) with 107 infective promastigotes of L. i. chagasi. Promastigotes were cultured in M199 medium, supplemented with 10% foetal bovine serum and 5% human male urine at 24 °C. Metacyclic promastigotes were obtained from cultured stationary phase promastigotes by density sedimentation on a Ficoll gradient.29 Metacyclic promastigotes were centrifuged (3000 rpm, 10 min, Beckman tabletop centrifuge), the supernatant was discarded and the pellet was resuspended and washed in M199 medium, then 1 × 107 metacyclic promastigotes were used for the animal infection. Thirty five days post infection one animal from each group (5 animal total) was euthanized and the liver and spleen were imprinted to confirm infection and verify the parasite burden, and then the treatment was started.
The animals were treated once a day for 30 days. The groups (n = 5) were formed by the compounds acetyl-thymol, benzoyl-thymol, acetyl-eugenol, benzoyl-eugenol (100 mg/kg intra peritoneal), to reach this dose an acute toxicity was preciously conducted (data not shown). The animal during the acute toxicity started to show signs of toxicity such as disorientations, bristly hair and quiet behavior at 500 mg/Kg. The controls groups were glucantime (80 mg/Kg intra muscular) and water treated group.
None of the animals died during the experiment, and the volume injected was 0.5 mL. After the treatment period all animals were euthanized and their liver and spleen collected to verify the parasite burden.
All procedures involving animals in this study were reviewed and approved by the Ceará State University Ethics Committee (CEUA-UECE).
2.7. Qualitative immunohistochemistry
The presence or absence of Leishmania after treatment (qualitative histological events) was determined by the presence of amastigote forms, Leishmania antigens detected by immunohisto chemistry.
Silanized slides containing sections of fragments from BALB/c liver and spleen obtained after the treatment of leishmaniasis were submitted to immunohistochemistry for the detection of amastigotes forms. The tissues were deparaffinized in histological sections (4 mm) with xylene, rehydration in a decreasing ethanol series. Immunohistochemistry was performed with anti-Leishmania antibodies produced in rabbits reacted with peroxidase-conjugated goat anti-rabbit IgG (Sigma Chemical Co.). All reactions were developed in the same way using a diaminobenzidine chromogen solution (Sigma Chemical Co., MO, USA-D5637), which precipitates a brown product and counterstaining was performed with Harris hematoxylin. The slides were dehydrated in a growing ethanol series and mounted with Permount resin (Fisher Chemicals, NJ, USA).
2.8. Statistical analysis
The number of living promastigotes was determined using the luciferase assay (OD—620 nm) representing the percentage of survival. The EC50 values were calculated using a nonlinear regression curve using the statistical software GhaphPad Prism 4.0. The data were initially tested for Shapiro–Wilk and Bartlett for confirmation of normal distribution and homogeneity of variance between treatments, respectively. As they were not verified for homoscedasticity, the results were compared using the nonparametric Kruskal–Wallis test, using statistical software SAS (2002). Differences were considered significant when p < 0.05 and the results were expressed as mean ± standard deviation.
