Abstract
Background
The pathogenesis of autoimmune hepatitis (AIH) is incompletely understood. Macrophage migration inhibitory factor (MIF) is an inflammatory cytokine implicated in the pathophysiology of multiple autoimmune diseases. We recently reported that MIF expression was increased in a US AIH cohort. MIF expression in non-Western AIH patients is unknown. A −173 GC single nucleotide polymorphism in the MIF promoter (rs755622) is clinically associated with steroid-resistance in several inflammatory disorders but has not been evaluated in AIH.
Aim
To compare MIF polymorphisms and their relationship to clinical parameters in AIH patients from the US and Japan.
Methods
DNA and matched sera from AIH patients and healthy controls from Japan (N=52) were compared to the US group. Serum concentrations of MIF and its circulating receptor CD74 were measured by ELISA. MIF −173 GC (rs755622) and −794 CATT5-8 (rs5844572) polymorphisms were analyzed by standard methods. MIF genotypes were correlated with serum ALT and steroid requirements.
Results
Serum MIF was increased in Japanese AIH patients vs. local controls, in agreement with the US AIH patients. In both groups, ALT was higher in CC/GC vs. GG patients. Further, the steroid requirement was higher in AIH patients with GC/CC genotype from both groups. In the Japanese patient group, the GC/CC genotype also was associated with acute symptomatic presentation.
Conclusions
The MIF −173 CC/GC genotypes may be associated with both higher ALT and maintenance steroid requirements in AIH patients from the US and Japan. This polymorphism could be a marker of disease severity in AIH patients.
Keywords: Hepatitis, Liver Disease, Autoimmune Hepatitis, Macrophage Migration Inhibitory Factor, MIF, Polymorphism
Introduction
Autoimmune hepatitis (AIH) is a chronic, relapsing disease of hepatocellular injury resulting from loss of immune tolerance [1]. Disease manifestation likely requires a combination of genetic predisposition [2] and idiopathic environmental triggers [3]. The nature of, and the relationship between, key autoimmune inflammatory pathways in AIH are not fully understood. In order to advance the management of AIH there is a need for immune-based biomarkers that can predict severity and disease severity.
Macrophage migration inhibitory factor (MIF) is a pro-inflammatory cytokine that critically modulates key innate and adaptive immune pathways [4]. Its unique properties, including T-cell induction and counter-regulation of endogenous glucocorticoid activity [5], have prompted investigations of its role in various inflammatory and autoimmune disorders [6]. MIF expression is associated with disease severity in several autoimmune diseases, including systemic lupus erythematosus [7], rheumatoid arthritis [8], systemic sclerosis [9], and inflammatory bowel disease [10,11]. Based on this, we hypothesized a role for MIF in autoimmune liver disease and recently reported the first study of MIF and its cellular receptor, CD74, in patients with AIH and primary biliary cholangitis (PBC) [12]. We demonstrated elevated MIF expression in the serum and liver in patients with AIH, while significant differences in the serum level of a soluble form of MIF’s receptor (CD74) were identified between AIH and PBC cohorts. Further, the genetic profile of functional polymorphisms in MIF distinguished subjects with AIH from those with PBC. This data suggested that MIF plays a specific and important role in the inflammatory cascade of AIH. However, the functional significance of MIF polymorphisms in AIH clinical activity has not yet been previously studied.
The promoter region of the MIF gene contains two distinct polymorphisms: 1) a functional −794 CATT5-8 repeat sequence in which higher repeat numbers result in increased MIF expression, and 2) a GC single nucleotide polymorphism (SNP) at position −17313. The −173C allele, which is in linkage disequilibrium with CATT , is associated with earlier disease onset and severity in diverse disorders including inflammatory polyarthritis, pediatric nephrotic syndrome, alopecia areata and inflammatory bowel disease [14-17]. Furthermore, the C allele has been shown to associate with steroid resistance in a range of diseases including colitis, juvenile arthritis, and nephrotic syndrome [18-20]. Therefore, the −173C allele in the MIF promoter is a candidate marker of steroid requirements and disease activity in autoimmune disease.
