Abstract
Introduction
It remains controversial whether transarterial chemoembolization (TACE) should be performed in patients with advanced-stage hepatocellular carcinoma (HCC). The present large retrospective cohort study aimed to define the survival outcome following TACE of advanced HCC and to identify the prognostic factors.
Methods
508 patients with Barcelona Clinic Liver Cancer (BCLC) C-stage HCC, Child-Pugh A/B were who were treated with TACE between November 1998 to December 2013 were identified.
Results
There was no significant difference in overall survival (OS) between patients with Eastern Cooperative Oncology Group (ECOG) 0 and those with ECOG ≥1 (10.5 months vs. 11.9 months, P=0.87). The median OS of patients without portal vein tumor thrombosis (PVTT) was longer than that of patients with PVTT (16.9 months vs. 6.1 months, P<0.001). Child-Pugh B class, PVTT, extrahepatic metastasis, tumor size ≥5 cm, number of tumors ≥3 and alpha-fetoprotein ≥ 400ng/dl were significantly associated with decreased survival and were used for determining the risk scores. All patients were divided into two groups (low-risk and high-risk groups) according to the cut-off value of 6.5 for risk scores. The patients with a value <6.5 (low-risk group) had significantly longer survival than those with >6.5 (high-risk group) (24.1 vs. 7.5 months, respectively; P<0.001).
Conclusions
TACE is an effective therapy for select patients with advanced stage HCC and may provide equal or improved survival as compared with reported outcomes with sorafenib. The results highlight the need for a differentiated approach to therapeutic recommendations for patients with BCLC C.
Keywords: Hepatocellular carcinoma, transarterial chemoembolization, overall survival, Barcelona Clinic Liver Cancer
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy and the second leading cause of cancer deaths worldwide with a rapidly growing incidence in the Western world primarily due to obesity-related non-alcoholic steatohepatitis 1, 2. Most patients with HCC are diagnosed at an intermediate to advanced-stage in which the chances of curative treatments are limited 3. According to the Barcelona Clinic Liver Cancer (BCLC) staging classification, patients with advanced-stage (BCLC stage C) disease have a dismal prognosis with expected median survival times of 6 months; in turn, sorafenib has been recommended as the standard treatment for these patients3–5. Transarterial chemoembolization (TACE), a catheter-based minimally invasive loco-regional therapy that is recommended for intermediate-stage patients (BCLC stage B), is, however, frequently applied to patients with advanced stage disease 6. In fact, the global BRIDGE study (the first multiregional large-scale, longitudinal survey on the real-life management of HCC) demonstrated that TACE was the most frequently recorded treatment for advanced-stage HCC patients 7. As such, the clinical applicability and “real world” implementation of the BCLC staging system has been questioned.
While the safety and feasibility of TACE in advanced-stage HCC patients have been established in published studies, whether TACE provides a survival benefit for patients with advanced HCC remains controversial 8–10. A retrospective case-control study reported improved overall survival (OS) among patients with advanced-stage HCC and portal vein invasion compared with supportive care, regardless of Child-Pugh A or B class 10. In a separate retrospective case-controlled study, TACE was reported to have comparable survival versus sorafenib among advanced-stage HCC patients 11. Most previous studies have been limited, however, as they excluded patients with extrahepatic metastasis and failed to provide information on the Eastern Cooperative Oncology Group (ECOG) performance status 12. In addition, few studies reported the prognostic factors associated with outcome following TACE therapy among patients with the advanced HCC. Therefore, the objective of the current study was to define the long-term survival following TACE of advanced HCC. In addition, we sought to identify prognostic factors associated with overall survival using a large single-center cohort of patients treated with advanced-stage HCC who underwent TACE therapy.
