Abstract
Importance
A 2009 randomized clinical trial (RCT) demonstrated that using CA-125 tests for routine surveillance in ovarian cancer increases chemotherapy use and decreases patients’ quality of life without improving survival, compared with clinical observation. The Society of Gynecologic Oncology guidelines categorize CA-125 testing as “optional” and discourage the use of radiographic imaging for routine surveillance. To date, few studies have examined their use in clinical practice.
Objective
To examine the use of CA-125 tests and CT scans in clinical practice before and after the 2009 RCT and estimate the economic impact of surveillance testing.
Design
Prospective cohort of women diagnosed with ovarian cancer between 2004-2011 and followed through 2012.
Setting
Six National Cancer Institute-Designated Cancer Centers.
Participants
1,241 women with ovarian cancer in clinical remission after completion of primary cytoreductive surgery and chemotherapy.
Main Outcome Measures
Use of CA-125 tests and CT scans before and after 2009 (n=1,241). Secondary outcomes included: the time from CA-125 doubling to retreatment among women who experienced a rise in CA-125 (n=511) before and after 2009, and the costs associated with surveillance testing using 2016 Medicare reimbursement rates.
Results
Use of CA-125 testing and CT scans was very similar over the study period. During 12 months of surveillance, the cumulative incidence of 3 or more CA-125 tests was 86% in 2004-2009 versus 91% in 2010-2012 (P=.95), and the cumulative incidence of more than 1 CT scan was 81% (2004-2009) versus 78% (2010-2012) (P=.50). Among women who experienced a CA-125 doubling (n=511), there was no significant difference in the time to retreatment with chemotherapy before and after 2009 (median: 2.8 months vs. 3.5 months, P=.40). Over a 12-month period, there were a mean of 4.6 CA-125 tests and 1.7 CT scans per patient, resulting in a United States population surveillance cost estimate of $1,999,029/year for CA-125 tests alone and $16,194,647/year with CT scans added.
Conclusion
CA-125 tests and CT scans are routinely employed for surveillance testing in patients with ovarian cancer, although their benefit has not been proven and their use may have significant quality of life and cost implications.
Keywords: ovarian cancer, quality of care, value-based cancer care, surveillance, CA125, CT scan, health care delivery
Introduction
The cost of cancer care is rising rapidly,1 prompting physicians and payors to identify “low-value” practices that increase costs without improving outcomes.2 In 2012 the American Board of Internal Medicine’s “Choosing Wisely” campaign challenged specialists to identify tests and treatments that are overused without providing meaningful clinical benefit. In response, the American Society of Clinical Oncology (ASCO) and Society for Gynecologic Oncology (SGO) recommended stopping surveillance testing in some asymptomatic patients.3
In the 2009 ASCO Annual meeting plenary session, Rustin et al, presented results from a clinical trial that randomized ovarian cancer patients in clinical remission to active surveillance with CA-125 testing or monitoring for symptoms of recurrent disease.4,5 There was no difference in survival between the groups, but women followed with CA-125 tests received more chemotherapy and had worse quality of life. The authors concluded that early detection and treatment of recurrent ovarian cancer did not improve clinical outcomes and recommended stopping surveillance testing.
Despite this, the 2015 National Comprehensive Cancer Center Network’s (NCCN) clinical guidelines recommend routine CA-125 testing for patients whose CA-125 levels were elevated at diagnosis and CT scans “if clinically indicated.”6 SGO guidelines categorize CA-125 testing as “optional,” and advise against routine radiographic surveillance.7 More recently, as part of the “Choosing Wisely” campaign, SGO explicitly recommended avoidance of routine imaging for ovarian cancer surveillance.3 To date, surveillance testing remains controversial and it is unclear how frequently is performed in patients with ovarian cancer.
The aim of this study was to examine the use of CA-125 surveillance testing at six National Cancer Institute-Designated Cancer Centers over time, and to determine whether the rates decreased after the Rustin data were presented in 2009. We hypothesized that CA-125 testing rates would not drop, but that the time to retreatment would increase, as providers delayed treatment to minimize reductions in patients’ quality of life while monitoring them closely for disease complications. We also examined the utilization of CT scans as few multi-institutional studies have described this previously. Finally, we sought to explore the economic impact of current ovarian cancer surveillance practices.