Based on this background, we hypothesized that MIF polymorphisms could be associated with potentially meaningful clinical parameters in AIH patients. To test this hypothesis we studied genotype-clinical correlations in two genetically distinct AIH patient groups, namely from Japan and also our previously reported cohort of AIH patients from the United States (US) [12].
Patients and Methods
Patient Populations
Two AIH patient groups from academic liver clinics were evaluated: one from Japan (Jikei University, Tokyo) and one the US (Yale University, New Haven). The US cohort, and respective healthy control group, were largely comprised of patients whose serum MIF values and genotype frequencies were reported in a separate publication [12]. Each group was comprised of patients diagnosed with type I AIH according to the International Autoimmune Hepatitis Group Score [21] and were followed by hepatologists at each center. Current demographic and clinical data were obtained from the medical record. In addition, laboratory data from the time of initial disease presentation were available in most cases. Control serum and isolated peripheral blood DNA were obtained from self-reported healthy persons from each country. The study was reviewed and approved by the institutional review boards from each center.
Serum measurements
Serum samples were collected at each center and stored at − 80°C. The Japanese samples then were shipped under strict dry ice conditions to the Yale Liver Center for testing and analysis. The serum protein concentrations of MIF and circulating CD74 were determined by sandwich ELISA at the Yale Liver Center, as previously described [12].
Genetic polymorphism analysis
Genomic DNA was extracted for each patient and analyzed at the Yale Liver Center. The MIF −794 CATT5-8 microsatellite repeat sequence (rs5844572) was determined by a fluorescence-based fragment analysis as previously described [13]. The −173 GC SNP sequence (rs755622), considered to be in linkage disequilibrium with the CATT microsatellite repeat sequence, was analyzed by pyrosequencing as previously described [13].
Statistical analysis
Calculators for Student’s t-test for normally distributed continuous variables, Fisher’s Exact Test for categorical data, and Pearson correlation analyses, were performed using Prism (GraphPad Software, La Jolla, CA) Version 6.0d.
Results
The demographic data for the Japanese AIH patients are presented in Table 1. There was no significant difference between this group and respective controls regarding age, sex, or ethnicity in comparison to the previously reported US cohort [12]. The AIH patients were predominantly female and had a mean age of 43-49 years at the time of diagnosis. Patients in both groups met criteria for definite AIH according to the International Autoimmune Hepatitis Group score [21].
Table 1.
Demographic data of the AIH cohort from Japan. The US AIH cohort demographic data was previously reported*.
| Japan | ||
|---|---|---|
| AIH | Control | |
| Patients | 52 | 30 |
| Mean age ± SD, years |
49±14 | 43.3±11 |
| Female, % | 89% | 80% |
| Mean IAIHG** Score ± SD |
16±3 | NA |
| ALT at diagnosis ± SD, U/L |
807.6±651.8 | NA |
| Bilirubin at diagnosis, ± SD, mg/dL |
2.9±2.9 | NA |
[12]
International Autoimmune Hepatitis Group Score.
Mean serum MIF concentrations were elevated in Japanese AIH patients compared to the Japanese healthy control group (Figure 1). Importantly, this agrees with our recent publication showing that MIF values were higher in the US AIH cohort vs. healthy controls (13.1±1.4 vs. 5.7±0.5 ng/mL, p<0.0001) [12]. There was no significant correlation between pre-treatment ALT, or bilirubin, and on-treatment MIF serum levels in the Japanese or the US cohorts (p=NS). However, there was a trend toward a correlation between serum MIF and on-treatment serum ALT levels in the US group (r 0.26, 95% Confidence Interval (CI) −0.03-0.52, p=0.08).
Figure 1. Serum MIF is elevated in Japanese AIH patients compared to Japanese controls.
