Abstract
Parasitic diseases are a serious global health concern. Many of the most common and most severe parasitic diseases, including Chagas’ disease, leishmaniasis, and schistosomiasis, are also classified as neglected tropical diseases and are comparatively less studied than infectious diseases prevalent in high income nations. The NLRs (nucleotide-binding domain leucine-rich-repeat-containing proteins) are cytosolic proteins known to be involved in pathogen detection and host response. The role of NLRs in the host response to parasitic infection is just beginning to be understood. The NLR proteins NOD1 and NOD2 have been shown to contribute to immune responses during Trypanosoma cruzi infection, Toxoplasma gondii infection, and murine cerebral malaria. The NLRP3 inflammasome is activated by T. cruzi and Leishmania amazonensis but also induces pathology during infection with schistosomes or malaria. Both the NLRP1 and NLRP3 inflammasomes respond to T. gondii infection. The NLRs may play crucial roles in human immune responses during parasitic infection, usually acting as innate immune sensors and driving the inflammatory response against invading parasites. However, this inflammatory response can either kill the invading parasite or be responsible for destructive pathology. Therefore, understanding the role of the NLR proteins will be critical to understanding the host defense against parasites as well as the fine balance between homeostasis and parasitic disease.
Keywords: Parasitology, NLR proteins, Innate immune defense, Inflammasomes
Introduction
The NLR family
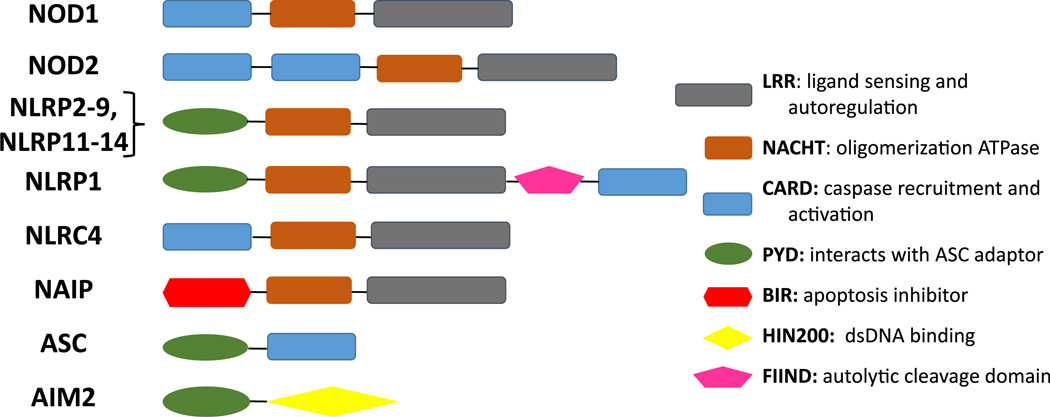
The NLR proteins are a family of 22 mammalian genes that have diverse functions in innate immunity and inflammation. The best characterized of these proteins, NLRP1, NLRP3, and NLRC4, act as cytosolic sensors and regulate cytokine secretion and cell death pathways. The family shares common structural motifs: a central nucleotide-binding domain (NACHT), a C-terminal leucine-rich-repeat (LRR) domain that has been implicated in ligand sensing, and an N-terminal effector domain that is either a CARD (caspase-1 activation and recruitment), BIR (baculovirus inhibitor of apoptosis protein repeat) domain or a PYD (pyrin) domain. The NLRP group members contain a PYD; the NAIPs include a BIR domain, and the NLRC group proteins contain a CARD domain (Fig. 1) [1].
Fig. 1.
NLR and related protein domains
At least three of these family members (NLRP1, NLRP3, and NLRC4) and the IFI20X/IFI16 family memberAIM2 are known to function in the assembly of a large molecular weight complex known as the inflammasome, where they act as a scaffold along with the adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD) leading to caspase-1 activation, and consequent activation and release of IL-1β and IL-18 [2, 3]. Other NLRs (NOD1, NOD2) are known to regulate inflammatory immune responses, whereas the molecular functions of many NLR proteins have yet to be discovered. Several NLRs, including some of the non-inflammasome-activating NLRs, play crucial roles in the host adaptive response to pathogens regulating cellular migration, goblet cell-induced mucous secretion, and negative regulation of inflammatory pathways [4–11].
Inflammasome activation
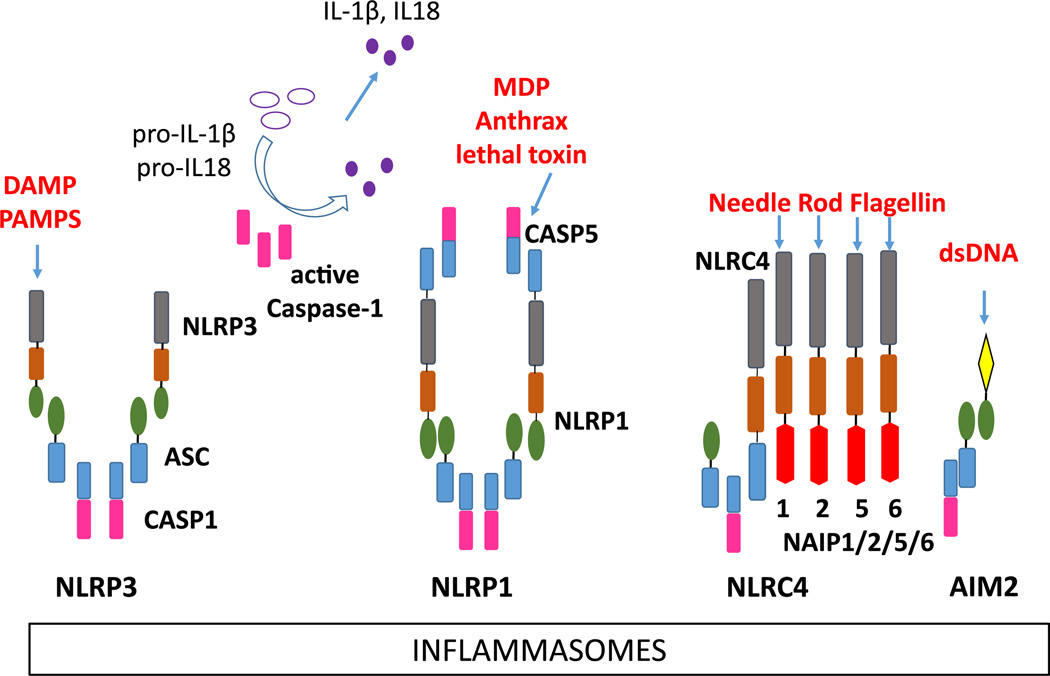
The formation of multiprotein inflammasome complexes constitutes a major pro-inflammatory pathway of the innate immune system. Signals that trigger activation are unique to each of the NLR proteins. Inflammasomes function in tandem with a priming signal, often ligation of a TLR, leading to production of pro-IL-1β. Upon activation by the appropriate ligand, the NLR couples with ASC and procaspase-1, converting caspase-1 to its active form that then cleaving the precursors of IL-1β and IL-18 into their mature secreted forms. Release of these cytokines leads to further inflammatory cascades in the host. All of the inflammasomes except the AIM2 inflammasome contain an NLR family protein that acts as pattern recognition receptor. NLRP1, NLRP3, NLRC4, and AIM2 have been confirmed to form inflammasome complexes with caspase-1 (Fig. 2) [1–3]. Other NLR proteins such as NLRP6 and NLRP12 have been proposed to form inflammasomes, although there is not conclusive evidence of their inclusion in an inflammasome complex [4, 12].