Patients and Methods
Patients
All consecutive patients with HCC treated using conventional TACE (cTACE) or drug-eluting beads TACE (DEB-TACE) between November 1998 and December 2013 were analyzed. Inclusion criteria included patients with HCC categorized as BCLC stage C, and Child-Pugh class A or B who had ECOG performance status 0–2. Three patients with Child-Pugh class C and 2 patients without detailed baseline information were excluded. A total of 508 consecutive HCC patients with BCLC stage C who underwent TACE were included in the final analytic cohort. Of these 508 patients, 16 (3.1%) patients received previously liver resection and 5 (1%) had undergone previous radiofrequency ablation. Among the total cohort, 44 patients received post-TACE surgical treatments including transplantation (n=34) and liver resection (n=10); 42 patients received sorafenib administration after TACE. HCC was diagnosed according to histologic examination or typical findings of early tumor enhancement followed by wash-out on dynamic cross-sectional liver imaging 13, 3. Last follow-up was on December 31st 2014.
Treatment
In our center, treatment decisions were routinely discussed in a multidisciplinary tumor board with medical oncologists, hepatologists, surgeons, pathologists, radiation oncologists, as well as interventional radiologists. After 2009, we started to perform DEB-TACE paralleling the growing evidence of its improved safety profile compared to cTACE. For DEB-TACE, LC Bead (BTG, Surrey, United Kingdom) with a diameter of 100–300 μm were loaded with 100 mg of doxorubicin hydrochloride (25 mg/mL) and mixed with an equal volume of nonionic contrast material (Oxilan, 300 mg of iodine/mL; Guerbet, Bloomington, Indiana, USA). Doxorubicin-eluting beads (up to 100 mg) were administered by alternating aliquot injections of the beads and contrast material until complete delivery was achieved or the blood flow of the feeding artery slowed down substantially 14, 15. For cTACE, an emulsion containing 50 mg doxorubicin (Adriamycin; Pharmacia & Upjohn, Peapack, NJ), and 10 mg mitomycin C in a 1:1 mixture with lipiodol (Lipiodol; Guerbet, Paris, France) was infused and followed by the infusion of gelatin-coated tris-acryl microspheres (Embosphere Microspheres; Merit Medical Systems, South Jordan, Utah, USA) until arterial inflow was substantially reduced as seen on fluoroscopy 15. In all cases, either a selective of super-selective approach was chosen. No patient received Yttrium-90 radioembolization.
Statistical analysis
Continuous variables were summarized as means and ranges. Categorical variables were expressed as frequencies and percentages. Survival was assessed according to the Kaplan-Meier method and differences in survival estimates were compared using the log-rank test. OS was calculated from the date of the first TACE until death; patients who were still alive at the end of observation were censored. Univariate and multivariate analyses were conducted using the Cox proportional hazards model to identify risk factors associated with survival. On univariate analysis, survival was analyzed according to baseline features including age, gender, etiology, Child-Pugh classification, ECOG, presence of portal vein tumor thrombosis (PVTT), presence of extrahepatic metastasis, tumor size, number of HCC nodules, and alpha-fetoprotein levels. Variables with a P-value <0.1 in the univariate Cox models were subsequently included in the multivariate model 16. Risk scores