Abstract
A 5-year old Massachusetts resident developed hard tick-borne relapsing fever caused by Borrelia miyamotoi. A partially engorged Ixodes scapularis tick was removed from her scalp and identified as infected with B. miyamotoi using PCR. Two weeks later, she developed an illness compatible with B. miyamotoi infection that included fatigue and recurrent fever. The diagnosis was confirmed by B. miyamotoi seroconversion.
Keywords: Borrelia miyamotoi, relapsing fever, child, hard tick, Ixodes scapularis
INTRODUCTION
Hard tick relapsing fever is caused by Borrelia miyamotoi, a recently discovered pathogen which is transmitted by the Ixodes species of ticks that also transmit the causative agents of Lyme disease, babesiosis, and anaplasmosis.1–5 Two large case series and a number of individual B. miyamotoi cases have been reported from Russia, the United States, Holland, and Japan but all cases have been in adults.4–13 We recently encountered the disease in a 5-year old child in Massachusetts who was bitten by a B. miyamotoi-infected tick and subsequently developed a relapsing febrile illness.
CASE REPORT
In late April, 2015 a 5-year old female resident of Northampton, Massachusetts was bitten on the scalp by a tick that was identified 72 hours later as a partially engorged adult female Ixodes scapularis (Laboratory of Medical Zoology, Amherst, MA) that was infected by Borrelia miyamotoi. Borrelia miyamotoi DNA was amplified from the tick by PCR, whereas Anaplasma phagocytophilum, Babesia microti, and Borrelia burgdorferi DNA were not detected. Two weeks later the patient developed intermittent fever of 38.4° C to 40.5° C. She was seen by her pediatrician on the third day of illness. There were no infectious exposures at home or in her preschool, no recent travel, and no history of rash, joint pain, headache or muscle pains. Past medical history was unremarkable. The child had an oral temperature of 39.4° C. The examination was otherwise normal except for a small red papule on the scalp just above the left ear and bilateral posterior cervical adenopathy. Laboratory data obtained that day included a CBC showing a white blood cell count of 2,600/mm3 with 18% band forms, 23% polymorphonuclear leukocytes, 47% lymphocytes, 10% monocytes, 1% eosinophils, and 1% basophils; Hgb 12.9 g/dL, Hct 35.4%, and platelets 84,000/µL. Other tests included CRP 0.52 mg/L; LDH 494 U/L, AST 119 U/L, ALT 83 U/L; and total protein 5.9 g/dL. No spirochetes, Anaplasma, Babesia, or Plasmodium were visualized on thin blood smear. PCR of blood for B. miyamotoi, B. microti, and A. phagocytophilum DNA were all negative. No IgM or IgG antibodies against B. miyamotoi GlpQ antigen or B. burgdorferi antigens were detected in serum. The child was given ibuprofen and the symptoms resolved by day 5 of illness.
Twelve days after the onset of the initial symptoms, she developed rhinorrhea and cough that progressively worsened so that three days later she had difficulty breathing. On physical examination she was alert with an oral temperature of 37.9° C, heart rate of 148, respiratory rate of 30, and oxygen saturation of 95%. Expiratory wheezes were noted in both lung fields. Albuterol was administered by nebulizer and she significantly improved but rales and inspiratory wheezes were noted in the left lower lobe. A chest radiograph showed a left retrocardiac density consistent with left lower lobe pneumonia. She was treated with azithromycin for five days, albuterol nebulizers as needed, and a fluticasone propionate inhaler for two weeks. A repeat blood sample on day 30 after the initial onset of symptoms revealed IgM and IgG antibody reactive against the B. miyamotoi GlpQ antigen by Western blot (Figure 1) but no reactivity against B. burgdorferi antigens. B. miyamotoi DNA was not amplified in a serum sample. The leukopenia, thrombocytopenia and elevated liver enzymes had resolved. No further therapy was administered. The child has remained afebrile one year after the initial febrile illness.
Figure 1. Immunoblot of the B. miyamotoi GlpQ.