Fig. 2.
Inflammasome assembly. Left to right NLRP3 inflammasome with the ASC adaptor and caspase-1; NLRP1 inflammasome assembly with ASC, and caspase-1 or caspase-5; NLRC4 inflammasome with NAIP1, NAIP2, NAIP5, or NAIP6, ASC (the human NAIP is equivalent to the murine NAIP1) and caspase-1; AIM2 inflammasome with the ASC adaptor and caspase-1
The NLRP1 inflammasome activation is activated by anthrax lethal toxin and by muramyl dipeptide (MDP), a bacterial peptidoglycan motif. Although there is no direct evidence of MDP-NLRP1 binding, it is proposed that pathogen-derived muramyl dipeptide binds NLRP1 in the cytosol, altering the NLRP1 conformation and allowing oligomerization and caspase-1 inflammasome assembly. Human NLRP1 has its own CARD domain enabling its direct binding to caspase-1; thus, the presence of the ASC adaptor is not essential [13, 14]. Mice have three paralogs of NLRP1. Among these, NLRP1b is activated by Bacillus anthracis lethal toxin and is required by the host to combat infection, i.e., animals with the lethal toxin-sensitive Nlrp1b allele have increased resistance to B. anthracis infection [15].
The NLRC4 inflammasome involves at least two NLR proteins, NLRC4 and a partner NAIP, to detect cytosolic bacterial proteins. In humans, a single NAIP detects the needle structure of the bacterial TSS3 system [16]. In mice, NAIP1 detects the needle, NAIP2 detects the rod of TSS3, and NAIP 5 and 6 detect bacterial flagellin directly [16–21]. The exact mechanism of the interaction between the NAIP and NLRC4 is unknown, although NLRC4 must be phosphorylated for inflammasome activation [22].
The AIM2 inflammasome, while not containing an NLR protein, assembles in a similar manner as the NLRP3 inflammasome. Thus, AIM2 recruits the ASC adaptor and caspase-1, which autolytically activates and cleaves pro-IL-1β and pro-IL-18 into their mature and secreted forms. The AIM2 HIN-200 domain functions as a sensor for cytosolic double-stranded DNA [23–25]. This allows the AIM2 inflammasome to sense a variety of threats that introduce dsDNA to the cytosol, including bacteria, DNA viruses, parasites, and internal damage [23, 26–28].
The NLRP3 inflammasome is the best-studied of the inflammasomes with the largest and most diverse list of agonists. Activation requires NLRP3 and the ASC adaptor as well as caspase-1. Pathogen-derived products, sterile crystalline molecules, and endogenous stimuli such as ATP are all capable of activating the NLRP3 inflammasome. The precise mechanism of NLRP3 activation by these diverse agonists is uncertain, although many agonists cause cation flux (including K+ efflux and increased cytosolic Ca2+ concentration) that may cause mitochondrial disruption, which is sensed by NLRP3 [29–31]. NLRP3 inflammasomes are activated by viral, bacterial, fungal, and parasitic pathogens. The precise function of the NLRP3 inflammasome in these infections is varied. Production of IL-1β and IL-18 can trigger a protective adaptive immune response or a cascade of inflammatory cytokines and chemokines, which can result in pathology [3]. Indeed, pathologic consequences of unregulated inflammasome activation in autoinflammatory disease, such as the cryopyrin-associated periodic syndrome, are specifically treated with the recombinant IL-1 receptor antagonist anakinra. The ability of inflammasomes to induce sterile inflammation followed by a strong adaptive response has been exploited in the use of alum, a potent NLRP3 activator, and the NLRP1 agonist MDP as vaccine adjuvants [32].
Functions of non-inflammasome-forming NLRs
The non-inflammasome-forming NLRs, including NOD1, NOD2, NLRC3, NLRC5, NLRP2, NLRP6, NLRP10, NLRP12, and NLRX1, have diverse functions in immunity.
NOD1 and NOD2 have been well studied. Human mutations in NOD1 are linked to atopic disease and asthma, and in NOD2 are closely linked with the autoinflammatory disorder Crohn’s disease and Blau syndrome [33]. Both NOD1 and NOD2 function in sensing bacteria, and it is hypothesized that improper recognition of gut microbiota due to the absence or malfunction of NOD1 and NOD2 leads to inappropriate immune responses within the gastrointestinal tract [34]. NOD1 is ubiquitously expressed, whereas NOD2 is expressed in hematopoietic cells. Both are cytosolic sensors that recognize bacterial peptidoglycan motifs and activate NF-κB signaling by interacting with several pathways using the RIPK2 adaptor (Fig. 1). NOD1 and NOD2 have been implicated in additional roles although the molecular signals and pathways triggering these functions are not known. These roles include induction of macroautophagy and regulation of adaptive immune responses [35, 36].
Other NLR proteins that are not known to incorporate into inflammasomes have also been implicated in antimicrobial function and immunity. NLRC5 has been reported to have an anti-inflammatory function via regulation of NF-κB and type I IFN responses, and its absence enhances antiviral immunity [37, 38]. Both NLRP10 and NLRP12 may be anti-inflammatory and participate in dendritic cell migration and formation of an adequate adaptive immune response [6, 7, 9].
NLR proteins during infection with Trypanosomatid protozoa
The Trypanosoma spp. and Leishmania spp. protozoa (Order Kinetoplastidae, Family Trypanosomatidae) are vector-borne protozoan parasites which are pathogenic for humans and other mammals. Trypanosoma cruzi is a cause of American trypanosomiasis or Chagas’ disease. In its acute form, Chagas’ disease manifests as a self-limited febrile illness. The severe forms of disease ensue years to decades after initial infection in 30–40 % of infected persons, causing either chronic enlargement of the heart and heart failure or chronic enlargement of the esophagus and intestines. Both chronic disease forms are progressive and untreatable. The two subspecies of Trypansoma brucei (T. brucei rhodesiense, T. brucei gambiense) cause African sleeping sickness (also called human African trypanosomiasis or HAT). More than 20 species of Leishmania cause a variety of human disease syndromes, the most common of which are local skin ulcers during cutaneous leishmaniasis, and visceral leishmaniasis associated with systemic spread of the parasite and chronic immunocompromise. The most important factor determining which clinical syndrome occurs is the infecting species of Leishmania.
Trypanosoma cruzi, NOD1, and NLRP3
Between seven and eight million people are infected with T. cruzi worldwide, and 30 % of chronically infected individuals will develop cardiac disease [39, 40]. Although it is unknown whether long-term chronic manifestations can be prevented by immune responses, murine models and severe infections in humans with immunocompromise have illuminated mechanisms by which the immune system contains the parasite. Immune control of the infection requires both innate and adaptive immune responses [41]. Innate immune responses implicated in parasite recognition include pattern recognition receptors TLR2, TLR4, TLR7, and TLR9 [42–44]. An inflammatory response to T. cruzi occurs only in hosts with intact MyD88 and ultimately drives the development of the adaptive response [45]. The NLR proteins NOD1 and the NLRP3/ASC/caspase-1 inflammasome also appear to be important in parasite sensing and inflammatory response [36, 46, 47].