for individual patients were calculated by combining the prognostic indicators weighted according to the corresponding regression coefficients. For ease of use, the regression coefficients were multiplied by 10 and then rounded to the nearest integer. The receiver operating characteristic (ROC) curve was used to evaluate the discriminatory ability of categorizing patients with advanced-stage into subgroups- low risk group and high risk group. The c-statistic may range from 0 to 1 and models with the value >0.7 are generally considered to be useful models 17. Cut-off values for risk scores were determined according to ROC curves. A two-tailed P-value of <0.05 was considered statistically significant. Statistical analyses were performed using SPSS (SPSS Inc., version 17.0, Chicago, IL)
Results
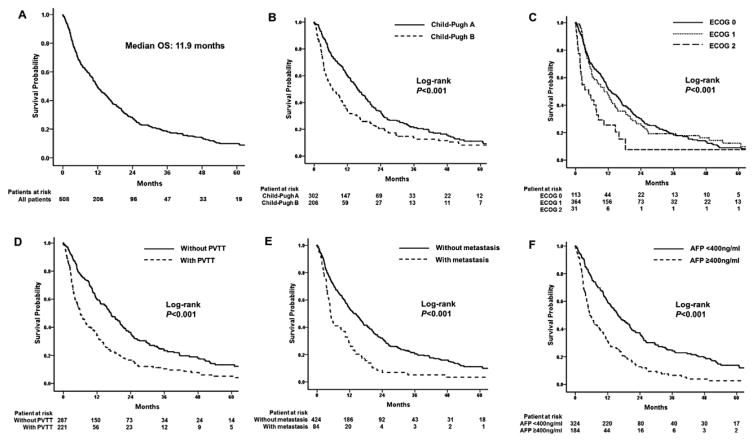
Baseline patient baseline clinical characteristics are noted in Table 1. Mean patient age was 63 (range, 19–90 years) and 79.3% of patients were male. Hepatitis virus C was the predominant cause of HCC (45.3%). 303 patients (59.6%) were categorized as Child-Pugh A class and 395 patients (77.8%) had an ECOG performance score ≥1. The distribution of stage-defining characteristics across the ECOG scores is detailed in Table 2. Specifically, 221 patients (43.5%) had PVTT and 84 patients (16.5%) had extrahepatic metastasis. The median duration of follow-up was 9.3 months (range 0.1–153.6). At the last follow-up, 377 (74.2%) patients had died. Median OS was 11.9 months (95%CI 10.1–13.7) (Figure 1A). The 1-, 3-, and 5-year survival was 40.6%, 9.1% and 3.7%, respectively.
Table 1.
Baseline patient demographics and clinical characteristics (n=508).
| Variable | No. | % |
|---|---|---|
| Age/years, mean (range) | 63 (19–90) | |
| Gender | ||
| Male | 403 | 79.3 |
| Female | 105 | 20.7 |
| Etiology | ||
| Hepatitis C infection | 230 | 45.3 |
| Hepatitis B infection | 83 | 16.3 |
| Alcohol | 173 | 34.1 |
| Child-Pugh class | ||
| A | 303 | 59.6 |
| B | 205 | 40.4 |
| ECOG performance status | ||
| 0 | 113 | 22.2 |
| 1 | 364 | 71.7 |
| 2 | 31 | 6.1 |
| Disease Burden | ||
| Portal vein tumor thrombosis | 221 | 43.5 |
| Extrahepatic metastasis | 84 | 16.5 |
| Tumor size (cm) | 7.9 ± 4.6 (1–22) | |
| No. of HCC nodules (1–2/≥3) | 231/277 | 45.5/54.5 |
| AFP | ||
| <400ng/mL | 324 | 63.8 |
| ≥400ng/mL | 184 | 36.2 |
| Ascites | ||
| Yes | 135 | 26.6 |
| No | 373 | 73.4 |
| Baseline laboratory values, mean (range) | ||
| International normalized ratio | 1.1 (0.7–4.2) | |
| Albumin, g/dL | 3.6 (1.8–5) | |
| Total bilirubin, mg/dL | 1.3 (0.2–16.7) | |
ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; AFP, alpha-fetoprotein.
Table 2.
508 HCC patients with different ECOG score.
| Variable | N | P | ||
|---|---|---|---|---|
|
| ||||
| ECOG 0 (n=113) | ECOG 1 (n=364) | ECOG 2 (n=31) | ||
| Child-Pugh A/B | 70 (61.9%) / 43 (27.1%) | 219 (60.2%) / 145 (29.8%) | 13 (41.9%) / 18 (58.1%) | 0.116 |
| Portal vein tumor thrombosis (yes/no) | 74 (65.4%) / 39 (34.6%) | 134 (36.8%) / 230 (63.2%) | 13 (41.9%) / 18 (58.1%) | <0.001 |
| Extrahepatic metastasis (yes/no) | 30 (26.5%) / 83 (73.5%) | 45 (12.4%) / 319 (87.6%) | 9 (29%) / 22 (71%) | <0.001 |
| Tumor size (≥5/<5) | 82 (72.6%) / 31(27.4%) | 235 (64.6%) / 129 (35.4%) | 23 (74.2%) / 8 (25.8%) | 0.194 |
| No. of HCC nodules (>2/1–2) | 70 (61.9%) / 43 (38.1%) | 193 (53%) / 171 (47%) | 14 (45.2%) / 17 (54.8%) | 0.140 |
HCC, hepatocellular carcinoma; ECOG, Eastern Cooperative Oncology Group.