Materials and Methods
Data source
The NCCN Ovarian Cancer Outcomes Database contains data collected prospectively on all patients diagnosed with ovarian, fallopian or primary peritoneal cancers, and treated at six institutions between January 2004 and December 2011, including: City of Hope Comprehensive Cancer Center, Dana-Farber/Brigham & Women’s Cancer Center, Fox Chase Cancer Center, the Ohio State University Comprehensive Cancer Center, the University of Texas MD Anderson Cancer Center and the University of Michigan Comprehensive Cancer Center. Patients who received all or part of their treatment at these centers were included; those seen for a single consultation were excluded. Medical record abstraction was performed to obtain patient characteristics (e.g. demographics, comorbidities, performance status), tumor characteristics (histology, grade, stage), treatments (e.g., surgical procedures, chemotherapy), surveillance studies (CA-125 and CT scans), and vital status. Data on race/ethnicity were collected at initial presentation to the NCCN institution and analyzed because of documented disparities in the quality of ovarian cancer care by race.8 Additional audits were undertaken at all six institutions prior to analysis for any patients with missing data on key variables (CA-125 values, CT scans) to further verify the accuracy of the database. The Institutional Review Boards (IRB) at each center approved the overall project, or deemed it exempt from review.
Study Cohorts
We identified two cohorts of patients. In Cohort 1, we examined the cumulative incidence of CA-125 testing and CT scans for all patients (n=1,241) who presented with newly diagnosed epithelial ovarian cancer to an NCCN center between January 1, 2004 and December 31, 2011, underwent cytoreductive surgery and chemotherapy, and met the Rustin study criteria for remission defined as: (1) normal CA-125 concentration, and (2) no additional chemotherapy or hormonal treatments within 4 months after completion of chemotherapy. We excluded patients enrolled in clinical trials (n=272) because their surveillance testing would be dictated by the trial protocol. In Cohort 2, which included a subset of patients from Cohort 1, we examined the time to retreatment with chemotherapy among 511 patients whose CA-125 doubled relative to the post-treatment nadir during the surveillance period. Patients were excluded from Cohort 2 if they initiated secondary therapy prior to a doubling of the CA-125 (suggesting clinical signs of recurrence; n=60) or had radiographic evidence of recurrence prior to the doubling of the CA-125 (n=31). All patients were divided into two groups: (1) “pre-Rustin” diagnosed between 2004-2009, and (2) “post-Rustin” diagnosed between 2010-2011. Data were collected on all patients through December 31, 2012.
Outcomes
The primary outcome was the use of CA-125 testing and CT scans in the first year after remission (Cohort 1). We also examined patient factors associated with testing. Secondary outcomes included: (1) time to retreatment with chemotherapy or radiation after a documented CA-125 doubling (Cohort 2), and (2) the costs associated with surveillance testing using 2016 Medicare reimbursement rates.
Independent variables
The primary independent variable of interest was era of diagnosis (pre-Rustin: 2004-2009 vs. post-Rustin: 2010-2011). Additional covariates included: age, race, Hispanic ethnicity, GOG performance status, Charlson comorbidity score,9 stage, grade, histology, CA-125 at diagnosis, and institution.
Statistical Analyses
Post-treatment CA-125 tests and CT scans were quantified using the cumulative incidence of patients with 1, 2, and 3+ surveillance tests plotted over time for patients diagnosed in the pre- and post-Rustin periods (Cohort 1). Patients were categorized as “in remission” after documentation of the first normal CA-125 value within 4 months of completion of primary chemotherapy. Patients were censored at the earlier of: 1) initiation of second-line therapy, 2) CA-125 doubling, 3) recurrence, or 4) death or loss to follow-up. The time to retreatment after a CA-125 doubling was also quantified using the cumulative incidence of retreatment plotted over time (Cohort 2).
Unadjusted associations between patient and disease characteristics and the time to post-treatment surveillance and retreatment were assessed using the log rank test. Multivariable associations were assessed with a Cox proportional hazards model including all covariates, except for GOG performance status and ethnicity due to collinearity with the institution variable. Due to limited variability in post-treatment surveillance testing with CA-125 tests (95% of patients were tested within 6 months), multivariable associations were not assessed for that outcome.
We performed several sensitivity analyses. First, we examined the cumulative incidence of CA-125 testing, use of CT scans, and time to re-treatment before and after the publication of the Rustin et al. study in The Lancet (October 2, 2010), instead of upon presentation of the data (June 1, 2009). Second, we examined whether individual institutions responded differently to the Rustin trial by plotting the cumulative incidence curves within institutions by era of diagnosis. While no substantive difference by era of diagnosis were observed within institutions for surveillance (data not shown), we observed two general practice patterns for time to retreatment. Specifically, there was no evidence of a practice change after 2009 for four institutions, but there was evidence of a practice change after 2009 for two institutions. We then combined institutions with similar empirical practice patterns and performed an interaction analysis to test formally for a change in time to retreatment in one group but not the other.