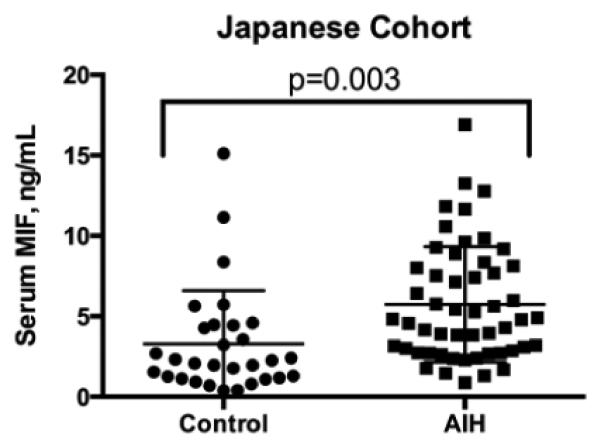
The mean serum MIF concentration was 3.3±0.6 (Control) vs. 5.7±0.5 ng/mL (AIH), p=0.003.
We recently reported the discovery that a truncated form of the MIF transmembrane receptor, CD74, is detectable in the circulation [12]. Interestingly, circulating CD74 can neutralize MIF bioactivity in vitro, suggesting a role for this soluble receptor in the modulation of MIF cytokine activity in vivo. We therefore measured circulating CD74 levels (Figure 2) in the Japanese AIH cohort and compared it to our previous finding in the US cohort. We found that, unlike the US group where the mean CD74 serum concentration in the US AIH group was higher than controls, in the Japanese AIH and control groups serum CD74 did not differ (p=NS). The reason for the differential concentrations of the MIF-neutralizing soluble receptor CD74 in the two control groups is unclear, and a genetically-driven difference in CD74 expression between US and Japanese patients cannot be ruled out.
Figure 2. Serum CD74 in AIH from Japan.
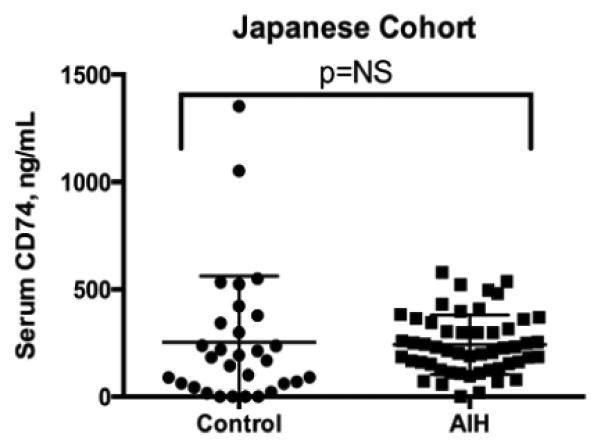
The mean serum CD74 concentration was 253.5±56.4 (Control) vs.242.2±19.2 ng/mL (AIH), p=NS.
We next analyzed the MIF genetic polymorphisms, −794 CATT5-8 and −173 GC SNP in the Japanese AIH and in a modified US AIH group from the previously published study (Table 2). There was no difference in the frequency distributions of MIF polymorphisms between the two AIH groups or between the Japanese patients vs. controls with regard to −794 CATT, and also with regard to the distribution of the −173C allele (Fisher ’s exact test), (p=NS). However, genetic-clinical analysis revealed that the subgroup of US AIH patients with at least one −173C allele (CC or GC) had a mean on-treatment serum ALT significantly higher than the subgroup with without −173C (GG), (Figure 3A). In the US AIH group, the non-GG genotype conferred an Odds Ratio (OR) of having on-treatment ALT >30 U/L of 5.2 (CI 1.5-18.5, p=0.014). Furthermore, we found a similar genetic-clinical correlation in the Japanese AIH patients, with a higher pre-treatment ALT in the −173 CC/GC subgroup compared to the −173 GG subgroup (Figure 3B). Serum ALT and the C allele also were correlated in these patients (r 0.35, CI 0.074-0.58, p=0.015). In addition, in the Japanese AIH group, a non-GG genotype conferred an OR of having pre-treatment ALT > 1000 U/L of 8.9 (CI 1.8-44.5, p=0.008).