Figure 1.
(A) Overall survival of 508 patients with advanced-stage HCC treated using TACE. (B) Comparison of survival times based on Child-Pugh class, (C) based on Eastern Cooperative Oncology Group (ECOG) performance score, (D) based on presence of Portal vein tumor thrombosis (PVTT), (E) based on presence of extrahepatic metastasis, and (F) based on alpha-fetoprotein level.
The median number of TACE sessions per patient was 2 (range, 1–10) for a total of 906 procedures; 296 (58.3%) patients received conventional TACE, while 152 (29.9%) patients received DEB-TACE and 60 (11.8%) patients received a combination of both treatments over time. There was no difference in OS among patients who received conventional TACE and those patients who received DEB-TACE [11.1 months (95%CI 9.6–12.6) vs. 10.4 months (95%CI 6.3–14.5), respectively; P=0.896]. In addition, there was no difference in OS among patients who received TACE alone versus patients who received TACE plus sorafenib [11.2 months (95%CI 9.6–12.6) vs. 16.5 months (95%CI 10.8–22.2), respectively; P=0.278].
The median OS among Child-Pugh class A patients was longer compared with Child-Pugh class B patients [15.7 months (95%CI 13.1–18.3) vs. 6.7 months (95%CI 4.1–9.3), respectively; P<0.001] (Figure 1B). In addition, there was no difference in OS among patients who were ECOG 0 versus those patients who had an ECOG ≥1 [10.5 months (95%CI 6.7–14.3) vs. 11.9 months (95%CI 9.6–14.2), respectively; P=0.87]. In contrast, patients with an ECOG ≤1 had a longer OS compared with patients who were ECOG ≥2 [12.3 months (95%CI 10.4–14.2) vs. 4.8 months (95%CI 0.6–9.0), respectively; P<0.001] (Figure 1C). Furthermore, patients without PVTT had a longer OS compared with patients who had PVTT [16.9 months (95%CI 14.3–19.5) vs. 6.1 months (95%CI 4.5–7.7), respectively; P<0.001] (Figure 1D). In addition, the median survival of the patients without extrahepatic metastasis was 13.6 months (95%CI 11.2–16.0) compared with only 5 months (95%CI 4.0–6.0) for patients who had metastasis (P<0.001) (Figure 1E). Baseline alpha-fetoprotein was also associated with OS, as patients with alpha-fetoprotein ≥400ng/ml had a median survival three times longer than patients who had a baseline alpha-fetoprotein <400ng/ml [15.9 months (95%CI 13.2–18.6) vs. 5.3 months (95%CI 3.8–6.8), respectively; P<0.001] (Figure 1F).
On univariate analysis, variables that were associated with an increased likelihood of death included Child-Pugh class, presence of PVTT, presence of extrahepatic metastasis, tumor size, number of HCC nodules and alpha-fetoprotein level (all P<0.05). On multivariate analysis, after controlling for competing risk factors, Child-Pugh B class (HR=1.5, 95%CI 1.2–1.9), PVTT (HR=1.5, 95%CI 1.2–1.9), extrahepatic metastasis (HR=1.8, 95%CI 1.4–2.4), tumor size ≥5 cm (HR=1.4, 95%CI 1.1–1.7), number of tumors ≥3 (HR=1.4, 95%CI 1.1–1.7) and alpha-fetoprotein ≥400ng/dl (HR=1.7, 95%CI 1.4–2.1) remained associated with survival (Table 3).
Table 3.