One study implicates NOD1 in resistance to T. cruzi infection. This study of knockout mice lacking either NOD1 or NOD2 proteins showed that the absence of NOD1, but not NOD2, led to an exacerbation of T. cruzi parasite load and greater mortality. Although not as severe as the defects in mice lacking either NOS2 or MyD88, the study linked the defect to poor response to IFN-γ in infected macrophages. The absence of NOD1 did not impair peripheral cytokine levels, implying the role of NOD1 in murine resistance to T. cruzi is independent of systemic cytokines [36].
The role of the NLRP3 inflammasome in T. cruzi infection has been described in two studies demonstrating that activation of the NLRP3 inflammasome by T. cruzi is protective in the mouse model of Chagas’ diseases. The Silva group showed activation of inflammasome-related genes in the tissue of T. cruzi-infected mice and secretion of IL-1β in infected murine macrophages. Furthermore, they showed that the secretion of IL-1β after T. cruzi infection was dependent upon NLRP3, ASC, and caspase-1 using knockout mice and macrophages. In murine macrophages, this NLRP3 inflammasome activation was dependent upon K+ efflux, ROS production, and lysosomal damage. The authors posit that the exit of parasites from the macrophage phagosome to become cytosolic amastigotes releases damage-associated molecular patterns (DAMPs) that trigger NLRP3 inflammasome assembly, although this assertion remains to be demonstrated. Evidence that an active inflammasome was protective for mice infected with T. cruzi was derived from study of ASC and caspase-1 knockout mice, which developed more severe illness and higher parasite burdens in end organs compared to wild-type controls [47]. Another study using NLRP3 and caspase-1 knockout mice confirmed that murine infection with T. cruzi activates the NLRP3 inflammasome resulting in production of IL-1β. This activation was protective; that is, mice lacking the NLRP3 inflammasome had increased parasite burdens. NLRP3-deficient macrophages were severely defective in killing T. cruzi, but surprisingly neutralization of IL-1β or IL-18 did not abrogate the protective effects of NLRP3 inflammasome activation in vitro. Instead, nitric oxide production was severely compromised in infected NLRP3- and caspase-1-deficient macrophages compared to wild type. This caspase-1-dependent nitric oxide release was required to control macrophage infection with T. cruzi, as shown with the use of caspase-1 inhibitors. This may point to a novel cytokine-independent function of inflammasome-activated caspase-1 [46].
The above studies lead to the conclusion that the NLR protein NOD1, but not NOD2, partially protects against acute T. cruzi infection of mice. The NLRP3 inflammasome also plays a role in protecting against T. cruzi infection in a murine model. NLRP3-mediated control of T. cruzi was suggested to be through caspase-1-dependent NO production, but independent of caspase-1-induced IL-1β/IL18 secretion. The mechanisms through which both of these NLR proteins control the parasite involve macrophage-mediated microbicidal responses, although the specific pathways need to be further elucidated.
Leishmania species and NLRP3
Leishmania species parasites cause leishmaniasis, a group of diseases affecting approximately 0.2–0.4 million individuals with new cases of visceral leishmaniasis and 0.7–1.2 million persons with new cases of cutaneous leishmaniasis cases [48, 49]. The worldwide distribution includes 98 endemic countries [50]. Most cases of leishmaniasis are transmitted by a Phlebotomus spp. or Lutzomyia spp. sand fly. Parasites develop in the sand fly gut to virulent metacyclic promastigotes which are then inoculated into mammalian skin. Promastigotes are rapidly taken up through receptor-mediated phagocytosis by local phagocytic cells such as macrophages, dendritic cells, or neutrophils, where they transform into the obligate intracellular amastigotes that replicate within the phagosome [51–54]. The Leishmania spp. parasites cause chronic infections whose symptoms range from cutaneous localized disease in which the pathology is caused by a vigorous inflammatory immune response, to a fatal visceral disease characterized by profound immunosuppression. Disease manifestations depend upon the species of parasite as well as the individual innate and adaptive immune response of the host [55, 56].
A role for NLR proteins is just beginning to be explored in models of the many species and varieties of leishmaniasis. Surprisingly little has been reported to date. The most studied model of leishmaniasis is the L. major model of cutaneous leishmaniasis. A notable characteristic of cutaneous leishmaniasis is the fact that “disease” symptoms are caused by a vigorous inflammatory response, which often but not always corresponds to the magnitude of parasite load in tissues surrounding a site of cutaneous ulceration. BALB/c mice are susceptible and fail to control the infection due to a predominant T helper 2-type (Th2-type) response to an immunodominant parasite antigen LACK [48]. This contrasts with resistant C57BL/6 mice which control the infection by mounting a T helper 1-type (Th1-type) immune response associated with IFNγ and IL-12 production [57]. Inflammation plays a central role in the pathogenesis of cutaneous leishmaniasis, but the role of cytokines produced by inflammasomes is not fully defined in models of cutaneous leishmaniasis. Two simultaneous reports of IL-18 in murine L. major infection documented a synergistic role for the cytokine in resistance to infection. One report found that IL-18 and IL-12 synergized in promoting resistance of BALB/c mice to pathology and protective immunity, and the other reported that the absence of IL-18, accomplished through either antibody-mediated depletion of BALB/c or genetic deletion in C57BL/6 mice, led to increased pathology [58]. The second group reported IL-18 plays a role only in protection early in L. major infection of IL-18 knockout C57BL/6 mice [59]. These reports suggest that, although IL-18 contributes to parasite control, it is not absolutely essential for protective immunity.
There is also an established role for IL-1β in leishmaniasis. Two species causing cutaneous leishmaniasis, L. major and L. amazonensis, differ in that the former leads to self-curing infections in all but BALB/c mice, whereas the latter causes progressive non-curing infection. Xin et al. [60] attributed this difference to differential induction of dendritic cell maturation. In particular, murine dendritic cells infected with L. amazonensis were less mature and released less IL-1β than those infected with L. major. Antigen presentation by dendritic cells led to a non-curative and inflammatory CD4? T cell phenotype producing IL-10 and IL-17, as opposed to a curative Th1 response induced by dendritic cells infected with L. major. A subsequent study was conducted with knockout mice lacking IL-1α, IL-1β, or IL-1Ra (IL-1 receptor antagonist) on a susceptible BALB/c background. The authors reported that IL-1α/β promotes the development of lesions due to L. major infection. Consistent with this, the lack of IL-1Ra, leading to unchecked IL-1 activity, leads to exacerbated parasite loads, and the authors suggest there is a divergence between disease and parasite load. [61]. Another study reported that L. major infection of IL-1α/β knockout mice on a resistant C57BL/6 background led to a delay in L. major lesion resolution rather than an overall decrease in lesions, and double knockout DCs were fully capable of inducing a protective immune response [62]. These results are further complicated because IL-1α, which uses the same receptor as IL-1β, is only partially dependent upon the inflammasome [63]. The effects of IL-1α may be distinct from IL-1β in leishmaniasis. IL-1α administration was found to be protective in L. major infection in both susceptible [64] and resistant mice, although ablation of both IL-1α and IL-1β did not cause increased disease in resistant mice [62]. Overall, these results suggest that IL-1 contributes to the pathology of cutaneous leishmaniasis and may also contribute to parasite control in some models. Nonetheless at this juncture, there is an unclear definition of the full role of IL-1β in murine models of cutaneous leishmaniasis, as these results could implicate different functions of IL-1β depending on the inoculum dose, innate resistance of the host model, and the species of Leishmania.