Costs of routine surveillance tests were estimated from 2016 Medicare reimbursement rates using CPT codes: CA-125 test (86304)=$28.35, CT chest with contrast (71260) and CT abdomen and pelvis with contrast (74177)=$544.75.10 To calculate the cost of surveillance testing over a 12-month period, the mean and median number of tests conducted were calculated and 2016 Medicare reimbursement rates applied. A population estimate of women entering surveillance testing annually was calculated: 21,290 women were diagnosed with ovarian cancer in 2015 of which approximately 90% were epithelial ovarian cancers and 80% of whom should achieve a clinical remission, resulting in approximately 15,329 women entering surveillance.11
A two-sided p value of less than 0.05 was considered to indicate statistical significance. Statistical analysis was performed using Stata version 13.1 (StataCorp).
Results
Use of CA-125 and CT scans
There were 2,861 patients diagnosed with ovarian cancer at NCCN institutions between 2004-2011. Among these, 2,082 women underwent cytoreductive surgery and chemotherapy, excluding clinical trial participants, and 1,241 (pre-Rustin n=905, post-Rustin n=336) women met criteria for clinical remission (Figure 1).
Figure 1.
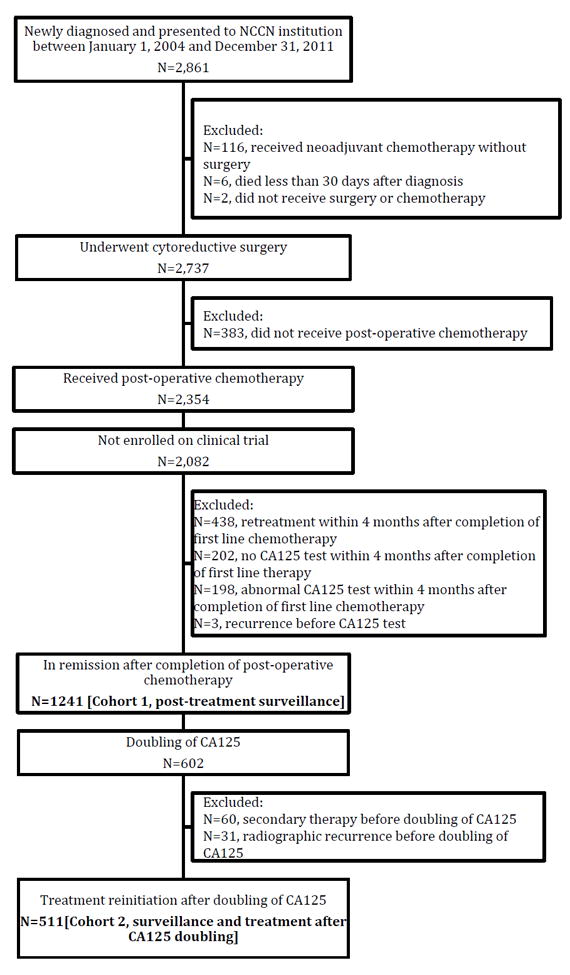
Flow diagram of inclusion and exclusion criteria
Figure 2 shows the cumulative incidence curves of CA-125 testing and CT scans before and after the Rustin study presentation. There was no significant difference in surveillance testing in the pre- vs. post-Rustin periods; 95% vs. 96% of patients received ≥1 CA-125 test within 6 months of remission, and 86% vs. 91% received ≥3 within 12 months. Similarly, 68% vs. 64% of patients received ≥1 CT scan within 6 months, while 30% vs. 29% received ≥3 CT scans within 12 months.
Figure 2.
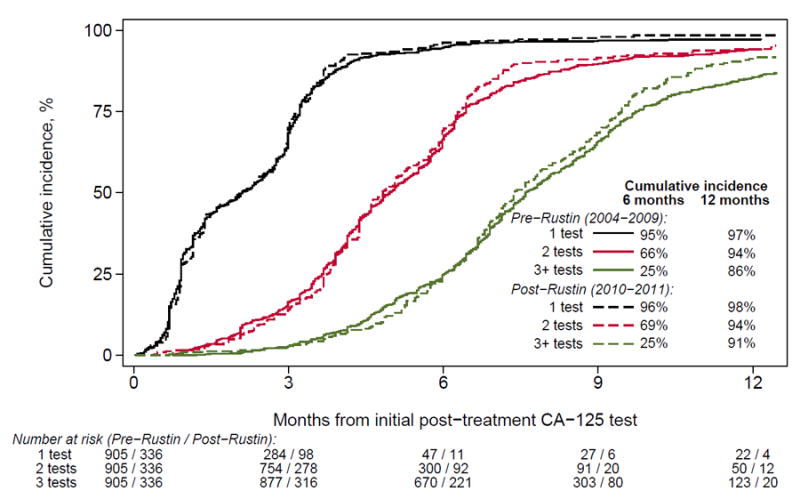
Cumulative incidence of post-treatment CA-125 in the year following remission by era of diagnosis. Patients were censored at the earliest sign of recurrence, including; doubling of CA-125, documentation of recurrence, initiation of retreatment, last follow-up, and death.