Predictors for overall survival in 508 HCC patients treated with TACE.
| Variable | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.005 | 0.997–1.014 | 0.217 | |||
| Gender (male/female) | 0.966 | 0.748–1.249 | 0.793 | |||
| Etiology (hepatitis infection/other) | 0.896 | 0.73201.098 | 0.291 | |||
| Child-Pugh (B/A) | 1.574 | 1.283–1.933 | <0.001 | 1.544 | 1.248–1.909 | <0.001 |
| ECOG (≥1/<1) | 0.98 | 0.769–1.249 | 0.871 | |||
| PVTT (yes/no) | 1.907 | 1.553–2.341 | <0.001 | 1.523 | 1.218–1.905 | <0.001 |
| Extrahepatic metastasis (yes/no) | 1.988 | 1.530–2.583 | <0.001 | 1.828 | 1.396–2.395 | <0.001 |
| Size (≥5/<5) | 1.826 | 1.458–2.286 | <0.001 | 1.359 | 1.058–1.747 | 0.016 |
| No. of HCC nodules (>2/1–2) | 1.751 | 1.421–2.158 | <0.001 | 1.380 | 1.103–1.725 | 0.005 |
| AFP (≥400/<400) | 2.082 | 1.680–2.580 | <0.001 | 1.707 | 1.365–2.135 | <0.001 |
HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; HR, hazard ratio; CI, confidence interval; PVTT, portal vein tumor thrombosis; ECOG, Eastern Cooperative Oncology Group; AFP, alpha-fetoprotein.
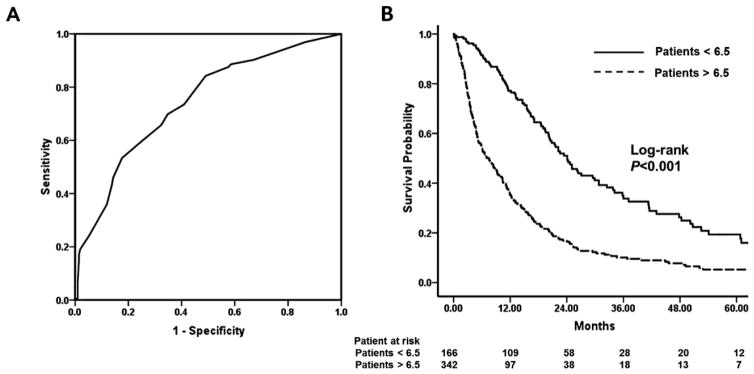
Risk scores for individual patients were then calculated by combining the six factors that were prognostic on multivariate analysis (Child-Pugh class, PVTT, extrahepatic metastasis, tumor size, number of HCC nodules and alpha-fetoprotein) with the corresponding regression coefficients. An equation was utilized to determine the risk score: 6×(metastasis: 0 if no, 1 if yes) + 5×(alpha-fetoprotein: 0 if <400ng/dl, 1 if ≥400ng/dl) + 4×(PVTT: 0 if no, 1 if yes) +4×(Child-Pugh: 0 if A, 1 if B) + 3×(tumor size: 0 if <5cm, 1 if ≥5cm) + 3×(number of lesions: 0 if 1–2, 1 if ≥3). Utilizing this score, the AUC to predict 1 year survival was 0.7 (95%CI 0.7–0.8) (Figure 2A). Giving equal weight to sensitivity and specificity, 6.5, the risk score cut-off value, was to achieve the maximum sensitivity and specificity (sensitivity=84.2%, specificity=51%). The patients with risk score <6.5 were classified as low risk group and the patients with risk score >6.5 were classified as high risk group. All risk scores were integer according to the equation. Thus there was no patient whose score was 6.5. After stratifying patients into two groups according to the 6.5 cut-off value, Kaplan-Meier analyses demonstrated a marked difference in the survival of patients with advanced HCC undergoing TACE. Specifically, the median survival among patient in the low risk group was over three times longer than patients in the high risk group [24.1months (95%CI 20.2–28.0) vs. 7.5months (95%CI 5.8–9.2), respectively; P<0.001] (Figure 2B).
Figure 2.
(A) Receiver operating characteristic (ROC) curves of the low risk and high risk groups; (B) Comparison of survival times between low risk and high risk groups.