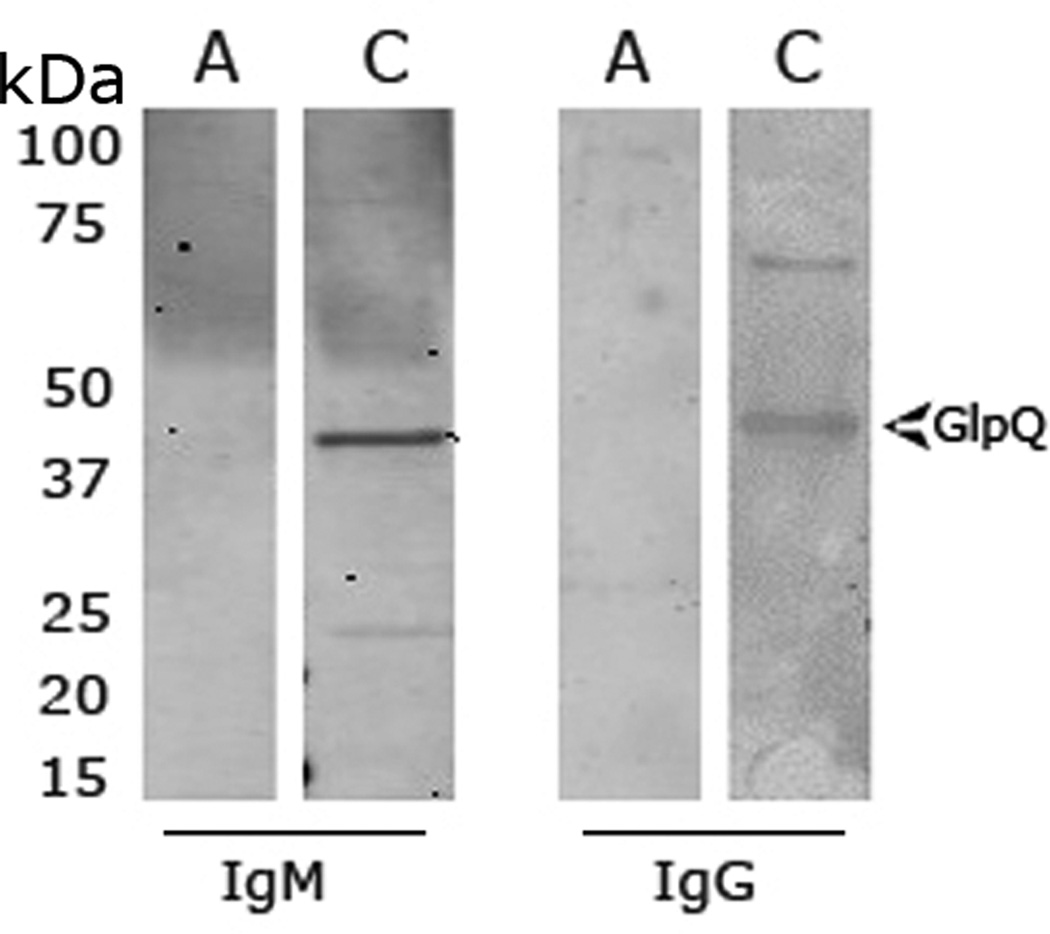
Sera specimens obtained during the patient’s acute illness and at one-month convalescence were tested using a B. miyamotoi GlpQ ELISA followed by immunoblot. An acute serum sample (A) reacted against the GlpQ protein in the ELISA assay but was negative by Western blots for both IgM and IgG. A convalescent serum (C) was positive by ELISA and also exhibited IgM and IgG reactivity to GlpQ. The arrows indicate the GlpQ (~39kDa) protein band. The molecular masses (kDa) based on the Precision-plus protein standards (BioRad, CA) are indicated.
LABORATORY ASSAYS
Borrelia miyamotoi PCR
We used a B. miyamotoi PCR as previously described to amplify B. miyamotoi DNA from sera.2 The Taqman assay for B. miyamotoi in whole tick extracts to detect a specific glpQ gene fragment was performed using the primers and probes: miyamotoi-F: 5-GACATAGTTCTAACAAAGGACAATATTCC-3, miyamotoi-R: 5-TCCGTTTTCTCTAGCTCGATTGG-3, and miyamotoi-Probe: HEX-TGCACGACCCAGAAATTGACACAACCACAA-BHQ-1. Forty cycles of 15s denaturation at 95°C and 1 min annealing-extension at 60°C was performed in 20 µl volumes using the Brilliant II QPCR Master Mix in a Stratagene MX3000P QPCR System.
Borrelia miyamotoi antibody
Sera were analyzed for B. miyamotoi antibody as previously described.7 For Western blot, replicate rGlpQ strips were individually incubated with human serum at a 1:250 dilution in PBS, washed and incubated with Infrared dye (IRdye 800 CW) goat anti-human IgG (LiCor Biosciences, NE) or biotinylated goat anti-human IgM (Sigma-Aldrich, MO) and IR 680 CW-Streptavidin (LiCor Biosciences, NE). Bound antibodies were detected with a LiCor Odyssey gel imaging scanner.
Borrelia burgdorferi antibody
We used a whole-cell sonicate ELISA and Western blot two-tier assay as described.7 Specimens were considered positive according to the criteria of the US Centers for Disease Control and Prevention (http://www.cdc.gov/lyme/diagnosistesting/LabTest/TwoStep/index.html).