Table 2.
MIF Polymorphism frequencies for the −173 G/C SNP and −794 CATT5-8 genotypes among Japanese AIH patients and controls, and of a modified US AIH cohort analyzed for ALT and steroid correlations. There were no significant differences in polymorphism frequencies between AIH all with regard to −794 CATT data, or between Japanese AIH patients vs. Controls with regard to GG vs. non-GG genotypes, p=NS).
| US | Japan | ||
|---|---|---|---|
| AIH (N=53) | Control (N=30) | AIH (N=52) | |
| −173 SNP % | |||
| GG | 69.8 | 60 | 69.2 |
| GC | 28.3 | 40 | 26.9 |
| CC | 1.9 | 0 | 3.9 |
| −794 CATT5-8 % | |||
| 55 | 13.2 | 20 | 13.5 |
| 56 | 24.5 | 30 | 34.6 |
| 57 | 1.9 | 13.3 | 11.5 |
| 58 | 1.9 | 0 | 0 |
| 66 | 32 | 26.7 | 26.9 |
| 67 | 13.2 | 10 | 11.5 |
| 68 | 1.9 | 0 | 0 |
| 77 | 9.5 | 0 | 1.9 |
| 78 | 1.9 | 0 | 0 |
| 88 | 0 | 0 | 0 |
Figure 3. Mean serum ALT is increased in non-GG MIF patients with AIH from the US and Japan.
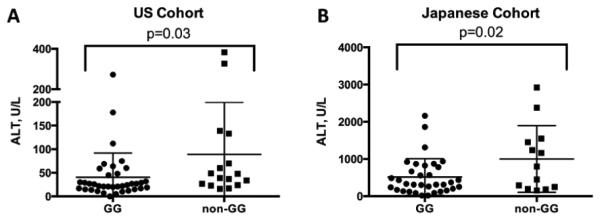
Panel A: In the US Cohort, the mean on-treatment serum ALT level was 40.6±8.4 (GG) vs. 89.1±27.6 (non-GG) U/L, p=0.03. Panel B: In the Japanese Cohort, the mean pre-treatment serum ALT level was 514.3±493.9 vs. 1000.5±895.7, p=0.02.
Chronic AIH generally responds to immunosuppression, and indeed treatment responsiveness is a component of the diagnostic criteria [21]. Steroids are a cornerstone of AIH therapy [1], however optimizing the steroid dose is challenging due to the risk of relapse with under-treatment and significant adverse effects with over-treatment [3]. Thus, a biomarker that is able to predict steroid responsiveness in AIH will have a clinical impact. Given the reported association between the MIF −173C polymorphism and steroid responsiveness in other disorders [17-19], we analyzed this polymorphism in relationship to prednisone requirements for the US and Japanese AIH cohorts. As with the serum ALT concentrations, prednisone requirements were increased in the AIH subgroup with a CC or GC genotype in both the US and in Japan (Figure 4A and 4B). Furthermore, in the US AIH group the prednisone dose positively correlated with the number of C allele copies (r 0.32, CI 0.052-0.54, p=0.02). This finding suggests that, along with increased serum ALT, the MIF −173C polymorphism in AIH patients may be a biomarker of increased disease activity including steroid requirements. In addition, the effect was of equivalent magnitude in two very different geographic settings.
Figure 4. Mean prednisone dose requirement is increased in non-GG MIF patients with AIH patients from the US and Japan.
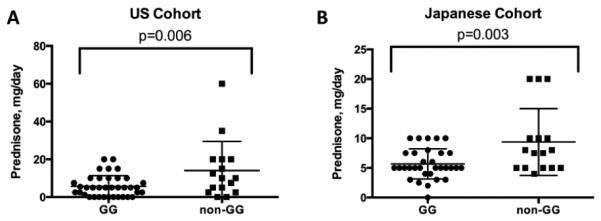
Panel A: In the US Cohort, the mean prednisone dose was 5.7±0.9 (GG) vs. 14.1±3.9 (non-GG) mg/day, p=0.006. Panel B: In the Japanese Cohort, the mean prednisone dose was 5.7±2.5 (GG) vs. 9.4±5.6 (non-GG) mg/day, p=0.003.