Discussion
The main finding of our study is that TACE may be considered an effective therapy for select advanced-stage HCC patients, potentially outperforming reported outcomes in patients treated with sorafenib. Specifically, the multi-variate analysis identified Child-Pugh class, presence of PVTT, extrahepatic metastasis, tumor size, number of HCC nodules and alpha-fetoprotein value as strong predictors of therapeutic outcomes. This data gathered from a large cohort reflects a real-life clinical experience of a North-American tertiary care center which highlights the need to further improve the BCLC staging system primarily by further stratifying among therapeutic allocation for patients classified as BCLC C. The herein proposed risk score does just that by offering an improved allocation of those patients into the TACE treatment arm with an overall life expectancy which would exceed the current standard of sorafenib.
Although TACE is not recommended for patients with advanced-stage HCC in the BCLC staging system, two guidelines from Asia give different opinions. The consensus-based clinical practice guidelines proposed by the Japan Society of Hepatology and the treatment algorithms proposed by the Asia-Pacific Association for the Study of Liver argued that vascular invasion is not an absolute contraindication to TACE 18, 19. Xu, et al. compared different staging systems in a cohort with 647 patients and concluded that the BCLC staging system was limited in the prognosis of survival 20. The BCLC staging system was based on the prognostic analysis of several small cohorts with early-stage HCC and a cohort of 102 patients with untreated intermediate- or advanced-stage HCC 21, 22. Thus, both the applicability and accuracy of BCLC in allocating treatment strategy for intermediate- or advanced-stage HCC may be limited. Yau et al. established the Hong Kong Liver Cancer (HKLC) classification based on a large cohort of 3856 patients and showed that the HKLC treatment algorithm yielded better survival discrimination compared to the BCLC system. As a result of their well-designed and statistically very robust data analysis, they suggested that the survival benefit of TACE over systemic therapy was significant in BCLC-C patients who were classified as HKLC-III 22.
The results in the current study support the opinions in the HKLC system to some degree. First, as showed in the multivariate analysis, extrahepatic metastasis was the most important prognostic factor. Similarly in HKLC system, extrahepatic metastasis plays the most important role in prognosis of patients with Child-Pugh A/B class. Second, in the BCLC staging system, the cut-off point of ECOG is 1 and all patients with ≥1 are classified into stage C. However in our study, ECOG ≥1 was found to be not an independent prognostic factor, which is in agreement with the results of the HKLC system. As showed in table 2, even patients with ECOG 1 had less PVTT and metastasis compared with patients with ECOG 0. The PVTT was present in 74 (65.4%) patients with ECOG 0, 134 (36.8%) patients with ECOG 1 and 13 (41.9%) patients with ECOG 2, respectively. The metastasis was present in 30 (26.5%) patients with ECOG 0, 45 (12.4%) patients with ECOG 1 and 9 (29%) patients with ECOG 2, respectively. Moreover, Hsu C et al. proposed that patients with ECOG performance status 0 or 1 should be reassigned to BCLC stage B in order to enhance the prognostic ability of the BCLC system 23. Therefore we believe that patients with ECOG 1 should not lose the opportunity to get access to TACE therapy though they are clinically more symptomatic.
Moreover, the BCLC definition of advanced-stage is heterogeneous–consisting of patients with PVTT, ECOG ≥1 and/or extrahepatic metastasis 6. However, the only treatment recommendation for this category of patients is sorafenib which is known to have modest efficacy. In the Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) and Asia-Pacific study, the median OS in the sorafenib group was only 10.7 and 6.5 months, respectively, in spite of the fact that these studies had enrolled a small proportion of intermediate-stage HCC patients 5, 4. Reported OS in advanced-stage HCC patients after sorafenib treatment ranged from 5 to 10.7 months 8, 5. In our study, the median OS for the entire cohort reached a promising result of 11.9 months. Most importantly we found that the median OS of low risk advanced-stage patients could be as long as 24.1 months, which is a much better outcome than reported results with systemic therapy 5. Thus, this subgroup of patients may possibly benefit the most from TACE. On the contrary, the median OS of patients with high-risk score was only 7.5 months, which is similar as the reported OS in advanced-stage patients treated with sorafenib. We consider that TACE should not be performed to this subgroup of patients. Taken together, these results highlighted the importance of stratifying the BCLC stage C classification and remaking the treatment recommendations accordingly.