There has been one report indicating that at least one Leishmania species is capable of activating the NLRP3 inflammasome in macrophages in vitro, and in vivo evidence that NLRP3 inflammasomes influence the course of murine cutaneous or visceral disease. Knockout mice lacking NLRP3, ASC, or caspase-1 developed increased lesion size and increased parasite burden compared to wild-type control mice when infected with L. amazonensis. There was evidence that NLRP3 and inflammasome components limit the intracellular growth of L. amazonensis in macrophages. However, conflicting internal data in this report showing a decrease in IL-1β and caspase-1 cleavage in infected macrophages led to confusion about the exact role of the classical NLRP3 inflammasome. Nonetheless, in vitro data demonstrated that IL-1β release by macrophages infected with L. amazonensis, L. braziliensis, and L. mexicana required macrophages expressing NLRP3, ASC, and caspase-1, and an in vivo role for NLRP3 was shown for L. infantum [65]. Although not all data were consistent with direct activation of inflammasomes by parasite products, the study raises the very intriguing possibility that the NLRP3 inflammasome may become activated and provide an essential role in resistance to at least some Leishmania species, particularly L. amazonensis [65]. Careful comparison with other species is essential because of the very different pathogenesis and intracellular compartmentalization of L. amazonensis compared to other Leishmania species [66, 67].
Overall, these experiments suggest a protective role for the NLRP3 inflammasome against Leishmania parasite load, with increased host resistance to a high parasite load. There is conflicting evidence suggesting that inflammasome assembly or IL-1β could actually exacerbate the size of lesions of cutaneous leishmaniasis. The underlying mechanisms need further exploration, as does the universal nature of these mechanisms to other Leishmania species. Little is known about any putative role of the NOD1/2 proteins in leishmaniasis. Similarly, nothing is yet reported about the roles of the “non-inflammasome” NLRs in leishmaniasis, although their importance in inflammation, and developing adaptive immunity, suggests these pathways could be important.
Apicomplexan protozoa
Malaria, NLRP3, and NLRP12
Malaria is a fatal parasitic disease transmitted to humans by mosquitoes carrying one of the five species of Plasmodium. About 3.3 billion people, or half the world’s population, live in areas where they are at risk for malaria infection. Malaria is characterized by periodic paroxysms of high fever, anemia, and headache; associated complications include metabolic, renal, or cerebral derangements leading to death of an untreated host. The five Plasmodium species cause human malaria include Plasmodium ovale, P. vivax, P. malariae, P. falciparum, and P. knowlesi [68]. Most fatalities are caused by P. vivax and P. falciparum, particularly when malaria progresses to cerebral malaria caused by P. falciparum infection [69]. The parasites are transmitted by the bite of infected Anopheles mosquitos. The inoculated sporozoite forms travel to the liver and reproduce asexually within the hepatocytes (the liver stage of infection). Hepatocyte rupture releases merozoites into the bloodstream, which in turn bind receptors that enable their infection of red blood cells. Replication and maturation of circulating malaria in the blood stage of infection, proceeding through maturing trophozoite and schizont stages, results in generation of erythrocytic merozoites that are released and infect new red blood cells. The febrile symptoms of malarial disease correspond to the time of merozoite release during the blood stage of infection. P. falciparum has additional characteristics making it particularly virulent in the host. Notably, the parasite-derived PfEMP proteins are inserted in the erythrocyte membrane, leading to cytoadherance to the vascular endothelial cells and consequently local hypoxemia [70]. The most severe complication of falciparum malaria, cerebral malaria, is associated with excess systemic inflammation driven by inflammatory cytokines including TNF-α and IFN-γ [71].
Immune sensing of the intracellular parasites and the role of this response in control of infection as well as development of inflammatory complications are not completely understood. Parasites digest erythrocyte heme, producing the inflammatory inorganic crystal byproduct hemozoin (Hz). TLR2 and TLR9 recognize released malarial components including free GPI anchors and Plasmodium DNA complexed to Hz [72–75]. Recently, a potential role for NLR proteins in recognizing malaria parasites has been described. Unfortunately, the four Plasmodium species most commonly infecting humans do not infect mice, so murine models have been generated using alternate Plasmodium species to mimic human disease. Because of the observation that NOD1 and NOD2 are upregulated in human peripheral blood cells exposed to Plasmodium sporozoites [76], the P. berghei ANKA (PbA) model of cerebral malaria was studied in NOD1/NOD2 double knockout mice or wild-type C57BL/6 controls. The clinical course of malaria was unaltered in NOD1/NOD2 knockout mice compared to controls, but the levels of inflammatory cytokine levels, including IL-1β, KC, MCP-1, and IFN-γ, were significantly diminished. Although TNF-α and IL-6 did not change, the list of altered cytokines includes some cytokines contributing to human cerebral malaria [71]. It is so far unclear whether the NOD proteins have important roles in human disease [77].
A series of studies, cited below, have documented that the crystalline malarial byproduct hemozoin (Hz) activates the NLRP3 inflammasome and exacerbates symptoms of cerebral malaria in mouse models. Synthetic Hz activates the inflammasome in vivo without malarial infection [78]. Murine bone marrow macrophages from wild-type mice released IL-1β and activated caspase-1 when incubated in hemozoin, but the effect was absent in macrophages from NLRP3 or ASC knockout mice. In vivo, NLRP3 knockout mice had decreased Plasmodium berghei ANKA-induced cerebral malaria with unchanged parasite burden, demonstrating the involvement of NLRP3 in pathology but not necessarily infection [79].
Studies in another murine model of cerebral malaria due to P. chaboudi adami DS corroborated a role for the NLRP3 inflammasome in the pathogenesis of cerebral malaria. Using knockout mice, the investigators showed Hz-induced IL-1β activation release was dependent upon the presence of NLRP3, ASC, and caspase-1 in murine bone marrow macrophages. Lack of each of these improved survival of each of these knockout mouse strains upon infection with P. chaboudi adami. Furthermore, this study demonstrated that the tyrosine kinases Lyn and Syk were needed to activate the NLRP3 inflammasome [80]. Interestingly, another study of the P. berghei ANKA model reported that NLRP3 knockout mice had delayed onset of cerebral malaria, but mice lacking ASC, caspase-1, or IL-1RI had unchanged survival, leading to the suggestion that there may be a role for NLRP3 independent of the inflammasome [81]. Yet another contrasting report showed that murine P. berghei ANKA cerebral malaria was not altered in the absence of caspase-1, IL-1β, or IL-18 when infection was initiated with sporozoites rather than infected erythrocytes and that pathology was solely dependent on host TLR2/4 and MYD88. As such, the IL-1β inhibitor Anakinra was not protective against cerebral malaria in this model [82]. The difference between these studies and outcomes is not entirely evident, although it is possible that experimental factors (infecting parasite species and stage, subtle differences in protocol leading to more Hz–DNA complex in one model than another) could play a role and/or that there is indeed an inflammasome-independent role for NLRP3.
In contrast to the exacerbatory effect of the NLRP3 inflammasome on cerebral malaria, other investigators demonstrated that there is an adjuvant effect of Hz when administered with vaccine against P. falciparum, and this activity was entirely dependent upon MyD88 and not the NLRP3 inflammasome [83].