Clinical features at initial disease presentation were analyzed for each AIH patient group. In the Japanese AIH group, 56.3% (9 of 16) of the −173 CC/GC patients presented with acute, symptomatic hepatitis, while only 22.2% (8 of 36) of the GG patients presented with acute symptoms (p=0.03). There was no relationship between the −173 MIF genotype and acute symptomatic hepatitis presentation in the US AIH group. However, the mean serum bilirubin level at initial disease presentation was higher in US AIH patients compared to the Japanese AIH cohort (5.5±7.5 vs. 2.3±2.7 mg/dL, p=0.01). There was no relationship between MIF levels or genotypes and ANA or smooth muscle antibody titers in the AIH groups. Finally, there was no relationship between the MIF −794 CATT5-8 alleles and clinical disease in the US or Japanese AIH groups.
Discussion
In this novel study to address a genetic-clinical susceptibility across two ethnically distinct populations with AIH, we report that AIH patients from both the US and Japan who have the MIF promoter −173 C-containing genotypes had increased serum ALT and higher maintenance steroid requirements compared to patients without the C allele. Further, the clinical presentation of the Japanese AIH group with the −173 CC/GC genotypes was more acute with symptomatic hepatitis at the time of diagnosis. In light of our recent study of MIF in AIH [12], these novel findings further support a role of MIF in AIH and suggest that the MIF −173 SNP polymorphism may be a biomarker for disease severity across Western and Japanese AIH patient populations.
MIF is expressed centrally in the neuroendocrine system and also peripherally in monocytes/macrophages, T cells, epithelial and endothelial cells [4]. It is a constitutively expressed cytokine that can also be rapidly secreted in response to stress through release from stored reservoirs. The pro-inflammatory, cytokine action of MIF in autoimmunity has been investigated for over a decade6. Specific MIF physiologic pathways include critical interactions with key components of the innate immune response to pathogens such as TLR-4 up-regulation and TNF-α production by macrophages [22]. In addition, MIF secretion by T cells in response to antigenic or IFN-γ stimulation [23] and its promotional role for the induction of IL-12 synthesis demonstrates an important role in Th1 adaptive immune pathways [24].
MIF is now known to play an important role in a number of autoimmune disorders through both animal and human studies including systemic lupus erythematosus [7,25], rheumatoid arthritis [8,26,27], inflammatory bowel disease [15,16], and nephrotic syndrome [15,20]. Furthermore, a relationship between the MIF −173C polymorphism and disease severity has been detected across various autoimmune disorders. Radstake et al. [27] demonstrated in a patient cohort with rheumatoid arthritis that the −173C allele correlated with advanced radiologic joint damage. In addition, Zhang et al. [17] recently conducted a meta-analysis of over 4,000 patients with inflammatory bowel disease and concluded that the C allele was a disease risk factor (OR 1.25).
Our findings are in agreement with the emerging, multi-disciplinary literature of MIF immunogenetics, specifically suggesting that the MIF −173C polymorphism may be an immune-based biomarker of AIH disease severity. There is a continued need for evidence-based tools to guide the management and prognostication of patients with AIH. This is illustrated by the recent finding that over 70% of AIH patients relapse within 2 years of immunosuppression discontinuation despite initially achieving remission [28]. There is also a need for predictors of steroid responsiveness to help guide AIH therapy and avoid unnecessary over-treatment, particularly given the negative effects of steroids on metabolic and psychological health. Our data suggesting that the MIF −173C polymorphism may be associated with an elevated liver inflammatory marker, ALT, and steroid resistance, provides the rationale for further evaluation of this polymorphism as a candidate biomarker in AIH management strategies.