3. Results and discussion
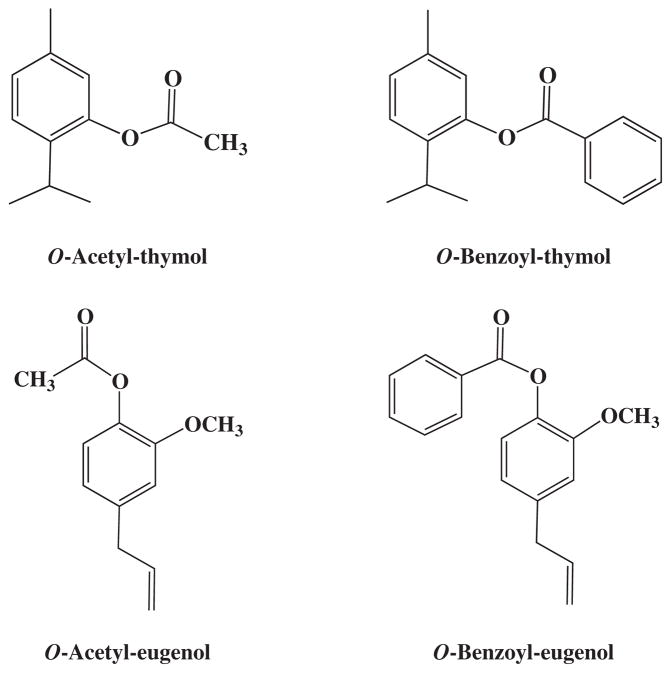
In the search for new compounds with lesser toxicity and greater activity, the compounds thymol and eugenol were chemically modified by acetylation and benzoylation processes. At the end of the process four derivatives were obtained, acetyl-thymol, benzoyl-thymol, acetyl-eugenol and benzoyl-eugenol (Fig. 1). Both thymol (C10H14O2) and eugenol (C10H12O2) have ten carbon atoms. In the acetylation process, two carbon atoms are introduced (CH3-C=O) which corresponds to carbons 11 (carbonyl) and 12 (methyl). Benzoyl derivatives gave an additional seven carbons (carbonyl and benzene ring) to this moiety. Thymol is a p-cymene type monoterpene phenol with many pharmaceutical properties including antimicrobial,8 anti-inflammatory and healing10, fungicidal12, and antioxidant.14 It has been found that some p-cymene derivatives have anti-leishmanial activity with low toxicity and greater inhibition than thymol.19,30
Figure 1.
Representation of chemical structures of thymol and eugenol derivatives.
The thymol derivatives demonstrated better activity against promastigotes, between the tested compounds. Benzoyl-thymol showed the highest degree of inhibitor of promastigoten growth, with an EC50 of 8.67 μg/mL (SD ± 0.28), and acetyl-eugenol was lower with an EC50 of 23.21 μg/mL (SD ± 3.46). All compounds, when compared to the control Pentamidine, were statistically different. When compared to the pure substances, acetyl-thymol demonstrated better antileishmanial activity against promastigotes than thymol, and eugenol presented an activity that was worse than both derivatives (Table 2).
Table 2.
Leishmanicidal activity of thymol and eugenol derivatives and toxicity against L. i. chagasi and RAW 264.7 cells
| Compounds | Promastigote LC50 (μg/mL) | Amastigote (μg/mL) | Toxicity% RAW 264.7 cells survival at 100 μg/mL (±SD) |
|---|---|---|---|
| Acetyl eugenol | 23.21 ± 3.46* Ab | 18.53 ± 4.79Aab | >100 |
| Acetyl thymol | 9.07 ± 0.06* Ae | 10.95 ± 3.00* Ab | >100 |
| Benzoyl eugenol | 10.58 ± 0.18* Ad | 14.93 ± 4.50Aab | 97.7 ± 5.6 |
| Benzoyl thymol | 8.67 ± 0.28* Af | 15.09 ± 3.65Bab | 63.6 ± 5.3 |
| Eugenol | 56.13 ± 2.09* Aa | 20.81 ± 1.59Ba | 29 ± 1.3 |
| Thymol | 12.85 ± 1.61* Ac | 23.93 ± 6.29Ba | 36.5 ± 0.5 |
| Amphotericin B | Nd | 20.44 ± 0.98 | 98.5 ± 7.5 |
| pentamidine | 5.30 ± 0.81 | Nd | 85.6 ± 6.9 |
Nd, not determined.
Represents significative difference in relation to the control. Different capital letters represent differences between columns (action of each drug on different forms). Lowercase letters represent significant differences between different lines (action of different drugs within each form).
The thymol derivatives acetyl-thymol and benzoyl-thymol exhibited an EC50 = 9.07 μg/mL (SD ± 0.06) and EC50 = 8.67 μg/mL (SD ± 0.28) against promastigote forms, respectively. Acetyl-thymol demonstrated the greatest activity against intracellular amastigote forms (EC50 = 10.95 μg/mL, SD ± 3.0). The next highest was benzoyl-thymol (EC50 = 14.93 μg/mL, SD ± 4.5) (Table 1). Medeiros et al.21 reported the antileishmanial activity of thymol-rich essential oil from Lippia sidoides and pure thymol against promastigote of Leishmania amazonensis, demonstrating an EC50 of 44.3 and 19.4 μg/mL, respectively. The magnitude of inhibition in these reported studies was lower than that observed using benzoyl-thymol or acetyl-thymol in this study. Another study demonstrated inhibition of Leishmania panamensis promastigote growth by thymol and derivatives, with five of the eight derivatives showing significantly more activity than thymol.19 The difference between the activity of essential oil and the pure substance can be explained by the fact that the essential oil has other elements and the concentration of the constituents is not equivalent to the pure substance. Thus, the greater inhibition using the thymol derivatives compared to thymol is an indication that the acetylation and benzoylation can improve the growth inhibition and lower the toxicity.