Factors Associated with Testing
Patient characteristics associated with CA-125 testing are shown in Supplemental eTable 1. There was no difference in testing rates among patients who had a CA-125 of <35 at diagnosis, compared with those who had a CA-125 ≥35 (cumulative incidence: 94% vs. 95%, P=.86). Institutions differed in their rates of testing at 6 months, although the absolute differences were small (range: 93% to 98%, P<.001). CA-125 testing did not vary significantly by diagnosis year (range 2004-2011: 94% to 96%, P=.95).
Patient characteristics associated with CT scans after completion of treatment are shown in Supplemental eTable 2. Patients diagnosed with advanced stage disease were more likely to have CT scans, compared with patients diagnosed with early stage disease. There were also significant differences in CT scan utilization among institutions (range at 6 months: 49% to 81%, P<.001). However, use of CT scans did not differ significantly by year (P=.29). We found no evidence of change in CT scan use pre- vs. post-Rustin within institutions (data not shown).
Time to retreatment
Figure 2 shows the cumulative incidence of the time to retreatment after a CA-125 doubling before and after 2009. The median time to retreatment was 2.8 months before 2009 and 3.5 months after (P=.40); 71% of patients re-initiated treatment within 6 months during both periods. In adjusted analyses, patients diagnosed with advanced stage disease had shorter times to retreatment compared with stage I disease (AOR=6.54, 95% CI=3.31-12.95), although there was no significant difference in the time to retreatment by era overall.
A sensitivity analysis was performed to compare the use of CA-125, CT scans and the time to retreatment before and after the date of study publication (October 2010) instead of the ASCO plenary presentation (June 2009), and there were no significant differences in our findings. In a second sensitivity analysis, we examined whether individual institutions responded differently to the Rustin study results, and found substantive differences in the time to retreatment by institution (Figure 5). Specifically, while there was no change in the time to retreatment within 4 institutions (Institution Group 1: median time to treatment before and after 2009: 3.9 months, log rank p=.70), there was evidence of a delayed time to retreatment within two institutions (Institution Group 2: median time to retreatment before and after 2009: 1.4 months vs. 3.2 months, log rank p=.01). As shown Supplemental eTable 3 (see model 2), an interaction analysis confirmed that patients treated at Institutions in Group 2 were more likely to be re-treated earlier than patients treated within Institution Group 1 before 2009 (HR 1.69, p<.001), but not after (HR=1.06, p=.69).
Figure 5.
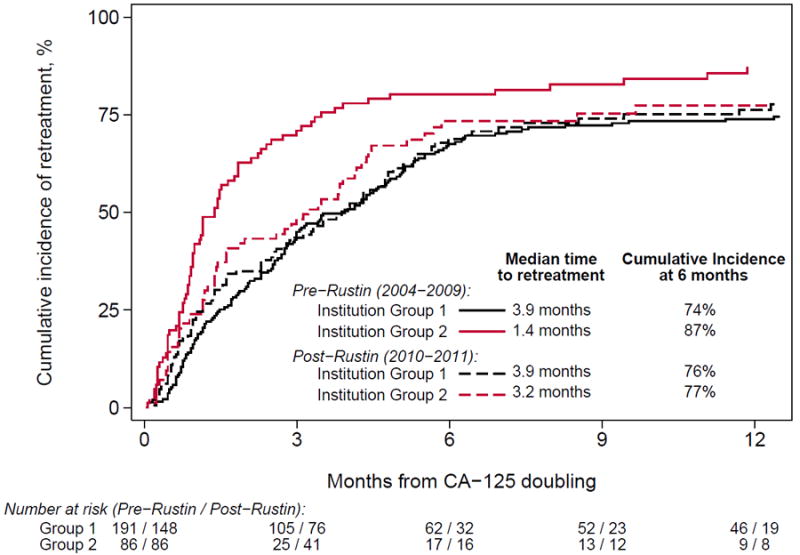
Cumulative incidence of time to retreatment after doubling of CA-125 by era of diagnosis, separately for institutions grouped according to empirical practice patterns. Patients were censored at last follow-up and death. Two practice patterns were observed: in group 1 (N=4 institutions), the time to retreatment was similar both pre- and post-Rustin (log rank P=.70); in group 2 (N=2 institutions), the time to retreatment was longer post-Rustin (log rank P=.01).