Our recent publication compared MIF polymorphisms in AIH vs. PBC, finding increased frequency of the high-expression −794 CATT7 allele in AIH vs. PBC and a borderline increased frequency of the −794 CATT77 genotype in AIH vs. Controls [12]. Further, we reported an increased serum level of MIF in AIH vs. controls, a finding also found here in the Japanese group. In contrast to our previous findings in the US group, the CATT7 frequency was lower in the Japanese patients and controls. The reason for this difference between US and Japanese groups is likely due to the well-known under-representation of CATT7 allele in the Japanese population [16]. Since CATT7 is a high-expression MIF variant, the lower frequency in Japan is likely the cause for the lower MIF concentrations observed in Japanese patients and controls (Figure 1) compared the previously described US cohort. Further, it is know that the frequency of AIH in Japan is lower than in the US, primarily a function of the decreased frequency of HLA-DR3 in that population [29].
There are limitations to this study, most notably the sample size of our populations, which are limited by the low prevalence of AIH in both the US and Japanese populations. However, the strength of this genotypic-clinical analysis includes the concordant results observed in two genetically diverse populations with regard to MIF polymorphism and relevant clinical findings in AIH. Future studies should be performed to validate these findings utilizing larger control groups, other liver disease control groups, AIH patients from other geographic areas, and in comparison to other associated genetic variants in AIH. Furthermore, it will be important to establish the functional consequence of the C allele for MIF secretion in the context of AIH itself. While there is literature demonstrating increased MIF section in the context of C allele containing haplotype as compared to the G allele [30], this has not yet been confirmed in hepatic tissues. The frequency of CC homozygotes was low in our populations, and while this is expected given the known frequency, adequate comparisons with homozygotes were not possible in this study.
A large GWAS study of AIH patients from Europe recently showed that the HLA-DRB1*0301 variant was most strongly associated with AIH type I with an OR of 2.9 [2]. A subsequent study of this cohort showed that the presence of HLA-DR3 correlated with increased IAIHG score at diagnosis and IgG levels, although steroid dosing was not assessed and ALT levels were not significantly different [31]. This illustrates the need for, and potential clinical application of, the MIF −173C polymorphism as a biomarker for use in steroid modulating algorithms. Further, the −173 GC SNP sequence (rs755622) reported in our study is not included in the GWAS immunochip used in that study (Illumina CytoSNP 12.0 platform, San Diego CA). As humanized anti-MIF pharmacotherapy advances into early clinical testing, its application to AIH management may benefit from using the −173 GC genotype to identify individuals with a MIF-dependent form of the disease. Before this, however, there is a need for larger, longitudinal studies to evaluate the −173C allele in AIH. It is also necessary to better understand the mechanisms underlying the observed relationship between MIF and AIH to determine to what degree MIF expression in AIH is disease-specific.
In summary, in our present study a MIF genotype containing the MIF −173C polymorphism was associated with clinically-meaningful parameters of AIH including increased serum ALT concentrations and prednisone dose requirements, a finding noted in patients from the US and Japan. This finding is also consistent with the literature on genetic-clinical correlations of MIF promoter polymorphisms in other autoimmune diseases. Our results suggest but do not yet confirm a potential relationship between the pro-inflammatory cytokine MIF and AIH, and the additional possibility that MIF polymorphisms could serve as markers of disease severity or progression. This remains to be confirmed and further studied through investigation in other cohorts and through longitudinal studies in AIH.
Acknowledgments
Funding
This work was supported by the Yale Center for Clinical Investigation Junior Faculty Development Award, the Yale Rheumatic Diseases Core Center Pilot/Feasibility Grant Assis), the NIH K08 Award DK099412 (D.N. Assis) the NIH RO1 AR049610 (R. Bucala), and the Yale Liver Center (DK P30-34989).
Footnotes
Disclosures:
Dr. Bucala is a co-inventor on patent applications describing the utility of MIF genotype determination. The authors have no additional financial or personal conflicts of interests.