DISCUSSION
We report hard tick relapsing fever due to B. miyamotoi in a 5-year old child residing in a B. miyamotoi enzootic region in western Massachusetts. To our knowledge, this is the first description of hard-tick relapsing fever in a child. The recurrent fever and pneumonia cannot be proven to be a consequence of B. miyamotoi even though it was coincident with the patient’s seroconversion. The respiratory abnormalities may have been due to another infectious agent and reactive airway disease, however, no one else in the family experienced respiratory illness. If in fact the pneumonia was a result of B. miyamotoi infection, this may be the first report of B. miyamotoi-associated pneumonia. Pulmonary consolidation, wheezing, and ARDS have been described in patients experiencing louse-borne and soft tick-borne relapsing fever.14–20
Relapsing fever can be caused by spirochetes transmitted by soft ticks or body lice.13–14 In 1994 B. miyamotoi was discovered in Japan in a hard-bodied Ixodes tick species that also transmits the agents of Lyme disease, babesiosis, and anaplasmosis.1–2,13 Human cases were first reported in 2011 in Russia and subsequently in the US, Europe, and Japan.4–13 The frequency of human B. miyamotoi infection in the northeastern US appears to be comparable to that of A. phagocytophilum and B. microti, the causative agents of anaplasmosis and babesiosis, respectively.6–7,11 Clinical findings of B. miyamotoi infection generally are non-specific and include fever, fatigue, headache, chills, myalgia, arthralgia and nausea.4–11 Recurrent febrile episodes lasting two to five days with periods of wellness in between are a distinctive feature.4,12 In two large case series, the frequency of fever relapse was four and 10 percent, respectively, but might have been greater if these patients had not been treated with doxycycline or ceftriaxone shortly after the diagnosis was established.4,12 Two elderly immunocompromised patients have been reported with meningoencephalitis.5,8 Although hard tick relapsing fever can cause severe disease requiring hospital admission, the full clinical spectrum remains to be defined.4,12
The diagnosis of B. miyamotoi requires residency or travel to an area enzootic for the infection, which appears to exist wherever Lyme disease is endemic.13 Laboratory evidence of B. miyamotoi infection includes identification of the spirochete on thin blood smear or in cerebrospinal fluid (CSF) of patients with neurologic disease. Unlike B. burgdorferi sensu stricto, relapsing fever spirochetes typically can be visualized on blood smear.13,21 A diagnosis of B. miyamotoi infection can be confirmed by amplification of B. miyamotoi DNA in blood or CSF using PCR and/or a four-fold rise in B. miyamotoi antibody titer in convalescent sera.5–7
The diagnosis in this case was established by the clinical presentation and documentation of seroconversion to B. miyamotoi GlpQ after the bite of a B. miyamotoi-infected tick. Feeding ticks can be evaluated for tick-borne pathogens using PCR, however, pathogen identification does not mean that transmission of infection has occurred.22 None the less, tick testing does provide information about exposure risk, can alert health care personnel to the possibility of a specific tick-borne infection, and might support the diagnosis, as was the case in our patient.23 We did not detect B. miyamotoi DNA in blood samples obtained on days three and 30 of illness. The duration of B. miyamotoi spirochetemia is uncertain and the patient had returned to her normal state of health by day 30. B. miyamotoi infection is known to resolve with the same antibiotics used for Lyme disease (doxycycline, amoxicillin, or ceftriaxone) administered for 7 to 14 days.4–6,8–10
Lyme disease and other tick-borne diseases are common in young children so it is likely that hard tick-relapsing fever will occur in children in this age group. Health care workers who practice in Lyme disease endemic areas should be aware of the possibility of hard tick-relapsing fever in children.
Acknowledgments
We thank Linda Bockenstedt, M.D. for performing the B. miyamotoi PCR on the human specimens and Cecilia Dumouchel for helping with the B. miyamotoi antibody assays.
Funding source: The authors acknowledge support by the National Institutes of Health (1R56AI114859-01 to P.J.K. and S.N.) and the Gordon and Llura Gund Foundation (P.J.K.).
Footnotes
Disclosures: The authors have no conflicts of interest or funding to disclose.