Economic Impact
The mean and median number of CA-125 tests during 12-month surveillance period was 4.6 and 4.0, while the mean and median number of CT scans was 1.7 and 1.0. The mean cost of testing using 2016 Medicare national reimbursement rates over a 1-year period was $1,056.49 per patient (CA-125 tests: $130.41 + CT Scans: $926.08). Assuming that 15,329 women achieve a clinical remission annually, the total cost of annual surveillance testing for this group is approximately $16,194,647/year ($1,999,029/year for CA-125 tests and $14,195,618 for CT scans). If the number of surveillance CT scans is increased to 3 (i.e. for one-third of patients in this study) the total cost of annual surveillance testing increases to $1,747.65 per patient with estimated population cost of $26,789,377.
Discussion
In this study, we found similar rates of surveillance testing at six academic centers before and after the presentation of an RCT which demonstrated that the use of surveillance CA-125 testing for early detection of recurrent disease decreased patients’ quality of life without improving their overall survival.5 After 2009, >90% of patients had ≥3 CA-125 tests over a 12-month period, suggesting that the Rustin study did not change practice at six NCCN academic centers in spite of widespread discussion.12-15 These results extend those from a prior study which employed hypothetical scenarios to survey United States (US) gynecologic oncologists about their surveillance practice patterns,16 and several single-institution retrospective studies.17-19
There are several reasons why the Rustin trial results may not have been widely embraced. National guidelines did not consistently advise against surveillance testing.6,7 Many patients received single-agent platinum for treatment of recurrent disease, instead of doublet therapy; thus survival outcomes may not reflect the US standard of care.14 CA-125 testing may also offer other benefits not examined in the Rustin study. Specifically patients and clinicians may be reluctant to stop CA-125 testing because it may offer a relatively inexpensive, patient-centered approach to maximize treatment options by identifying recurrent disease before irreversible disease complications ensue which might limit opportunities for clinical trials eligibility or secondary cytoreductive surgery.20-23 To that end, continued testing may be a rational, values-based decision to “do everything possible” to guard against the worst-case scenario. Alternatively, patients and physicians may share a bias towards therapeutic action. Earlier studies have documented that data from RCT supporting the adoption of new treatments and/or technologies change practice more rapidly than those supporting the de-adoption of existing practices.24,25
We hypothesized that physicians might delay the time to retreatment with chemotherapy as a strategy to minimize harm while continuing to monitor patients closely for disease complications. Although our overall results demonstrated no differences in the time to retreatment in the pre- and post-Rustin periods, we did observe differences by institution. Specifically, while four institutions had similar—and somewhat delayed--times to re-treatment throughout, two institutions demonstrated an increase in time to retreatment in the post-Rustin era. This was the only evidence of a change in practice patterns we observed. Notably, this delay post-Rustin brought these two institutions in line with the other four.
To date, few studies have examined the use of CT scans for surveillance testing in ovarian cancer. Although one small study found that patients who underwent routine CT scans had improved overall survival,26 another documented no difference.19 Although NCCN and SGO guidelines state CT scans should be used only when clinically indicated,6,7 and the Choosing Wisely® campaign explicitly states that providers should avoid routine radiographic surveillance,3 we found that two-thirds of patients had at least one CT scan within 6 months, while one-third of patients had ≥3 CT scans within 12 months of stopping treatment. Of note, there was significant variation in use of CT scans between institutions, suggesting that this may be a reasonable target for improving value. Future studies should prospectively examine whether CT scans improve survival, given the significant variation and costs associated with this practice.
There is a growing focus on the costs of cancer care as physicians and payors try to maximize value in cancer care.1-3,27 Others have estimated the cost of ovarian cancer surveillance by modeling the NCCN guidelines, including costs of CA-125 tests, associated labs, physician visits and CT scans.17 Our findings extend these studies by quantifying the costs of actual surveillance testing at six cancer centers. Our data demonstrate that women undergo CA-125 tests approximately every three months and have ≥1 CT scan within the first year after entering remission. This results in an estimated mean population cost for 1 year of ovarian cancer surveillance testing of $16,194,647 per year, predominantly due to radiographic imaging. If the number of CT scans is increased to three (1/3 of patients received ≥3 CT scans), this calculation increases by $10 million dollars per year. Of note, these are conservative estimates, including only the costs of the tests, without consideration of expenditures on laboratory tests, physician visits, diagnostic evaluations, or societal costs.