References
- 1.Heneghan MA, Yeoman AD, Verma S, Smith AD, Longhi MS. Autoimmune hepatitis. Lancet. 2013;382:1433–44. doi: 10.1016/S0140-6736(12)62163-1. [DOI] [PubMed] [Google Scholar]
- 2.de Boer YS, van Gerven NM, Zwiers A, et al. Genome-wide association study identifies variants associated with autoimmune hepatitis type 1. Gastroenterology. 2014;147:443–52. doi: 10.1053/j.gastro.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 3.Czaja AJ, Manns MP. Advances in the diagnosis, pathogenesis, and management of autoimmune hepatitis. Gastroenterology. 2010;139:58–72. doi: 10.1053/j.gastro.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 4.Calandra T, Bernhagen J, Metz CN, et al. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- 5.Calandra T, Bucala R. Macrophage migration inhibitory factor (MIF): a glucocorticoid counter-regulator within the immune system. Crit Rev Immunol. 1997;17:77–88. doi: 10.1615/critrevimmunol.v17.i1.30. [DOI] [PubMed] [Google Scholar]
- 6.Bucala R. MIF, MIF alleles, and prospects for therapeutic intervention in autoimmunity. J Clin Immunol. 2013;33(Suppl1):S72–8. doi: 10.1007/s10875-012-9781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sreih A, Ezzeddiine R, Leng L, et al. Dual effect of the macrophage migration inhibitory factor gene on the development and severity of human systemic lupus erythematosus. Arthritis Rheum. 2011;63:3942–51. doi: 10.1002/art.30624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llamas-Covarrubias MA, Valle Y, Bucala R, et al. Macrophage migration inhibitor factor (MIF): genetic evidence for participation in early onset and early stage rheumatoid arthritis. Cytokine. 2013;61:759–65. doi: 10.1016/j.cyto.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu SP, Leng L, Feng Z, et al. Macrophage migration inhibitory factor promoter polymorphisms and the clinical expression of scleroderma. Arthritis Rheum. 2006;54:3661–9. doi: 10.1002/art.22179. [DOI] [PubMed] [Google Scholar]
- 10.Murakami H, Akbar SM, Matsui H, Onji M. Macrophage migration inhibitory factor in the sera and at the colonic mucosa in patients with ulcerative colitis: clinical implications and pathogenic significance. Eur J Clin Invest. 2001;31:337–43. doi: 10.1046/j.1365-2362.2001.00796.x. [DOI] [PubMed] [Google Scholar]
- 11.Nishihira J. Molecular function of macrophage migration inhibitory factor and a novel therapy for inflammatory bowel disease. Ann N Y Acad Sci. 2012;1271:53–7. doi: 10.1111/j.1749-6632.2012.06735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assis DN, Leng L, Du X, et al. The role of macrophage migration inhibitory factor in autoimmune liver disease. Hepatology. 2014;59:580–91. doi: 10.1002/hep.26664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong XB, Leng L, Beitin A, et al. Simultaneous detection of microsatellite repeats and SNPs in the macrophage migration inhibitory factor (MIF) gene by thin-film biosensor chips and application to rural field studies. Nucleic Acids Res. 2005;33:e121. doi: 10.1093/nar/gni123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barton A, Lamb R, Symmons D, et al. Macrophage migration inhibitory factor (MIF) gene polymorphism is associated with susceptibility to but not severity of inflammatory polyarthritis. Genes Immun. 2003;4:487–91. doi: 10.1038/sj.gene.6364014. [DOI] [PubMed] [Google Scholar]