Studies of human immune responses during malaria confirm a putative role for inflammasomes. Human primary peripheral blood cells incubated with P. falciparum confirmed that the inflammasome is activated and cytokines are secreted by human macrophages in response to FcγR-meditated phagocytosis of infected RBCs. Inflammasome activation was confirmed by cleavage of caspase-1 and production of IL-1β, both of which were abrogated by caspase-1 inhibition [84]. Overlap or synergy between TLR- and NLRP3-induced inflammasome responses during the innate immune response to malaria is likely. A study of both human and murine cells showed that P. falciparum or P. berghei ANKA malaria hemozoin–DNA complexes activate TLR9 in human or murine macrophages, respectively. Injection of infected erythrocytes into mice also resulted in inflammasome activation and release of IL-1β. Parallel experiments with gene knockout mouse erythrocytes infected with P. berghei ANKA revealed that both the NLRP3 inflammasome responding to Hz and the AIM2 inflammasome responding to Plasmodium DNA are activated and produce IL-1β [26].
A recent murine study suggests that both NLRP12 and NLRP3 promote inflammasome formation in response to Plasmodium chabaudi AS infection with bacterial superinfection. Wild-type mice infected with P. chabaudi produced high levels of IL-1β in response to a secondary challenge with bacteria or LPS, and mice superinfected with bacteria succumbed to septic shock. In contrast, knockout mice lacking ASC, caspase-1, NLRP3, or NLRP12 had improved survival during a parallel bacterial superinfection. The authors proposed a dual role for NLRP3 and NLRP12 in forming inflammasomes during dual malarial–bacterial infection [85]. However, they stopped short of showing that NLRP12 is incorporated into inflammasomes and did not distinguish between responses to the parasite alone versus the parasite and bacteria. Since NLRP12 has been reported by other investigators to be anti-inflammatory through mechanisms that do not involve inflammasomes [8, 9], this model warrants revisiting.
Overall, the above studies suggest a role for NLRP3-induced inflammasomes and for TLR9 in the response to malaria hemozoin with or without complexed malaria DNA. They also implicate TLR9 in the pathologic responses to cerebral malaria in murine models. Potential involvement of AIM2-induced inflammasomes and the role of NLRP12 will require further investigation. It remains to be seen whether the NLR proteins play a role in exacerbating human malarial symptoms, including the deadly complication of cerebral malaria.
Toxoplasma, NOD2, NLRP1, and NLRP3
Toxoplasma species infect a broad host range of animals and birds including humans. T. gondii infection rates in humans are as high as 30–50 % worldwide [86]. The parasite is transmitted both horizontally and vertically in humans. Most immune competent human hosts are asymptomatic during toxoplasmosis, although occasionally a mononucleosis-like syndrome can occur. Immune-compromised individuals, however, can develop serious disease symptoms due to toxoplasmosis, including encephalitis and organ failure. Some of the severe conditions are congenital toxoplasmosis and CNS toxoplasmosis [87].
Toxoplasma gondii trophozoites can infect any nucleated cell. Robust cellular immunity, including inflammatory monocytes, a Th1 adaptive immune response and an NK cell response are needed to control infection [41, 45, 88, 89]. The importance of NOD2 in forming a protective Th1 immune response during toxoplasmosis was shown in a study utilizing NOD2 knockout mice. NOD2-deficient mice are unable to clear T. gondii and fail to mount an appropriate adaptive response. Dendritic cells are fully functional, and the NOD2 defect in immunity was shown to be due to a T cell intrinsic defect with impaired nuclear localization of c-Rel and defective Il2 transcription [90]. A search for genetic factors that influence susceptibility to toxoplasmosis led to even more exciting results. Using case–parent trios and transmission disequilibrium testing, human susceptibility to congenital toxoplasmosis was found to be significantly associated with SNPs in the NLRP1 gene locus. The role of NLRP1 in toxoplasmosis was investigated using RNAi knockdown of NLRP1 in a transfected human cell line. This revealed that Toxoplasma infection of cells with NLRP1 knockdown failed to induce production of inflammatory cytokines including IL-1β, IL-18, and IL-12 compared to control cells. NLRP1 knockdown cells also underwent accelerated cell death upon T. gondii infection [90, 91]. Using a murine model, Boothroyd’s group showed the NLRP1b inflammasome was activated during T. gondii infection of mouse and rat models, leading to protective immunity against oral challenge infection [92]. The parasite “ligand” for the inflammasome forming NLR, presumably NLRP1, was clearly shown in human monocytes or a human cell line infected with T. gondii. In this study, IL-1β release was dependent on mammalian cell caspase-1 and ASC, and on the parasite protein GRA15, the presumed agonist for NLRP1 [93]. Recently, Grigg’s group implicated the NLRP3 inflammasome as well as the NLRP1 inflammasome in murine resistance to T. gondii infection. In vivo, T. gondii activated the NLRP3 inflammasome in macrophages. Mice lacking NLRP3, ASC, and NLRP1 all displayed increased parasite burden and mortality. These studied further indicated that IL-18 may be a key cytokine in inflammasome-mediated resistance to T. gondii infection [94].
Thus, in contrast to malaria, NLRP1 and NLRP3 inflammasome activation induced by T. gondii infection limits the parasite load and dissemination. The role of NOD2 seems to be important as a sensor necessary for development of an effective adaptive response to Toxoplasma.
Schistosomes and NLRP3
The parasites discussed so far are unicellular protozoa, most of which can become intracellular and interact with NLRs or other intracellular host microbicidal pathways. In contrast, the Schistosoma species are a group of multicellular helminthic parasites which live in the human host as intravascular adult worms releasing inflammatory eggs into tissues. Schistosomiasis is transmitted in fresh bodies of water that are contaminated with human excrement, and which support growth of the intermediate snail host. Parasites directly penetrate through human skin and become a larval form before converting to adult worms. Eggs released by these adults not only can be released back into the environment to complete the life cycle, but also they can enter tissues causing a granulomatous response and severe inflammatory disease. More than 240 million people are infected with schistosomiasis worldwide, and more than 700 million are living in endemic areas [95]. As they are not intracellular, it is not immediately evident how these parasites would interact with the NLR proteins.
Among the species known to infect humans, a common parasite causing gastrointestinal or hepatic schistosomiasis is S. mansoni. Murine models show the immune response is characterized by a strong initial Th1 response to inoculated parasites, which later switches to a strong Th2 response to schistosomal egg antigen. Pathologic responses in S. mansoni disease are secondary to T-cell granulomas in the liver and intestines in response to eggs that become embedded in the tissue [96, 97]. It has previously been reported that components of soluble egg antigen (SEA) suppress TLR9-mediated dendritic cell response and release of TNF-α and IL-6 [98]. The da Costa group extended this observation to note that SEA dampens signaling through TLR7, TLR8, and TLR9, while simultaneously activating the NLRP3 inflammasome and triggering IL-1β secretion from mouse macrophages. Cells from knockout animals showed that this activation is dependent upon caspase-1 and NLRP3. The mechanism of activation was through an SEA component binding to Dectin-2, which associates with the FcRγ chain, activating Syk kinase and leading to ROS production and K+ efflux. Interestingly, however, during S. mansoni infection of knockout mice lacking either NLRP3 or ASC yielded a milder disease and decreased Th1, Th2, and Th17 cytokine production compared to wild-type mice [99].
Schistosomiasis is another example in which the inflammasome seems to promote pathology as opposed to cure or prevention of parasitic disease. Even though SEA triggers NLRP3 and IL-1β secretion leading to immunopathology in the liver, SEA may also be important for production of antigen-specific adaptive immune response.