REFERENCES
- 1.Fukunaga M, Takahashi Y, Tsuruta Y, et al. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Internat J System Bacteriol. 1995;45:804–810. doi: 10.1099/00207713-45-4-804. [DOI] [PubMed] [Google Scholar]
- 2.Scoles GA, Papero M, Beati L, Fish D. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001;1:21–34. doi: 10.1089/153036601750137624. [DOI] [PubMed] [Google Scholar]
- 3.Barbour AG, Bunikis J, Travinsky B, et al. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. American J Trop Med Hyg. 2009;81:1120–1131. doi: 10.4269/ajtmh.2009.09-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Platonov AE, Karan LS, Kolyasnikova NM, et al. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816–1823. doi: 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gugliotta JL, Goethert HK, Berardi VP, Telford SR., 3rd Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. New Engl J Med. 2013;368:240–245. doi: 10.1056/NEJMoa1209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krause PJ, Narasimhan S, Wormser GP, et al. Human Borrelia miyamotoi Infection in the United States. New Engl J Med. 2013;368:291–293. doi: 10.1056/NEJMc1215469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krause PJ, Narasimhan S, Wormser GP, et al. Borrelia miyamotoi sensu lato seroreactivity and seroprevalence in the northeastern United States. Emerg Infect Dis. 2014;20:1183–1190. doi: 10.3201/eid2007.131587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hovius JW, de Wever B, Sohne M, et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet. 2013;382:658. doi: 10.1016/S0140-6736(13)61644-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdri HR, Gugliotta JL, Berardi VP, et al. Borrelia miyamotoi infection presenting as human granulocytic anaplasmosis: a case report. Ann Intern Med. 2013;159:217. doi: 10.7326/0003-4819-159-1-201307020-00005. [DOI] [PubMed] [Google Scholar]
- 10.Sato K, Takano A, Konnai S, et al. Human infections with Borrelia miyamotoi, Japan. Emerg Infect Dis. 2014;20:1391–1393. doi: 10.3201/eid2008.131761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jahfari S, Herremans T, Platonov AE, et al. High seroprevalence of Borrelia miyamotoi antibodies in forestry workers and individuals suspected of human granulocytic anaplasmosis in the Netherlands. New Microbes New Infect. 2014;2:144–149. doi: 10.1002/nmi2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molloy PJ, Telford SR, 3rd, Chowdri HR, et al. Borrelia miyamotoi disease in the United States: a case series. Ann Intern Med. 2015;21(163):91–98. doi: 10.7326/M15-0333. [DOI] [PubMed] [Google Scholar]
- 13.Krause PJ, Fish D, Narasimhan S, Barbour AG. Borrelia miyamotoi infection in nature and in humans. Clin Microbiol Infect. 2015;21:631–639. doi: 10.1016/j.cmi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dworkin MS, Schwan TG, Anderson DE, Jr, Borchardt SM. Tick-borne relapsing fever. Infect Dis Clin North Am. 2008;22:449–468. doi: 10.1016/j.idc.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salih SY, Mustafa D, Abdel Wahab SM, Ahmed MA, Omer A. Louse-borne relapsing fever: I. A clinical and laboratory study of 363 cases in the Sudan. Trans R Soc Trop Med Hyg. 1977;71:43–48. doi: 10.1016/0035-9203(77)90206-1. [DOI] [PubMed] [Google Scholar]
- 16.Borgnolo G, Hailu B, Ciancarelli A, Almaviva M, Woldemariam T. Louse-borne relapsing fever. A clinical and an epidemiological study of 389 patients in Asella Hospital, Ethiopia. Trop Geogr Med. 1993;45:66–69. [PubMed] [Google Scholar]
- 17.Borgnolo G, Denku B, Chiabrera F, Hailu B. Louse-borne relapsing fever in Ethiopian children: a clinical study. Ann Trop Paediatr. 1993;13:165–171. doi: 10.1080/02724936.1993.11747641. [DOI] [PubMed] [Google Scholar]
- 18.Melkert PW. Fatal-Jarisch Herxheimer reaction in a case of relapsing fever misdiagnosed as lobar pneumonia. Trop Geogr Med. 1987;39:92–93. [PubMed] [Google Scholar]
- 19.Morb Mortal Weekly Report. Acute respiratory distress syndrome in persons with tickborne relapsing fever- three states 2004–2005. 2007;56:1073–1076. [PubMed] [Google Scholar]
- 20.Badger MS. Tick Talk: Unusually Severe Case of Tick-Borne Relapsing Fever With Acute Respiratory Distress Syndrome—Case Report and Review of the Literature. Wilderness Environmental Med. 2008;19:280–286. doi: 10.1580/07-WEME-CR-140.1. [DOI] [PubMed] [Google Scholar]
- 21.Pritt BS, Mead P, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. 2016 doi: 10.1016/S1473-3099(15)00464-8. www.thelancet.com/infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nadelman RB, Nowakowski J, Fish D, et al. Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. New Engl J Med. 2001;345:79–84. doi: 10.1056/NEJM200107123450201. [DOI] [PubMed] [Google Scholar]
- 23.Hofhuis A, Herremans T, Notermans DW, et al. A prospective study among patients presenting at the general practitioner with a tick bite or erythema migrans in the Netherlands. Plos one. 2013;8:e64361. doi: 10.1371/journal.pone.0064361. [DOI] [PMC free article] [PubMed] [Google Scholar]