Table 1.
Anti-leishmanial activity of thymol or eugenol derivatives: Activity against axenically cultured promastigotes or intracellular amastigotes in RAW 264.7 cells, and toxicity of compounds for RAW 264.7 cells
| Compounds | Promastigote EC50 (μg/mL) | Amastigote EC50 (μg/mL) | p valueb | Toxicity % RAW 264.7 cells survival at 100 μg/mL (±SD) |
|---|---|---|---|---|
| Acetyl-Eugenol | 23.21 ± 3.46* Ab | 18.53 ± 4.79Aab | p < 0.3796 | >100 |
| Acetyl-Thymol | 9.07 ± 0.06* Ae | 10.95 ± 3.00* Ab | p < 0.4687 | >100 |
| Benzoyl-Eugenol | 10.58 ± 0.18* Ad | 14.93 ± 4.50Aab | p < 0.3454 | 97.7 ± 5.6 |
| Benzoyl-Thymol | 8.67 ± 0.28* Af | 15.09 ± 3.65Bab | p < 0.0437 | 63.6 ± 5.3 |
| Eugenol | 56.13 ± 2.09* Aa | 20.81 ± 1.59Ba | p < 0.0027 | 29 ± 1.3 |
| Thymol | 12.85 ± 1.61* Ac | 23.93 ± 6.29Ba | p < 0.0319 | 36.5 ± 0.5 |
| Amphotericin B | Nd | 20.44 ± 0.98 | Nd | 98.5 ± 7.5 |
| Pentamidine | 5.30 ± 0.81 | Nd | Nd | 85.6 ± 6.9 |
| p valueb | p < 0.001 | p < 0.0476 | Nd | Nd |
Nd, not determined.
Represents significant difference in relation to the control. Different capital letters represent differences between columns (action of each drug on different forms). Lowercase letters represent significant differences between different lines (action of different drugs within each form).
Significance level of the test (probability of Type I error, i.e., rejection of a true null hypothesis).
Eugenol is a phenylpropanoid with antifungal,13 anthelmintic,16 insecticide,31 and anti-leishmanialactivity.22 This compound is the main constituent of essential oil of Ocimum gratissimum and Eugenia caryophyllus.32,33 Many phenylpropanoids demonstrate growth inhibition34,35 but there are no studies on derivatives of eugenol. Similar to the thymol derivatives, we hypothesized that the eugenol derivative could also provide greater antileishmanial activity than nonderivatized compound.
Comparing the two eugenol derivatives, benzoyl-eugenol had the greatest antileishmanial activity against promastigotes (EC50 = 10.58 μg/mL, SD ± 0.18), followed by acetyl-eugenol (EC50 = 23.21 μg/mL, SD ± 3.46) All compounds presented similar inhibitory capacities against the amastigote form. Ueda-Nakamura et al.22 tested the eugenol-rich essential oil of Ocimum gratissimum and its main constituent against L. amazonensis amastigotes and promastigotes, and observed that the purified eugenol had a greater inhibition than the essential oil. Pessoa et al.16 measured the percentage of the constituents in O. gratissimum essential oil and found that only 43.7% is eugenol. Comparing the activity of O. gratissimum essential oil and pure eugenol, the eugenol derivatives tested in this study revealed a greater activity than the eugenol-rich essential oil and the pure compound. Arango et al.20 evaluated the ant-leishmanial activity of six quinolone-eugenol hybrids and pure eugenol and demonstrated that the hybrids had a greater inhibition than eugenol, indicating that derivatives can potentiate the inhibition activity. Other studies demonstrate that parasitic activity of eugenol is superior to eugenol-rich essential oil. Santoro et al.36 reported that the trypanocidal activity of Ocimum basilicum essential oil was lower than its main constituent, eugenol; another study measured the anti-giardia activity of Syzygium aromaticum and its major compound eugenol, and observed that the eugenol itself had a greater growth inhibition of Giardia lamblia,37 and these activities might improve with the use of compound derivates.