Conclusions
Parasitic pathogens affect large segments of the global population and result in significant morbidity and mortality worldwide, yet are comparatively less studied than bacterial and viral pathogens. For this reason, many parasitic diseases are included in the list of “neglected tropical diseases,” including malaria, Chagas’ disease, leishmaniasis, and schistosomiasis [40, 50, 69, 95]. The host immune response and the parasites’ manipulation of that response are key areas of study that must be understood to achieve the goals of developing safe and effective vaccines and treatment regimens to control these important diseases.
The NLR proteins are crucial in sensing and responding to pathogens. Their functions in parasitic diseases are beginning to be described, particularly NOD1, NOD2, and the inflammasome forming NLRP3 and NLRP1 molecules. The NLR protein NOD1 is protective in T. cruzi infection, NOD1 and NOD2 function in inflammatory cytokine production in cerebral malaria, and NOD2 may be necessary to developing a T cell response to T. gondii. The NLRP1 and NLRP3 inflammasomes act as essential sensors of T. gondii and cause inflammation but are needed to limit parasite burden and dissemination. The NLRP3 inflammasome is protective to the host in T. cruzi infections, controlling parasite burdens and limiting end organ damage and may also be protective against Leishmania parasite burden, although it may also contributed to the inflammation that occurs during cutaneous leishmaniasis. The NLRP3 inflammasome, and possibly the AIM2 inflammasome, may increase pathogenesis and decrease survival in at least some models of cerebral malaria. NLRP3 causes immunopathology in the liver in response to S. mansoni egg antigens but may also be important for production of an adaptive immune response.
The investigation of NLR proteins in parasitic disease models is shedding light on some hitherto unknown functions and new mechanisms of actions of these proteins. For example, the functions of both NOD1 and NLRP3 in the T. cruzi mouse model appear to be novel, with NOD1 acting independent of NOD2 and the NLRP3 inflammasome acting primarily through enhanced nitric oxide production rather than through IL-1β [36, 46]. The great evolutionary diversity of parasitic disease-causing organisms is a strong argument for investigating NLR proteins in the broadest array of parasitic pathogens, as there will likely be unexpected interactions and evasion mechanisms that can shed light on the mechanisms by which the host senses pathogens and invokes inflammatory pathways.
Some NLR proteins may be needed for the host response to parasite infection by acting as innate immune sensors and driving the inflammatory response. However, the inflammatory response driven by these same proteins can also result in pathology during some parasitic diseases. Unregulated NLR-induced pathways can lead to organ failure and death. Dissecting the roles of NLR proteins in host defense and inflammatory pathology due to parasitic diseases will be invaluable background for the development of treatments and vaccines that must balance the roles of inflammation in protective immunity versus inflammatory injury.
Acknowledgments
This work was support in part by Grants R01 AI076233 (MEW) and NIH R01 AI087630 (F.S.S.) from the National Institutes of Health and by and grants 1i01BX001983 and 5I01BX000536 from the Department of Veterans’ Affairs.
Biography

Mary E. Wilson
Contributor Information
Gwendolyn M. Clay, The Interdisciplinary Program in Molecular and Cellular Biology, University of Iowa, 400 EMRB, 500 Newton Rd., Iowa City, IA 52242, USA
Fayyaz S. Sutterwala, Department of Internal Medicine, University of Iowa, SW54-GH, 200 Hawkins Dr., Iowa City, IA 52242, USA Veterans’ Affairs Medical Center, Iowa City, IA 52246, USA.
Mary E. Wilson, Email: mary-wilson@uiowa.edu, Veterans’ Affairs Medical Center, Iowa City, IA 52246, USA; Departments of Internal Medicine, Microbiology and Epidemiology, University of Iowa, SW34-GH, 200 Hawkins Dr., Iowa City, IA 52242, USA.
References
- 1.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 2.Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13(4):325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elinav E, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145(5):745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wlodarska M, et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell. 2014;156(5):1045–1059. doi: 10.1016/j.cell.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenbarth SC, et al. NLRP10 is a NOD-like receptor essential to initiate adaptive immunity by dendritic cells. Nature. 2012;484(7395):510–513. doi: 10.1038/nature11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joly S, et al. Cutting edge: Nlrp10 is essential for protective antifungal adaptive immunity against Candida albicans. J Immunol. 2012;189(10):4713–4717. doi: 10.4049/jimmunol.1201715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen IC, et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-κB signaling. Immunity. 2012;36(5):742–754. doi: 10.1016/j.immuni.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arthur JC, et al. Cutting edge: NLRP12 controls dendritic and myeloid cell migration to affect contact hypersensitivity. J Immunol. 2010;185(8):4515–4519. doi: 10.4049/jimmunol.1002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaki MH, et al. The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell. 2011;20(5):649–660. doi: 10.1016/j.ccr.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, et al. NLRC3, a member of the NLR family of proteins, is a negative regulator of innate immune signaling induced by the DNA sensor STING. Immunity. 2014;40(3):329–341. doi: 10.1016/j.immuni.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vladimer GI, et al. The NLRP12 inflammasome recognizes Yersinia pestis. Immunity. 2012;37(1):96–107. doi: 10.1016/j.immuni.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faustin B, et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25(5):713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 14.Hsu LC, et al. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1β secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci USA. 2008;105(22):7803–7808. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terra JK, et al. Cutting edge: resistance to Bacillus anthracis infection mediated by a lethal toxin sensitive allele of Nalp1b/Nlrp1b. J Immunol. 2010;184(1):17–20. doi: 10.4049/jimmunol.0903114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, et al. Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. Proc Natl Acad Sci USA. 2013;110(35):14408–14413. doi: 10.1073/pnas.1306376110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477(7366):596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 18.Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477(7366):592–595. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miao YC, Liu CJ. ATP-binding cassette-like transporters are involved in the transport of lignin precursors across plasma and vacuolar membranes. Proc Natl Acad Sci USA. 2010;107(52):22728–22733. doi: 10.1073/pnas.1007747108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tenthorey JL, et al. Molecular basis for specific recognition of bacterial ligands by NAIP/NLRC4 inflammasomes. Mol Cell. 2014;54(1):17–29. doi: 10.1016/j.molcel.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rayamajhi M, et al. Cutting edge: mouse NAIP1 detects the type III secretion system needle protein. J Immunol. 2013;191(8):3986–3989. doi: 10.4049/jimmunol.1301549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qu Y, et al. Phosphorylation of NLRC4 is critical for inflammasome activation. Nature. 2012;490(7421):539–542. doi: 10.1038/nature11429. [DOI] [PubMed] [Google Scholar]