There are several limitations to our analysis. We were not able to definitively distinguish between CA-125 tests and imaging ordered in response to patients’ symptoms versus surveillance testing in asymptomatic patients, despite careful audits of medical records. We expect that our estimates are conservative, however, because some patients received care at outside facilities which we could not capture. We also restricted the surveillance window to include the time between the first normal CA-125 and a CA-125 doubling, excluding “baseline” CT scans obtained after chemotherapy and CT scans performed after a doubling of the CA-125. Therefore, our results may underestimate actual surveillance testing. The analysis is also limited to six comprehensive cancer centers through 2012 and may not be representative of practices outside of academic institutions or in later time periods. Finally, the cost calculations are rough population estimates, reliant upon assumptions of the number of women entering surveillance annually.
In closing, we found that CA-125 tests and CT scans are routinely employed in ovarian cancer patients who are in clinical remission. Although a RCT demonstrated that surveillance testing results in worse quality of life without improvements in survival, our results demonstrate that the recommendation to avoid routine surveillance testing has not been adopted into clinical practice in the US. Similarly, although the routine use of CT scans has been strongly discouraged by guideline committees, CT scans appear to be routinely utilized at significant cost. These practices have significant, but poorly understood psychosocial and cost implications, and no benefit on survival to date. Future studies should examine which patient populations benefit most from surveillance testing to improve the value of cancer care in this patient population.
Supplementary Material
Figure 3.
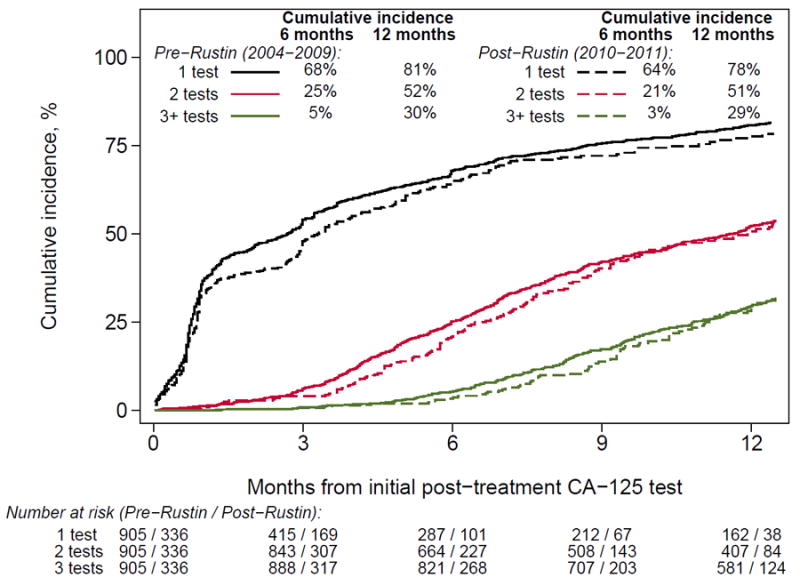
Cumulative incidence of post-treatment and CT scans in the year following remission by era of diagnosis. Patients were censored at the earliest sign of recurrence, including; doubling of CA-125, documentation of recurrence, initiation of retreatment, last follow-up, and death.
Figure 4.
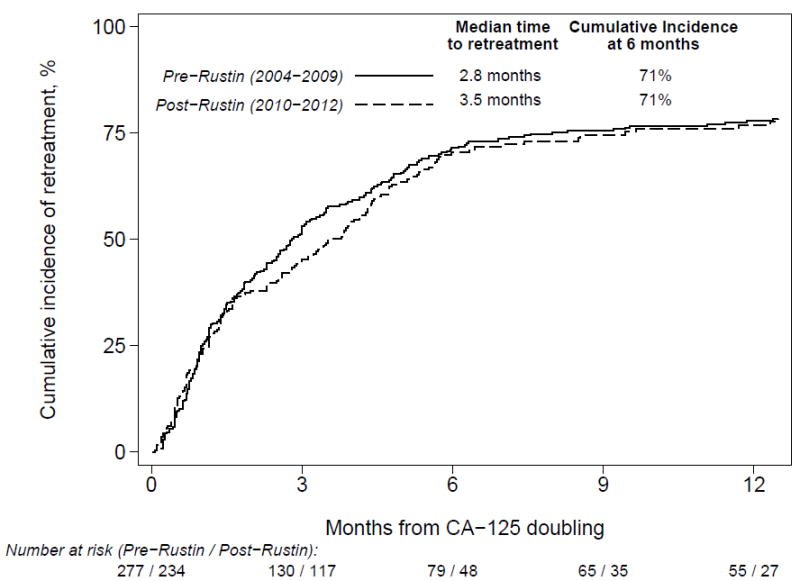
Cumulative incidence of time to retreatment after doubling of CA-125 by era of diagnosis with all institutions combined (log rank P=.40). Patients were censored at last follow up and death.