The amastigote is the parasitic form persisting in the host and causing symptomatic disease. Thus this form should be the major chemotherapeutic target in studies of new antileishmanial agents, although promastigotes and amastigotes share many metabolic pathways making it valid to screen both parasite forms.19 All the compounds tested in this study were statistically similar to the positive control drug Amphotericin B against amastigotes, with the exception of acetyl-thymol which had a greater activity than amphtericin B. Additionally, only the acetyl-eugenol was significantly different between its activity against the promastigote versus the amastigote forms. In this case the inhibition of promastigotes was greater than inhibition of amastigotes. The activity of the other compounds was statistically similar against either promastigotes or amastigotes. Thus, these compounds are active against both the intracellular and the extracellular parasite stages (Table 1).
The toxicity of drugs against the murine RAW 264.7 macrophage-like cell line at revealed that 100 μg/mL acetyl-thymol was not toxic, although 100 μg/mL benzoyl-thymol decreased macrophage survival to 63.6%, a low but worrisome level of toxicity. There was no detectable toxicity of either eugenol derivative against RAW 264.7 macrophages in this study. All derivatives had lower toxicity than their parent compounds eugenol or thymol (Table 1). This is an excellent demonstration that the acetylation and benzoylation processes can decrease the toxicity of chemical compounds for mammalian cells.
The antileishmanial mechanism of these compounds could be explained by the ability of ergosterol to interact with the Leishmania membrane,38,39 causing an increase in cell permeability and loss of small cations such as K+. Eugenol also induces apoptosis, which could inhibit both stages of Leishmania parasites,7 an observation that could be related to the membrane effects.
A few published studies have demonstrated that essential oils rich in thymol and eugenol have anti-parasitic activity as well as toxicity for mammalian cells. However, no studies were found that tested the activity of derivatives of these compounds, such as the compounds studied in the current report, acetyl-thymol, benzoyl-thymol, acetyl-eugenol and benzoyl-eugenol.
Most reports measure in vitro growth inhibition and toxicity of essential oils and their major compounds, but most have not been followed up with in vivo studies, with the exception of a few that evaluate Leishmania species leading to cutaneous disease. In the current study we evaluated the activity of thymol and eugenol derivatives in vivo in BALB/c mice infected with L. i. chagasi, the main cause of visceral leishmaniasis in Latin America. Preliminary acute toxicity studies were conducted and the optimal safe treatment dose was reached (data not shown). Subsequently male BALB/c mice were infected with 107 promastigotes IP, and the treatment started 30 days post-infection. Thymol and eugenol derivatives were administered at 100 mg/kg dose once a day for an additional 30 days. Control groups of mice received either Glucantime or water in the same volume as drugs. All groups of animals were euthanatized, and spleen and liver tissue samples were collected for histopathology and immunohistochemistry analyses.
Immunohistochemical stains with rabbit polyclonal antisera to total Leishmania lysate were performed to detect qualitatively whether Leishmania amastigote forms were present or absent. All stains were compared with control animals that received either no drug or Glucantime, the standard therapy for visceral leishmaniasis. The data revealed that amastigotes were only visible in the spleens of animals treated with no drug (water), Glucantime, or acetyl-thymol. Even though the immunohistochemical assay is a qualitative assay, it was possible to notice the difference between the amount of amastigotes present in the Glucantime and the acetyl-thymol and water treated stains. The first one, only a few amastigotes was found, and the last two it was possible to find a regular amount of parasites spread in the stain. Parasites could not be visualized in spleens of mice treated with acetyl-eugenol, benzoyl-eugenol or benzoyl-thymol. Amastigotes were visualized in the livers of mice that did not receive drug treatment (water treated animals), but not in the livers of mice treated with any of the drugs. The histopathological findings of the liver and spleen samples stained with HE showed no significant alterations in the spleen, all the livers presented moderate hydropic degeneration. L. i. chagasi infection in mice leads to an initial rise of parasites in the liver followed by local resolution, after which parasites expand in the spleen during chronic infection. It is difficult to discern where in the kinetics of liver growth the treated mice are, although lower parasite loads the spleens would suggest an overall decrease in parasite load rather than merely a change in infection kinetics.