- 23.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458(7237):514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes-Alnemri T, et al. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458(7237):509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts TL, et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323(5917):1057–1060. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 26.Kalantari P, et al. Dual engagement of the NLRP3 and AIM2 inflammasomes by Plasmodium-derived hemozoin and DNA during malaria. Cell Rep. 2014;6(1):196–210. doi: 10.1016/j.celrep.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones JW, et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc Natl Acad Sci USA. 2010;107(21):9771–9776. doi: 10.1073/pnas.1003738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adamczak SE, et al. Pyroptotic neuronal cell death mediated by the AIM2 inflammasome. J Cereb Blood Flow Metab. 2014;34(4):621–629. doi: 10.1038/jcbfm.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cassel SL, Sutterwala FS. Sterile inflammatory responses mediated by the NLRP3 inflammasome. Eur J Immunol. 2010;40(3):607–611. doi: 10.1002/eji.200940207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen H, Miao EA, Ting JP. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity. 2013;39(3):432–441. doi: 10.1016/j.immuni.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horng T. Calcium signaling and mitochondrial destabilization in the triggering of the NLRP3 inflammasome. Trends Immunol. 2014;35(6):253–261. doi: 10.1016/j.it.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fitzgerald KA. NLR-containing inflammasomes: central mediators of host defense and inflammation. Eur J Immunol. 2010;40(3):595–598. doi: 10.1002/eji.201040331. [DOI] [PubMed] [Google Scholar]
- 33.Philpott DJ, et al. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol. 2014;14(1):9–23. doi: 10.1038/nri3565. [DOI] [PubMed] [Google Scholar]
- 34.Rubino SJ, et al. Nod-like receptors in the control of intestinal inflammation. Curr Opin Immunol. 2012;24(4):398–404. doi: 10.1016/j.coi.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Moreira LO, Zamboni DS. NOD1 and NOD2 Signaling in Infection and Inflammation. Front Immunol. 2012;3:328. doi: 10.3389/fimmu.2012.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva GK, et al. Cutting edge: nucleotide-binding oligomerization domain 1-dependent responses account for murine resistance against Trypanosoma cruzi infection. J Immunol. 2010;184(3):1148–1152. doi: 10.4049/jimmunol.0902254. [DOI] [PubMed] [Google Scholar]
- 37.Cui J, et al. NLRC5 negatively regulates the NF-κB and type I interferon signaling pathways. Cell. 2010;141(3):483–496. doi: 10.1016/j.cell.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benko S, et al. NLRC5 limits the activation of inflammatory pathways. J Immunol. 2010;185(3):1681–1691. doi: 10.4049/jimmunol.0903900. [DOI] [PubMed] [Google Scholar]
- 39.Hotez PJ, et al. The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl Trop Dis. 2008;2(9):e300. doi: 10.1371/journal.pntd.0000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanowitz HB, et al. Perspectives on Trypanosoma cruzi-induced heart disease (Chagas disease) Prog Cardiovasc Dis. 2009;51(6):524–539. doi: 10.1016/j.pcad.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teixeira MM, Gazzinelli RT, Silva JS. Chemokines, inflammation and Trypanosoma cruzi infection. Trends Parasitol. 2002;18(6):262–265. doi: 10.1016/s1471-4922(02)02283-3. [DOI] [PubMed] [Google Scholar]
- 42.Bafica A, et al. Cutting edge: TLR9 and TLR2 signaling together account for MyD88-dependent control of parasitemia in Trypanosoma cruzi infection. J Immunol. 2006;177(6):3515–3519. doi: 10.4049/jimmunol.177.6.3515. [DOI] [PubMed] [Google Scholar]
- 43.Oliveira AC, et al. Expression of functional TLR4 confers proinflammatory responsiveness to Trypanosoma cruzi glycoinositolphospholipids and higher resistance to infection with T. cruzi. J Immunol. 2004;173(9):5688–5696. doi: 10.4049/jimmunol.173.9.5688. [DOI] [PubMed] [Google Scholar]
- 44.Caetano BC, et al. Requirement of UNC93B1 reveals a critical role for TLR7 in host resistance to primary infection with Trypanosoma cruzi. J Immunol. 2011;187(4):1903–1911. doi: 10.4049/jimmunol.1003911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campos MA, et al. Impaired production of proinflammatory cytokines and host resistance to acute infection with Trypanosoma cruzi in mice lacking functional myeloid differentiation factor 88. J Immunol. 2004;172(3):1711–1718. doi: 10.4049/jimmunol.172.3.1711. [DOI] [PubMed] [Google Scholar]
- 46.Goncalves VM, et al. NLRP3 controls Trypanosoma cruzi infection through a caspase-1-dependent IL-1R-independent NO production. PLoS Negl Trop Dis. 2013;7(10):e2469. doi: 10.1371/journal.pntd.0002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silva GK, et al. Apoptosis-associated speck-like protein containing a caspase recruitment domain inflammasomes mediate IL-1β response and host resistance to Trypanosoma cruzi infection. J Immunol. 2013;191(6):3373–3383. doi: 10.4049/jimmunol.1203293. [DOI] [PubMed] [Google Scholar]
- 48.Julia V, Rassoulzadegan M, Glaichenhaus N. Resistance to Leishmania major induced by tolerance to a single antigen. Science. 1996;274(5286):421–423. doi: 10.1126/science.274.5286.421. [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization. Leishmaniasis. 2014 www.who.int/topics/leishmaniasis/en/ and www.who.int/mediacentre/factsheets/fs375/en/
- 50.Alvar J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blackwell JM, et al. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J Exp Med. 1985;162(1):324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Da Silva RP, et al. CR1, the C3b receptor, mediates binding of infective Leishmania major metacyclic promastigotes to human macrophages. J Immunol. 1989;143(2):617–622. [PubMed] [Google Scholar]
- 53.Wilson ME, Pearson RD. Roles of CR3 and mannose receptors in the attachment and ingestion of Leishmania donovani by human mononuclear phagocytes. Infect Immun. 1988;56(2):363–369. doi: 10.1128/iai.56.2.363-369.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mosser DM, Springer TA, Diamond MS. Leishmania promastigotes require opsonic complement to bind to the human leukocyte integrin Mac-1 (CD11b/CD18) J Cell Biol. 1992;116(2):511–520. doi: 10.1083/jcb.116.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson ME, Jeronimo SM, Pearson RD. Immunopathogenesis of infection with the visceralizing Leishmania species. Microb Pathog. 2005;38(4):147–160. doi: 10.1016/j.micpath.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 56.Olivier M, Gregory DJ, Forget G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clin Microbiol Rev. 2005;18(2):293–305. doi: 10.1128/CMR.18.2.293-305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol. 2011;9(8):604–615. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 58.Monteforte GM, et al. Genetically resistant mice lacking IL-18 gene develop Th1 response and control cutaneous Leishmania major infection. J Immunol. 2000;164(11):5890–5893. doi: 10.4049/jimmunol.164.11.5890. [DOI] [PubMed] [Google Scholar]
- 59.Ohkusu K, et al. Potentiality of interleukin-18 as a useful reagent for treatment and prevention of Leishmania major infection. Infect Immun. 2000;68(5):2449–2456. doi: 10.1128/iai.68.5.2449-2456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xin L, Li Y, Soong L. Role of interleukin-1β in activating the CD11c(high) CD45RB-dendritic cell subset and priming Leishmania amazonensis-specific CD4+ T cells in vitro and in vivo. Infect Immun. 2007;75(10):5018–5026. doi: 10.1128/IAI.00499-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Voronov E, et al. IL-1-induced inflammation promotes development of leishmaniasis in susceptible BALB/c mice. Int Immunol. 2010;22(4):245–257. doi: 10.1093/intimm/dxq006. [DOI] [PubMed] [Google Scholar]
- 62.Kautz-Neu K, et al. IL-1 signalling is dispensable for protective immunity in Leishmania-resistant mice. Exp Dermatol. 2011;20(1):76–78. doi: 10.1111/j.1600-0625.2010.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fettelschoss A, et al. Inflammasome activation and IL-1β target IL-1α for secretion as opposed to surface expression. Proc Natl Acad Sci USA. 2011;108(44):18055–18060. doi: 10.1073/pnas.1109176108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Von Stebut E, et al. Interleukin 1α promotes Th1 differentiation and inhibits disease progression in Leishmania major-susceptible BALB/c mice. J Exp Med. 2003;198(2):191–199. doi: 10.1084/jem.20030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lima-Junior DS, et al. Inflammasome-derived IL-1β production induces nitric oxide-mediated resistance to Leishmania. Nat Med. 2013;19(7):909–915. doi: 10.1038/nm.3221. [DOI] [PubMed] [Google Scholar]
- 66.Ji J, Sun J, Soong L. Impaired expression of inflammatory cytokines and chemokines at early stages of infection with Leishmania amazonensis. Infect Immun. 2003;71(8):4278–4288. doi: 10.1128/IAI.71.8.4278-4288.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soong L, et al. Role of CD4+ T cells in pathogenesis associated with Leishmania amazonensis infection. J Immunol. 1997;158(11):5374–5383. [PubMed] [Google Scholar]
- 68.Moyes CL, et al. Defining the geographical range of the Plasmodium knowlesi reservoir. PLoS Negl Trop Dis. 2014;8(3):e2780. doi: 10.1371/journal.pntd.0002780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.World Health Organization. World malaria report. 2012:1–249.