Prognostic factors and risk-groups play an essential role in the design, conduction and analysis of clinical trials. Our study identified six prognostic factors that affected the OS in patients with advanced-stage HCC after TACE treatment: Child-Pugh class, presence of PVTT, extrahepatic metastasis, tumor size, number of HCC nodules and alpha-fetoprotein value. Although alpha-fetoprotein is not included in the BCLC staging system, its importance has been highlighted in other studies 24. The Cancer of the Liver Italian Program (CLIP) staging system included alpha-fetoprotein value as an independent prognostic value 25. Our results suggested a 2-fold increased risk of death in patients with an alpha-fetoprotein baseline level ≥ 400ng/ml. A recent study based on 2938 patients also showed that the baseline alpha-fetoprotein value ≥ 400ng/ml was an independent risk factor of OS 20. These results strengthen the prognostic value of alpha-fetoprotein in advanced-stage patients treated using TACE. In addition, tumor-related factors are absent in the current advanced-stage BCLC classification. Our results demonstrated that the tumor burden in terms of tumor size and number play an important role in determining survival outcomes. These findings would also help the clinical community to pay attention to the tumor burden as the factor to balance the experimental group and control group in randomized controlled studies.
The strengths of our study were the large sample size and the consecutive enrollment of all advanced-stage HCC patients with PVTT, extrahepatic metastasis or ECOG ≥ 1 in the real clinical setting. However, this study has limitations. First, the selection bias may exist because of the retrospective nature and all the patients were enrolled from single center. Second, the lack of control arm prevents us from drawing a definite conclusion about the efficacy of TACE in advanced-stage HCC patients.
In conclusion, our study demonstrated that select patients with advanced-stage HCC may benefit from TACE treatment, providing a more comprehensive evidence to challenge the current definition and treatment recommendations in BCLC stage C. Moreover, Child-Pugh class, presence of PVTT, extrahepatic metastasis, tumor size, number of HCC nodules and alpha-fetoprotein value were found to be key indicators in the prognosis of advanced HCC patients after TACE treatment, and these indicators could be used as valuable factors for designing future randomized controlled studies in term of pretreatment stratification.
Acknowledgments
Funding: Our study was funded by NIH/NCI R01 CA160771, Philips Research North America, Cambridge, MA.
The authors have declared no conflicts of interest.
MingDe Lin is a Philips Employee; Jean-François Geschwind received a grant from Philips Healthcare.
Abbreviations
- HCC
hepatocellular carcinoma
- BCLC
Barcelona Clinic Liver Cancer
- TACE
transarterial chemoembolization
- OS
overall survival
- ECOG
Eastern Cooperative Oncology Group
- DEB
drug-eluting bead
- PVTT
portal vein tumor thrombosis
- ROC
Receiver Operating Characteristic
Footnotes
This work has been presented in Radiological Society of North America 2015, Chicago.
Authorship Statement:
Conceived and designed the study: Yan Zhao; Rafael Duran; Julius Chapiro; Collection and analysis of data: Yan Zhao, Jae Ho Sohn, Florian Fleckenstein, Li Zhao, Howard Lee; Manuscript writing: All authors; Critical revision of the manuscript: Rafael Duran, Julius Chapiro, Timothy M. Pawlik, Sonia Sahu, Rüdiger Schernthaner, Shuixiang He, MingDe Lin, Jean-François H. Geschwind.