Acknowledgments
The authors would like to acknowledge the following people for their contributions to this project: Jane C. Weeks, MD MSc, who was involved in the conception of this study and design of the database, but tragically did not live to see its completion; all of the study participants; the Ovarian Cancer Outcomes Consortium for material and administrative support; and the National Comprehensive Cancer Center Network for providing partial support for the outcomes database infrastructure and management. Project support was obtained from the National Cancer Institute K07 CA166210 (Wright), the Cancer Prevention and Research Institute of Texas RP140020 (Meyer). The funding organizations had no input into the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Authorship contributions: Wright and Cronin had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Esselen, Cronin, Wright
Analysis and interpretation of data: Esselen, Cronin, Wright
Drafting of the manuscript: Esselen, Wright
Critical revision of the manuscript for important intellectual content: All authors
Final approval of the manuscript: All authors
The following authors have confirmed that there are no potential conflicts of interest or disclosures to report pertaining to this submission: Katharine M. Esselen, Angel Cronin, Kristen Bixel, David E. Cohn, Michaela Cristea, Jennifer J. Griggs, Charles F. Levenback, Gina Mantia-Smaldone, Ursula A. Matulonis, Joyce C. Niland, Charlotte Sun, and Alexi A. Wright.
Disclosure Statement: The remaining authors have the following disclosures: Michael A. Bookman (McKesson, US Oncology Research, Genentech, Boehringer Ingelheim, Astra Zeneca, AbbVie, Sanofi, Novartis, Immunogen, Endocyte, Gradalis), Robert A. Burger (Amgen, Boehringer Ingelheim, Genentech, Janssen Pharmaceuticals, Oxygene, Roche), David M. O’Malley (AstraZeneca, Genentech, Janssen Pharmaceuticals, Eisai), Larissa A. Meyer (AstraZeneca, TRM Oncology)
References
- 1.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011 Jan 19;103(2):117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnipper LE, Davidson NE, Wollins DS, et al. American Society of Clinical Oncology Statement: A Conceptual Framework to Assess the Value of Cancer Treatment Options. J Clin Oncol. 2015 Aug 10;33(23):2563–2577. doi: 10.1200/JCO.2015.61.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.SGO - Routine imaging for cancer surveillance. Choosing Wisely. [February 8, 2016]; http://www.choosingwisely.org/clinician-lists/society-gynecologic-oncology-routine-imaging-for-surveillance-gynecologic-cancer/
- 4.Rustin GJ, vdB M. A randomized trial in ovarian cancer (OC) of early treatment of relapse based on CA125 level alone versus delayed treatment based on conventional clinical indicators (MRC OV05/EORTC 55955 trials) J Clin Oncol. 2009;27(18s) abstr 1. [Google Scholar]
- 5.Rustin GJ, van der Burg ME, Griffin CL, et al. Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet. 2010 Oct 2;376(9747):1155–1163. doi: 10.1016/S0140-6736(10)61268-8. [DOI] [PubMed] [Google Scholar]
- 6.Morgan RJ, Jr, Alvarez RD, Armstrong DK, et al. Ovarian cancer, version 2.2013. Journal of the National Comprehensive Cancer Network : JNCCN. 2013 Oct 1;11(10):1199–1209. doi: 10.6004/jnccn.2013.0142. [DOI] [PubMed] [Google Scholar]
- 7.Salani R, Backes FJ, Fung MF, et al. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. American journal of obstetrics and gynecology. 2011 Jun;204(6):466–478. doi: 10.1016/j.ajog.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Bristow RE, Powell MA, Al-Hammadi N, et al. Disparities in ovarian cancer care quality and survival according to race and socioeconomic status. J Natl Cancer Inst. 2013 Jun 5;105(11):823–832. doi: 10.1093/jnci/djt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 10.Overview of the Medicare Physician Fee Schedule Search. [January 19, 2016]; https://www.cms.gov/apps/physician-fee-schedule/overview.aspx.