- 70.Moxon CA, Grau GE, Craig AG. Malaria: modification of the red blood cell and consequences in the human host. Br J Haematol. 2011;154(6):670–679. doi: 10.1111/j.1365-2141.2011.08755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frevert U, Nacer A. Immunobiology of Plasmodium in liver and brain. Parasite Immunol. 2013;35(9–10):267–282. doi: 10.1111/pim.12039. [DOI] [PubMed] [Google Scholar]
- 72.Coban C, et al. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J Exp Med. 2005;201(1):19–25. doi: 10.1084/jem.20041836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krishnegowda G, et al. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J Biol Chem. 2005;280(9):8606–8616. doi: 10.1074/jbc.M413541200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gowda NM, Wu X, Gowda DC. The nucleosome (histone-DNA complex) is the TLR9-specific immunostimulatory component of Plasmodium falciparum that activates DCs. PLoS ONE. 2011;6(6):e20398. doi: 10.1371/journal.pone.0020398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parroche P, et al. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci USA. 2007;104(6):1919–1924. doi: 10.1073/pnas.0608745104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ockenhouse CF, et al. Common and divergent immune response signaling pathways discovered in peripheral blood mononuclear cell gene expression patterns in presymptomatic and clinically apparent malaria. Infect Immun. 2006;74(10):5561–5573. doi: 10.1128/IAI.00408-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Finney CA, et al. Disruption of Nod-like receptors alters inflammatory response to infection but does not confer protection in experimental cerebral malaria. Am J Trop Med Hyg. 2009;80(5):718–722. [PubMed] [Google Scholar]
- 78.Griffith JW, et al. Pure Hemozoin is inflammatory in vivo and activates the NALP3 inflammasome via release of uric acid. J Immunol. 2009;183(8):5208–5220. doi: 10.4049/jimmunol.0713552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dostert C, et al. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS ONE. 2009;4(8):e6510. doi: 10.1371/journal.pone.0006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shio MT, et al. Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS Pathog. 2009;5(8):e1000559. doi: 10.1371/journal.ppat.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reimer T, et al. Experimental cerebral malaria progresses independently of the Nlrp3 inflammasome. Eur J Immunol. 2010;40(3):764–769. doi: 10.1002/eji.200939996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kordes M, Matuschewski K, Hafalla JC. Caspase-1 activation of interleukin-1β (IL-1β) and IL-18 is dispensable for induction of experimental cerebral malaria. Infect Immun. 2011;79(9):3633–3641. doi: 10.1128/IAI.05459-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coban C, et al. Immunogenicity of whole-parasite vaccines against Plasmodium falciparum involves malarial hemozoin and host TLR9. Cell Host Microbe. 2010;7(1):50–61. doi: 10.1016/j.chom.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 84.Zhou J, et al. Opsonization of malaria-infected erythrocytes activates the inflammasome and enhances inflammatory cytokine secretion by human macrophages. Malar J. 2012;11:343. doi: 10.1186/1475-2875-11-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ataide MA, et al. Malaria-induced NLRP12/NLRP3-dependent caspase-1 activation mediates inflammation and hypersensitivity to bacterial superinfection. PLoS Pathog. 2014;10(1):e1003885. doi: 10.1371/journal.ppat.1003885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Flegr J, et al. Toxoplasmosis—a global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. PLoS ONE. 2014;9(3):e90203. doi: 10.1371/journal.pone.0090203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Robert-Gangneux F, Darde ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25(2):264–296. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Michailowsky V, et al. Pivotal role of interleukin-12 and interferon-γ axis in controlling tissue parasitism and inflammation in the heart and central nervous system during Trypanosoma cruzi infection. Am J Pathol. 2001;159(5):1723–1733. doi: 10.1016/s0002-9440(10)63019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cardillo F, et al. Regulation of Trypanosoma cruzi infection in mice by γ interferon and interleukin 10: role of NK cells. Infect Immun. 1996;64(1):128–134. doi: 10.1128/iai.64.1.128-134.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shaw MH, et al. T cell-intrinsic role of Nod2 in promoting type 1 immunity to Toxoplasma gondii. Nat Immunol. 2009;10(12):1267–1274. doi: 10.1038/ni.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Witola WH, et al. NALP1 influences susceptibility to human congenital toxoplasmosis, proinflammatory cytokine response, and fate of Toxoplasma gondii-infected monocytic cells. Infect Immun. 2011;79(2):756–766. doi: 10.1128/IAI.00898-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ewald SE, Chavarria-Smith J, Boothroyd JC. NLRP1 is an inflammasome sensor for Toxoplasma gondii. Infect Immun. 2014;82(1):460–468. doi: 10.1128/IAI.01170-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gov L, et al. Human innate immunity to Toxoplasma gondii is mediated by host caspase-1 and ASC and parasite GRA15. MBio. 2013;4(4):e0255-13. doi: 10.1128/mBio.00255-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gorfu G, et al. Dual role for inflammasome sensors NLRP1 and NLRP3 in murine resistance to Toxoplasma gondii. MBio. 2014;5(1):e01117-13. doi: 10.1128/mBio.01117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.World Health Organization. Geneva: World Health Organization; 2013. Schistosomiasis: progress report 2001–2011, strategic plan 2012–2020. http://apps.who.int/iris/handle/10665/78074#sthash.H3fFKrLo.dpuf. [Google Scholar]
- 96.Fairfax K, et al. Th2 responses in schistosomiasis. Semin Immunopathol. 2012;34(6):863–871. doi: 10.1007/s00281-012-0354-4. [DOI] [PubMed] [Google Scholar]
- 97.Maizels RM, et al. Regulation of pathogenesis and immunity in helminth infections. J Exp Med. 2009;206(10):2059–2066. doi: 10.1084/jem.20091903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jenkins SJ, et al. Schistosome larvae stimulate macrophage cytokine production through TLR4-dependent and -independent pathways. Int Immunol. 2005;17(11):1409–1418. doi: 10.1093/intimm/dxh319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ritter M, et al. Schistosoma mansoni triggers Dectin-2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proc Natl Acad Sci USA. 2010;107(47):20459–20464. doi: 10.1073/pnas.1010337107. [DOI] [PMC free article] [PubMed] [Google Scholar]