- 15.Berdeli A, Mir S, Ozkayin N, Serdaroglu E, Tabel Y, Cura A. Association of macrophage migration inhibitory factor −173C allele polymorphism with steroid resistance in children with nephrotic syndrome. Pediatr Nephrol. 2005;20:1566–71. doi: 10.1007/s00467-005-1930-9. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu T, Hizawa N, Honda A, et al. Promoter region polymorphism of macrophage migration inhibitory factor is strong risk factor for young onset of extensive alopecia areata. Genes Immun. 2005;6:285–89. doi: 10.1038/sj.gene.6364191. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Ma L, Dong LQ, Shu C, Xu JL. Association of the macrophage migration inhibitory factor gene—173G/C polymorphism with inflammatory bowel disease: a meta-analysis of 4296 subjects. Gene. 2013;526:228–31. doi: 10.1016/j.gene.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Nohara H, Okayama N, Inoue N, et al. Association of the −173G/C polymorphism of the macrophage migration inhibitory factor gene with ulcerative colitis. J Gastroenterol. 2004;39:242–6. doi: 10.1007/s00535-003-1284-7. [DOI] [PubMed] [Google Scholar]
- 19.Vivarelli M, D’Urbano LE, Insalaco A, et al. Macrophage migration inhibitory factor (MIF) and oligoarticular juvenile idiopathic arthritis (o-JIA): association of MIF promoter polymorphisms with response to intra-articular glucocorticoids. Clin Exp Rheumatol. 2007;25:775–81. [PubMed] [Google Scholar]
- 20.Vivarelli M, D’Urbano LE, Stringini G, et al. Association of the macrophage migration inhibitory factor −173*C allele with childhood nephrotic syndrome. Pedatr Nephrol. 2008;23:743–8. doi: 10.1007/s00467-007-0729-2. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez F, Berg PA, Bianchi FB, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–38. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 22.Cvetkovic I, Stosic-Grujicic S. Neutralization of macrophage migration inhibitory factor – novel approach for the treatment of immunoinflammatory disorders. Immunopharmacol. 2006:1527–34. doi: 10.1016/j.intimp.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Bacher M, Metz CN, Calandra T, et al. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci U S A. 1996;93:7849–54. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stosic-Gruficic S, Stojanovic I, Nicoletti F. MIF in autoimmunity and novel therapeutic approaches. Autoimm Rev. 2009;8:244–9. doi: 10.1016/j.autrev.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 25.Foote A, Briganti EM, Kipen Y, Santos L, Leech M, Morand EF. Macrophage migration inhibitory factor in systemic lupus erythematosus. J Rheumatol. 2004;31:268–73. [PubMed] [Google Scholar]
- 26.Kim HR, Park MK, Cho ML, et al. Macrophage migration inhibitory factor upregulates angiogenic factors and correlates with clinical measures in rheumatoid arthritis. J Rheumatol. 2007;34:927–36. [PubMed] [Google Scholar]
- 27.Radstake TR, Sweep FC, Welsing P, et al. Correlation of rheumatoid arthritis severity with the genetic functional variants and circulating levels of macrophage migration inhibitory factor. Arthritis Rheum. 2005;52:3020–9. doi: 10.1002/art.21285. [DOI] [PubMed] [Google Scholar]
- 28.van Gerven NM, Verwer BJ, Witte BI, et al. Relapse is almost universal after withdrawal of immunosuppressive medication in patients with autoimmune hepatitis in remission. J Hepatol. 2013;58:141–7. doi: 10.1016/j.jhep.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Zeniya M, Takahashi H. Characteristics of autoimmune hepatitis in the Asia-Pacific Region: historical review. Hepatol Int. 2012;6:342–9. doi: 10.1007/s12072-011-9313-8. [DOI] [PubMed] [Google Scholar]
- 30.Wang FF, Hugna XF, Shen N, et al. A genetic role for macrophage migration inhibitory factor (MIF) in adult-onset Still’s disease. Arthritis Res Ther. 2013;15:R65. doi: 10.1186/ar4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Gerven NM, de Boer YS, Zwiers A, et al. HLA-DRB1*03:01 and HLA-DRB1*04:01 modify the presentation and outcome in autoimmune hepatitis type-1. Genes Immun. 2015;16:247–52. doi: 10.1038/gene.2014.82. [DOI] [PubMed] [Google Scholar]