Data presented in this study documented antileishmanial activities of thymol, eugenol or their derivatives both in vivo and in vitro. The data shows the derivatives are active against the parasite in vitro, and demonstrate the ability of derivatives to reduce the parasite loads in the livers and spleens of mice. The modifications of thymol or eugenol to yield benzoylated or acetylated derivatives most likely was responsible for the decreased toxicity and increased antileishmanial activities of these compounds., The data suggest these modified compounds are promising candidates for further studies of antileishmanial drug development.
Acknowledgments
We are grateful to the CENAUREMN (Northeastern Center for the Application and Use of Nuclear Magnetic Resonance) of the Federal University of Ceará for the NMR spectra of the compounds.
References and notes
- 1.Gontijo CMF, Melo MN. Rev Bras Epidemiol. 2004;7:338. [Google Scholar]
- 2.Rath S, Trivelin LA, Imbrunito TR, Tomazela DM, Jesús MN, Marzal PC, Andrade HF, Tempone AG. Quim Nova. 2003;26:550. [Google Scholar]
- 3.Lindoso JAL, Costa JML, Goto ITQH. Res Rep Trop Med. 2012;3:69. doi: 10.2147/RRTM.S24764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. [access August of 2013]; http://www.who.int/zoonoses/diseases/leishmaniasis/en/
- 5.Anthony J, Fyfe L, Smith H. Trends Parasitol. 2005;21:462. doi: 10.1016/j.pt.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Gobbo-Neto L, Lopes NP. Quim Nova. 2007;30:374. [Google Scholar]
- 7.Mishra P, Kumar A, Khare P, Gupta S, Kumar N, Dube A. J Med Microbiol. 2009;58:1058. doi: 10.1099/jmm.0.009290-0. [DOI] [PubMed] [Google Scholar]
- 8.Guarda A, Rubilar JF, Miltz J, Galotto MJ. Int J Food Microbiol. 2011;146:144. doi: 10.1016/j.ijfoodmicro.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Catherine AA, Deepika H, Negi PS. J Essent Oil Res. 2012;24:481. [Google Scholar]
- 10.Riella KR, Marinho RR, Santos JS, Pereira-Filho RN, Cardoso JC, Albuquerque-Junior RLC, Thomazzi SM. J Ethnopharmacol. 2012;143:656. doi: 10.1016/j.jep.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 11.Bachiega TF, Sousa JPB, Bastos JK, Sforcin JM. J Pharm Pharm Sci. 2012;64:610. doi: 10.1111/j.2042-7158.2011.01440.x. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad A, Khan A, Yousuf S, Khan LA, Manzoor N. Fitoterapia. 2010;81:1157. doi: 10.1016/j.fitote.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 13.Nair VD, Gopi R, Mohankumar M, Kavina J, Panneersalvam R. Acta Physiol Plant. 2012;34:599. [Google Scholar]
- 14.Underger U, Basaran A, Degen GH, Basaran N. Food Chem Toxicol. 2009;47:2037. doi: 10.1016/j.fct.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 15.Fujisawa S, Atsumi T, Kadoma Y, Sakagami H. Toxicology. 2002;177:39. doi: 10.1016/s0300-483x(02)00194-4. [DOI] [PubMed] [Google Scholar]
- 16.Pessoa LM, Morais SM, Bevilaqua CML, Luciano JHS. Vet Parasitol. 2002;109:59. doi: 10.1016/s0304-4017(02)00253-4. [DOI] [PubMed] [Google Scholar]
- 17.Ali SM, Khan AA, Ahmed I, Musaddiq M, Ahmed KS, Polasa H, Rao LV, Habibullah CM, Sechi LA, Ahmed N. Ann Clin Microbiol Antimicrob. 2005;4:20. doi: 10.1186/1476-0711-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botelho MA, Nogueira NAP, Bastos GM, Fonseca SC, Lemos TL, Matos FJ, Montenegro D, Heukelbach J, Rao VS, Brito GA. Braz J Med Biol Res. 2007;40:349. doi: 10.1590/s0100-879x2007000300010. [DOI] [PubMed] [Google Scholar]