All authors approved the final manuscript submitted.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer Journal international du cancer. 2010;127(12):2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Global battle against cancer won’t be won with treatment alone--effective prevention measures urgently needed to prevent cancer crisis. Central European journal of public health. 2014;22(1):23, 8. [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology (Baltimore, Md) 2005;42(5):1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 4.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. The Lancet Oncology. 2009;10(1):25–34. doi: 10.1016/s1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. The New England journal of medicine. 2008;359(4):378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Journal of hepatology. 2012;56(4):908–43. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver international: official journal of the International Association for the Study of the Liver. 2015;35(9):2155–66. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y, Wang WJ, Guan S, Li HL, Xu RC, Wu JB, et al. Sorafenib combined with transarterial chemoembolization for the treatment of advanced hepatocellular carcinoma: a large-scale multicenter study of 222 patients. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2013;24(7):1786–92. doi: 10.1093/annonc/mdt072. [DOI] [PubMed] [Google Scholar]
- 9.Lee HS, Kim JS, Choi IJ, Chung JW, Park JH, Kim CY. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer. 1997;79(11):2087–94. [PubMed] [Google Scholar]
- 10.Chung GE, Lee JH, Kim HY, Hwang SY, Kim JS, Chung JW, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258(2):627–34. doi: 10.1148/radiol.10101058. [DOI] [PubMed] [Google Scholar]
- 11.Pinter M, Hucke F, Graziadei I, Vogel W, Maieron A, Konigsberg R, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263(2):590–9. doi: 10.1148/radiol.12111550. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Cai G, Zhou L, Liu L, Qi X, Bai M, et al. Transarterial chemoembolization in hepatocellular carcinoma with vascular invasion or extrahepatic metastasis: A systematic review. Asia-Pacific journal of clinical oncology. 2013;9(4):357–64. doi: 10.1111/ajco.12081. [DOI] [PubMed] [Google Scholar]
- 13.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. Journal of hepatology. 2001;35(3):421–30. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 14.Loffroy R, Lin M, Yenokyan G, Rao PP, Bhagat N, Noordhoek N, et al. Intraprocedural C-arm dual-phase cone-beam CT: can it be used to predict short-term response to TACE with drug-eluting beads in patients with hepatocellular carcinoma? Radiology. 2013;266(2):636–48. doi: 10.1148/radiol.12112316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liapi E, Geschwind JF. Transcatheter arterial chemoembolization for liver cancer: is it time to distinguish conventional from drug-eluting chemoembolization? Cardiovascular and interventional radiology. 2011;34(1):37–49. doi: 10.1007/s00270-010-0012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iavarone M, Cabibbo G, Piscaglia F, Zavaglia C, Grieco A, Villa E, et al. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology (Baltimore, Md) 2011;54(6):2055–63. doi: 10.1002/hep.24644. [DOI] [PubMed] [Google Scholar]
- 17.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology (Baltimore, Md) 2001;33(2):464–70. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 18.Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, et al. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Digestive diseases (Basel, Switzerland) 2011;29(3):339–64. doi: 10.1159/000327577. [DOI] [PubMed] [Google Scholar]
- 19.Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatology international. 2010;4(2):439–74. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu L, Peng ZW, Chen MS, Shi M, Zhang YJ, Guo RP, et al. Prognostic nomogram for patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. Journal of hepatology. 2015;63(1):122–30. doi: 10.1016/j.jhep.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 21.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Seminars in liver disease. 1999;19(3):329–38. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 22.Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146(7):1691–700. e3. doi: 10.1053/j.gastro.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 23.Hsu CY, Lee YH, Hsia CY, Huang YH, Su CW, Lin HC, et al. Performance status in patients with hepatocellular carcinoma: determinants, prognostic impact, and ability to improve the Barcelona Clinic Liver Cancer system. Hepatology (Baltimore, Md) 2013;57(1):112–9. doi: 10.1002/hep.25950. [DOI] [PubMed] [Google Scholar]
- 24.Riaz A, Ryu RK, Kulik LM, Mulcahy MF, Lewandowski RJ, Minocha J, et al. Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: oncologic marker of radiologic response, progression, and survival. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(34):5734–42. doi: 10.1200/jco.2009.23.1282. [DOI] [PubMed] [Google Scholar]
- 25.Investigators TCotLIPC. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients. Hepatology (Baltimore, Md) 1998;28(3):751–5. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]