- 11.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015 Jan-Feb;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 12.Early treatment based on rising CA125 did not improve survival in recurrent ovarian cancer. [February 8, 2016]; http://www.healio.com/hematology-oncology/gynecologic-cancer/news/online/%7Ba252b6b9-c487-4a54-9d86-5405ec8f9798%7D/early-treatment-based-on-rising-ca125-did-not-improve-survival-in-recurrent-ovarian-cancer.
- 13.CA125 to Monitor for Relapse in Epithelial Ovarian Cancer. ASCO Connection. [February 8, 2016]; https://connection.asco.org/magazine/current-controversies-oncology/ca125-monitor-relapse-epithelial-ovarian-cancer.
- 14.Chitale R. Monitoring ovarian cancer: CA125 trial stirs controversy. J Natl Cancer Inst. 2009 Sep 16;101(18):1233–1235. doi: 10.1093/jnci/djp316. [DOI] [PubMed] [Google Scholar]
- 15.Bast RC., Jr CA 125 and the detection of recurrent ovarian cancer: a reasonably accurate biomarker for a difficult disease. Cancer. 2010 Jun 15;116(12):2850–2853. doi: 10.1002/cncr.25203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harmandayan GZ, Gao F, Mutch DG, Virgo KS, Gibb RK, Johnson FE. Ovarian cancer patient surveillance after curative-intent initial treatment. Gynecol Oncol. 2011 Feb;120(2):205–208. doi: 10.1016/j.ygyno.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong A, Otvos B, Singh S, Debernardo R. Evaluation of the cost of CA-125 measurement, physical exam, and imaging in the diagnosis of recurrent ovarian cancer. Gynecol Oncol. 2013 Dec;131(3):503–507. doi: 10.1016/j.ygyno.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Rettenmaier CR, Rettenmaier NB, Wojciechowski T, et al. The utility of routine follow-up procedures in the surveillance of uterine cancer: a 20-year institutional review. Oncology. 2010;79(3-4):262–268. doi: 10.1159/000322502. [DOI] [PubMed] [Google Scholar]
- 19.Gadducci A, Fuso L, Cosio S, et al. Are surveillance procedures of clinical benefit for patients treated for ovarian cancer?: A retrospective Italian multicentric study. Int J Gynecol Cancer. 2009 Apr;19(3):367–374. doi: 10.1111/IGC.0b013e3181a1cc02. [DOI] [PubMed] [Google Scholar]
- 20.Parker PA, Kudelka A, Basen-Engquist K, Kavanagh J, de Moor J, Cohen L. The associations between knowledge, CA125 preoccupation, and distress in women with epithelial ovarian cancer. Gynecol Oncol. 2006 Mar;100(3):495–500. doi: 10.1016/j.ygyno.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 21.Jordens CF, Morrell B, Harnett P, Hobbs K, Mason C, Kerridge IH. Cancergazing? CA125 and post-treatment surveillance in advanced ovarian cancer. Soc Sci Med Nov. 2010;71(9):1548–1556. doi: 10.1016/j.socscimed.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 22.Ganz P. Beyond the Scans: What to Look for and How. [January 25, 2016];2016 [Internet]; Podcast. Available from: http://meetinglibrary.asco.org/content/117414?media=vm.
- 23.Markman M, Petersen J, Belland A, Burg K. CA-125 monitoring in ovarian cancer: patient survey responses to the results of the MRC/EORTC CA-125 Surveillance Trial. Oncology. 2010;78(1):1–2. doi: 10.1159/000292352. [DOI] [PubMed] [Google Scholar]
- 24.Soulos PR, Yu JB, Roberts KB, et al. Assessing the impact of a cooperative group trial on breast cancer care in the medicare population. J Clin Oncol. 2012 May 10;30(14):1601–1607. doi: 10.1200/JCO.2011.39.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCormick B, Ottesen RA, Hughes ME, et al. Impact of guideline changes on use or omission of radiation in the elderly with early breast cancer: practice patterns at National Comprehensive Cancer Network institutions. Journal of the American College of Surgeons. 2014 Oct;219(4):796–802. doi: 10.1016/j.jamcollsurg.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Tanner EJ, Chi DS, Eisenhauer EL, Diaz-Montes TP, Santillan A, Bristow RE. Surveillance for the detection of recurrent ovarian cancer: survival impact or lead-time bias? Gynecol Oncol. 2010 May;117(2):336–340. doi: 10.1016/j.ygyno.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Young RC. Value-Based Cancer Care. N Engl J Med. 2015 Dec 31;373(27):2593–2595. doi: 10.1056/NEJMp1508387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