- 19.Robledo S, Osorio E, Muñoz D, Jaramillo LM, Restrepo A, Arango G. Antimicrob Agents Chemother. 2005;49:1652. doi: 10.1128/AAC.49.4.1652-1655.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arango V, Dominguez JJ, Cardona W, Robledo SM, Muñoz DL, Figadere B, Sáez J. Med Chem Res. 2012;21:3445. [Google Scholar]
- 21.Medeiros MGF, Silva AC, Cito AMGL, Borges AR, Lima SG, Lopes JA. Parasitol Int. 2011;60:237. doi: 10.1016/j.parint.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Ueda-Nakamura T, Mendonça-Filho RR, Morgado-Díaz JA, Maza PK, Dias BP, Cortez DAG, Alviano DS, Rosa MSS, Lopes AHCS, Alviano CS, Nakamura CV. Parasitol Int. 2006;55:99. doi: 10.1016/j.parint.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 23.MS—Ministério da Saúde do Brasil. Manual of Vigilance and Control of Visceral Leishmaniasis. 2006. [Google Scholar]
- 24.Furniss B, Hannaford A, Smith P, Tatchell A. Vogel’s Textbook of Practical Organic Chemistry. Prentice Hall; London: 1989. [Google Scholar]
- 25.Thalhofer CJ, Graff JW, Love-Homan L, Hickerson SM, Craft N, Beverley SM, Wilson ME. J Vis Exp. 1980;2010:41. doi: 10.3791/1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang T, Goyard S, Lebastard M, Milon G. Cell Microbiol. 2005;7:383. doi: 10.1111/j.1462-5822.2004.00468.x. [DOI] [PubMed] [Google Scholar]
- 27.Piazza RMF, Andrade HF, Umezawa ES, Katzin M, Stolf AMS. Acta Trop. 1994;57:301. doi: 10.1016/0001-706x(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 28.Tempone AG, Borborema SET, Andrade HF, Gualda NCA, Yogi A, Cravalho CS, Bachiega D, Lupo FN, Bonotto SV, Fischer DCH. Phytomedicine. 2005;12:382. doi: 10.1016/j.phymed.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Spath GF, Stephen MB. Exp Parasitol. 2001;99:97. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- 30.Mikus J, Harkenthal M, Steverding D, Reichling J. Planta Med. 2000;66:366. doi: 10.1055/s-2000-8548. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y, Ho S, Lee H, Yap Y. J Stored Prod Res. 2002;38:403. [Google Scholar]
- 32.Interaminense LF, Jucá DM, Magalhães PJ, Leal-Cardoso JH, Duarte GP, Lahlou S. Fundam Clin Pharmacol. 2007;21:497. doi: 10.1111/j.1472-8206.2007.00514.x. [DOI] [PubMed] [Google Scholar]
- 33.Jirovetz L, Buchbauer G, Stoilova I, Stoyanova A, Krastonov A, Schmidt E. J Agr Food Chem. 2006;54:6303. doi: 10.1021/jf060608c. [DOI] [PubMed] [Google Scholar]
- 34.Kaur A, Singh R, Dey CS, Sharma SS, Bhutani KK, Singh IP. J Exp Biol. 2010;48:314. [PubMed] [Google Scholar]
- 35.Takahashi M, Fuchino H, Sekita S, Satake M. Phytother Res. 2004;18:573. doi: 10.1002/ptr.1502. [DOI] [PubMed] [Google Scholar]
- 36.Santoro GF, Cardoso MG, Guimarães LGL, Mendonça LZ, Soares MJ. Exp Parasitol. 2007;116:283. doi: 10.1016/j.exppara.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 37.Machado M, Dinis AM, Salgueiro L, Custódio JB, Cavaleiro C, Sousa MC. Exp Parasitol. 2011;127:732. doi: 10.1016/j.exppara.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Paila YD, Saha B, Chattopadhyay A. Biochem Biophys Res Commun. 2010;399:429. doi: 10.1016/j.bbrc.2010.07.099. [DOI] [PubMed] [Google Scholar]
- 39.Dinesh N, Kaur PK, Swamy KK, Singh S. Exp Parasitol. 2014;144:84. doi: 10.1016/j.exppara.2014.06.004. [DOI] [PubMed] [Google Scholar]